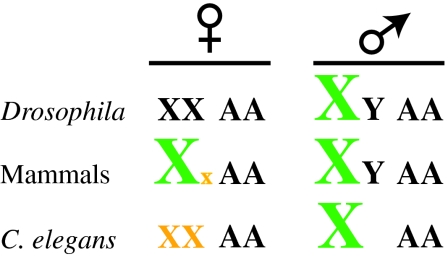Summary
Dosage compensation is the crucial process that equalizes gene expression from the X chromosome between males (XY) and females (XX). In Drosophila, the male-specific lethal (MSL) ribonucleoprotein complex mediates dosage compensation by upregulating transcription from the single male X chromosome approximately twofold. A key challenge is to understand how the MSL complex distinguishes the X chromosome from autosomes. Recent studies suggest that this occurs through a multi-step targeting mechanism that involves DNA sequence elements and epigenetic marks associated with transcription. This review will discuss the relative contributions of sequence elements and transcriptional marks to the complete pattern of MSL complex binding.
Introduction
The genetic control of sex determination is often associated with dimorphic sex chromosomes (see Glossary, Box 1). In the XY system, females are homogametic (XX), whereas males are heterogametic (XY) (see Glossary, Box 1). Although originally homologous to the X chromosome, the Y chromosome has degenerated over time, creating an imbalance in X-linked gene products between the two sexes. Dosage compensation mechanisms have evolved to equalize X-linked gene expression between males and females, thereby ensuring the appropriate balance of X-chromosomal and autosomal gene products in each sex (Charlesworth, 1996).
Box 1. Glossary
- Autosomes
Non-sex chromosomes.
- Chromocenter
Densely staining body in Drosophila polytene chromosomes where under-replicated heterochromatic regions from all chromosomes converge.
- Dimorphic sex chromosomes
Sex chromosomes that have two distinct forms, i.e. the X and the Y.
- Heterogametic sex
The sex that has different sex chromosomes, i.e. XY males.
- Homogametic sex
The sex that has identical sex chromosomes, i.e. XX females.
- Polytene chromosomes
Aligned chromosome fibers from the salivary glands of Drosophila larvae that are formed by multiple rounds of DNA replication without cell division. Euchromatic regions are present in as many as 1024 copies, whereas heterochromatin is generally under-replicated.
Dosage compensation has been best studied in worms, flies and mammals, revealing three distinct strategies for equalizing X chromosome expression (Lucchesi et al., 2005) (Fig. 1). The modulation of X-linked gene expression is essential for viability. In Drosophila males, dosage compensation globally upregulates expression from the single X chromosome twofold. In one step, this strategy equalizes X-linked gene expression between males and females and restores the balance between the X chromosome and autosomes (see Glossary, Box 1). By contrast, mammalian dosage compensation, also known as X-inactivation, silences gene expression from one of the two X chromosomes in females (Box 2). Dosage compensation in C. elegans also occurs in the homogametic sex (XX animals are hermaphrodites), but acts on both X chromosomes to downregulate gene expression twofold (Box 2). Although these mechanisms equalize X-linked gene expression between the sexes in C. elegans and mammals, X chromosome expression would be effectively half that of autosomes if these mechanisms stood alone. Therefore, in C. elegans and mammals, X chromosome repression in homogametic animals is thought to be accompanied by a twofold upregulation in the expression of the X chromosome(s) in both sexes, thereby restoring the balance between the X chromosome and autosomes (Gupta et al., 2006; Nguyen and Disteche, 2006). Even though the individual strategies differ, the dosage compensation machineries in these organisms share a central problem: how to distinguish the X chromosome(s) from autosomes? In a more general sense, this represents a fundamental challenge in genomics: how do chromatin regulators recognize their targets within a complex genome?
Fig. 1.
Dosage compensation strategies. Different organisms have evolved distinct dosage compensation strategies. In Drosophila, expression from the single X chromosome present in males is upregulated ∼twofold. In mammals, gene expression from one of the two X chromosomes present in females is silenced (a process also known as X-inactivation), whereas in the C. elegans XX sex, expression from both X chromosomes is halved. In mammals and C. elegans, a twofold upregulation of the X chromosomes in both sexes probably accompanies repression in XX animals to balance transcription between the X chromosome(s) and autosomes. Upregulated chromosomes are in green; chromosomes subject to transcriptional enhancement and repression are in yellow; the letter `A' denotes autosomes.
Box 2. Targeting dosage compensation in mammals and C. elegans
In placental mammals, X-inactivation requires the 17 kb non-coding Xist RNA, which coats the inactive X chromosome (Xi) to initiate silencing (Clemson et al., 1996). Parallels with Drosophila suggest that the evolution of long non-coding RNAs might be a general strategy for marking the X chromosome for dosage compensation. Xist spreading is restricted to the chromosome from which it is expressed (Lee et al., 1996; Herzing et al., 1997; Wutz and Jaenisch, 2000). Ectopic Xist transgenes on autosomes can spread and induce silencing to varying extents. The reduced efficiency of autosomal spreading is consistent with a model in which the X chromosome is enriched for `relay' or `booster' elements, such as long interspersed nuclear elements (LINEs), that facilitate Xist spreading (reviewed by Lyon, 2003). Even though the mechanism for spreading is unknown, Xist is thought to serve as a scaffold on the Xi to recruit repressive Polycomb complexes and to form a specialized silencing compartment within the nucleus (Chaumeil et al., 2006; Zhao et al., 2008).
The dosage compensation complex (DCC) in C. elegans modestly represses transcription from the X chromosome and is structurally related to the 13S condensin complex, which is required for chromosome condensation and segregation during mitosis and meiosis (reviewed by Meyer, 2005). Similar to the situation in Drosophila, discrete elements dispersed along the length of the X chromosome are proposed to mediate X chromosome recognition and to nucleate spreading to additional sites. recruitment element on X (reX) sites are sufficient for ectopic DCC recruitment (Csankovszki et al., 2004; McDonel et al., 2006). Crucial DNA motifs in reX sites are only modestly enriched on the X chromosome, but motif clustering may underlie the distinction of the X chromosomes from autosomes (McDonel et al., 2006; Ercan et al., 2007). A second set of sites are proposed to function as `way stations' that facilitate spreading, similar to LINEs in mammals (Blauwkamp and Csankovszki, 2009). ChIP-on-chip analysis suggests that the DCC favors binding to the 5′ ends of active genes, rather than displaying the 3′ bias seen with the Drosophila male-specific lethal (MSL) complex (Ercan et al., 2007).
This review will focus on the dosage compensation machinery in Drosophila, known as the male-specific lethal (MSL) complex, and on how this ribonucleoprotein complex recognizes the male X chromosome. The giant polytene chromosomes (see Glossary, Box 1) from larval salivary glands, consisting of up to 1024 copies of each chromosome aligned in register, have provided a powerful system for investigating MSL localization. Recently, high-resolution technologies have enabled the identification of MSL targets at the molecular level. Together, these approaches are beginning to uncover a complex X chromosome targeting mechanism that involves the recognition of DNA elements and transcriptionally active genes. Here, we explore the relative contributions of sequence elements and transcriptional activity to the complete pattern of MSL complex binding, and discuss how the individual RNA and protein components of the complex contribute to this process.
Dosage compensation in Drosophila: components and models
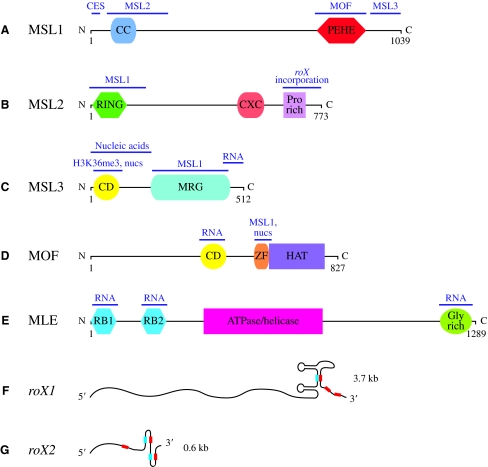
The MSL complex, which is essential for male viability, consists of at least five protein subunits and two non-coding RNAs (ncRNAs). These components co-localize to hundreds of discrete sites along the male X chromosome (Lucchesi et al., 2005) (Figs 2 and 3; Table 1). MSL1 and MSL2 form a core protein complex that targets a subset of sites on the X chromosome. (Fig. 3A,B). However, the protein components MSL3 (Fig. 3C), Males absent on the first (MOF) (Fig. 3D) and Maleless (MLE) (Fig. 3E), as well as the ncRNAs RNA on the X (roX) 1 and 2 (Fig. 3F,G), are required for the full MSL localization pattern on the X chromosome (Palmer et al., 1994; Lyman et al., 1997; Gu et al., 1998; Meller and Rattner, 2002). roX1 and roX2 are encoded on the X chromosome and are functionally redundant for male viability, despite significant differences in sequence and size (Amrein and Axel, 1997; Meller et al., 1997; Meller and Rattner, 2002). MLE and MOF are enzymes: MLE is an RNA/DNA helicase related to human RNA helicase A; MOF [also known as K-acetyltransferase (KAT) 8] is a histone acetyltransferase that specifically modifies histone H4 at lysine 16 (H4K16) (Hilfiker et al., 1997; Lee et al., 1997; Akhtar and Becker, 2000; Smith et al., 2000).
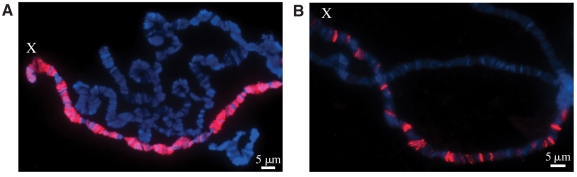
Fig. 2.
Localization of the MSL complex on the X chromosome. Drosophila polytene chromosomes from the salivary glands of third instar larvae, labeled with an antibody specific for male-specific lethal (MSL) 2 (red) and with Hoechst to stain DNA (blue). (A) The MSL complex component MSL2 localizes specifically to the X chromosome (X) in wild-type males. (B) Ectopically expressed MSL2 recognizes a subset of sites in msl3 mutant females. msl3 mutant male larvae display a similar pattern, but are too unhealthy for optimal cytology. Images courtesy of Art Alekseyenko and Andrey Gortchakov.
Fig. 3.
MSL complex components. (A) The MSL1 protein serves as the scaffold for the MSL complex, and its N-terminus is required for chromatin entry site (CES) recognition (Scott et al., 2000; Morales et al., 2004; Li et al., 2005). The coiled coil (CC) domain and PEHE domain (named for its characteristic amino acid composition) are shown (Marin, 2003). (B) The RING finger motif of the MSL2 protein is involved in interactions with MSL1, whereas its C-terminus (including a proline-rich domain) is required for the efficient incorporation of RNA on the X (roX) RNAs into the MSL complex (Copps et al., 1998; Li et al., 2008). MSL2 also contains a conserved cysteine-rich CXC domain of unknown function (Marin, 2003). (C) MSL3 interacts with MSL1 through its MRG domain, whereas its chromodomain (CD) is implicated in binding nucleosomes (nucs), nucleic acids and H3K36me3 (Morales et al., 2005; Buscaino et al., 2006; Sural et al., 2008). (D) The histone acetyltransferase Males absent on the first (MOF) and MSL3 interact with RNA in vitro (Akhtar et al., 2000). The histone acetyltransferase (HAT) domain of MOF is adjacent to a zinc-finger (ZF) domain that is required for MSL1 interaction and nucleosome binding (Akhtar and Becker, 2000; Smith et al., 2000; Akhtar and Becker, 2001; Morales et al., 2004). (E) Maleless (MLE) is an RNA/DNA helicase with multiple RNA-interaction domains, including its N-terminal double-stranded RNA-binding (RB) motifs and its C-terminal glycine-rich domain (Lee et al., 1997; Izzo et al., 2008). (F,G) The roX box elements (red) and inverse roX boxes (blue) are proposed to mediate the formation of alternative secondary structures in the non-coding RNAs roX1 and roX2 (Park et al., 2007; Kelley et al., 2008; Park et al., 2008).
Table 1.
MSL complex components
| Component | Protein/RNA type | Mutant phenotype | Proposed basis for chromosomal phenotype | References |
|---|---|---|---|---|
| MSL1 | Scaffold for MSL complex assembly | Male lethality; no X chromosome recognition | MSL1 and MSL2 are required together for CES recognition; MSL1 is unstable in the absence of MSL2 | (Palmer et al., 1994; Lyman et al., 1997; Chang and Kuroda, 1998; Scott et al., 2000; Morales et al., 2004) |
| MSL2 |
RING finger protein |
MSL2 is the limiting MSL component in males |
(Palmer et al., 1994; Kelley et al., 1995; Lyman et al., 1997) |
|
| MSL3 | Chromodomain protein | Chromodomain required for spreading | (Palmer et al., 1994; Buscaino et al., 2006; Sural et al., 2008) | |
| MOF | H4K16 acetyltransferase | Male lethality; partial MSL complex at CES | Histone acetyltransferase activity required for spreading | (Hilfiker et al., 1997; Gu et al., 1998; Akhtar and Becker, 2000; Gu et al., 2000; Smith et al., 2000) |
| MLE |
RNA/DNA helicase; prefers dsRNA or RNA/DNA hybrid substrates |
Helicase activity required for spreading |
(Palmer et al., 1994; Lee et al., 1997; Gu et al., 2000; Izzo et al., 2008; Morra et al., 2008) |
|
| roX1 | Non-coding RNAs; encoded on the X chromosome | Male lethality in double mutant; MSL complex relocalized to chromocenter | Internal redundancy within each RNA contributes to X chromosome specificity and spreading | (Meller and Rattner, 2002; Stuckenholz et al., 2003; Park et al., 2007; Kelley et al., 2008; Park et al., 2008) |
| roX2 |
CES, chromatin entry site; dsRNA, double-stranded RNA.
H4K16 acetylation by MOF is linked to transcriptional upregulation. Like other histone acetylation marks, H4K16 acetylation stimulates transcription on chromatin templates (Akhtar and Becker, 2000), and the MSL complex is required for the enrichment of this modification on the Drosophila male X chromosome (Lucchesi et al., 2005). H4K16 acetylation also has special properties: it antagonizes the ISWI family of ATP-dependent chromatin remodeling enzymes and is the only histone modification known to decondense chromatin structure globally (Corona et al., 2002; Shogren-Knaak et al., 2006; Robinson et al., 2008). Therefore, by depositing this histone mark, the MSL complex is thought to enhance transcription along the male X chromosome.
An alternative view is that the MSL complex acts in response to the increased expression of all chromosomes caused by monosomy of the X chromosome (the inverse dosage effect) (Birchler et al., 2003). The central idea of this model is that the MSL complex sequesters transcriptional activators such as MOF to the X chromosome to prevent upregulation of the autosomes, thus passively limiting increased expression to the X chromosome. However, the depletion of msl2 was recently shown to decrease X-linked expression with little effect on autosomes. Therefore, the model that the primary role of the MSL complex is to enhance transcription of genes on the X chromosome appears more plausible at present (Hamada et al., 2005; Straub et al., 2005a). It remains possible that the incorporation of MOF into the MSL complex limits the free pool of MOF that is available for MSL-independent functions at autosomal targets (Bhadra et al., 1999; Kind et al., 2008).
MSL targets: from polytenes to high resolution
Dosage compensation in Drosophila females is prevented by the repression of MSL2, the limiting component of the MSL complex. Ectopic expression of msl2 in females is sufficient to induce MSL complex localization to the X chromosomes and dosage compensation, suggesting that females carry all of the information necessary for MSL targeting (Kelley et al., 1995). This finding implies that specific sequence elements are associated with the X chromosome that distinguish it from other chromosomes. The sequence composition of the X chromosome is distinct from that of autosomes in its enrichment for simple repeat sequences (Stenberg et al., 2005; Gallach et al., 2007); however, their functional relevance in dosage compensation is unknown.
Cytological studies using polytene chromosomes have revealed a largely invariant pattern of MSL staining, consistent with a sequence-based model of MSL targeting (Kotlikova et al., 2006). In some cases, however, subtle changes in MSL staining were detected over developmental time and in different tissues (Sass et al., 2003). Specifically, the insertion of enhancer sequences on the X chromosome recruited the MSL complex to ectopic loci only in the presence of the corresponding transcriptional activator, providing the first evidence that transcription might be involved in attracting the MSL complex to sites along the X chromosome.
High-resolution analysis of MSL binding on the X chromosome
The recent molecular identification of MSL targets has provided novel insight into the mechanisms by which the MSL complex recognizes discrete sites along the X chromosome. Chromatin immunoprecipitation to enrich for MSL-associated DNA fragments was coupled with microarrays (a technique known as ChIP-on-chip) to determine the distribution of the MSL complex along chromatin at high resolution. The MSL complex was found to be specifically enriched on the X chromosome in embryos, larval salivary glands, and in two `male' cell lines, consistent with cytology (Alekseyenko et al., 2006; Gilfillan et al., 2006; Legube et al., 2006).
In contrast to many known transcriptional regulators, the MSL complex does not bind to promoters, nor does it generally bind over large domains along the X chromosome. Instead, the MSL complex binds specifically to genes, broadly associating with the middle and 3′ ends of active genes on the X chromosome (Alekseyenko et al., 2006; Gilfillan et al., 2006). Even though the mechanism of transcriptional enhancement is currently unknown, the localization of the MSL complex suggests that it acts downstream of initiation, potentially at the level of elongation or of the recycling of RNA polymerase II (RNAP II) back to the promoter for reinitiation (Smith et al., 2001; Alekseyenko et al., 2006; Gilfillan et al., 2006).
Although all ChIP-on-chip studies published to date report preferential MSL binding to active genes on the X chromosome, the extent of this correlation varies between studies, and two different interpretations have been put forward to explain how this localization is achieved. The sequence model posits that DNA elements play a gene-specific role in recruiting the MSL complex. In two studies, sequence motifs were derived from the MSL localization pattern, although these were not predictive of the full pattern of MSL binding (Gilfillan et al., 2006; Legube et al., 2006). On the basis of a third study, in which no sequence elements specific to MSL binding sites were identified, the transcription model was proposed instead, which postulates that the MSL complex localizes along the X chromosome by recognizing features of active genes (Alekseyenko et al., 2006).
The sequence and transcription models are not mutually exclusive. As described below, there is a general consensus that sequence-dependent high-affinity sites promote the distinction between the X chromosome and autosomes. Following the recognition of the X chromosome, the MSL complex may spread to active genes on the X chromosome by recognizing marks of transcription and possibly additional DNA elements. The roles of sequence elements and transcriptional marks in targeting the MSL complex to the X chromosome and in its spreading along this chromosome are considered in turn.
Targeting the MSL complex to the X chromosome
For over a decade, it has been known that partial MSL complexes can recognize a subset of sites along the X chromosome. No binding to the X chromosome is detected in msl1 or msl2 mutant backgrounds. In the absence of msl3, mle or mof, however, MSL1 and MSL2 co-localize to a reproducible set of ∼35-70 sites on the X chromosome (Palmer et al., 1994; Lyman et al., 1997; Gu et al., 1998) (Fig. 2B). A similar set of sites is observed when the availability of the MSL complex is limited, which suggests that they constitute high-affinity MSL binding sites (Kelley et al., 1997; Demakova et al., 2003). Termed chromatin entry sites (CESs), these were postulated to be the locations of MSL complex assembly and/or nucleation sites that enable the MSL complex to access the X chromosome (Lyman et al., 1997; Kelley et al., 1999). Interestingly, the roX1 and roX2 loci were the first of these sites to be identified, based on their ability to recruit the MSL complex to an autosomal insertion in an msl3 mutant background (Kelley et al., 1999).
MSL complex assembly along nascent roX RNAs
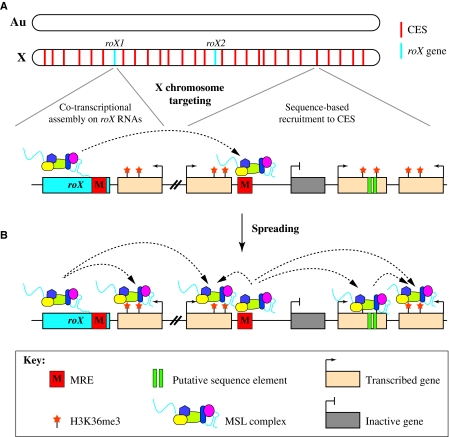
The fact that the ncRNAs that are associated with the MSL complex are themselves encoded on the X chromosome suggests that the roX loci play a role in X chromosome recognition (Fig. 4A). One model is that the roX RNAs are incorporated into the MSL complex as they are synthesized. This idea of co-transcriptional MSL complex assembly at roX genes is supported by autosomal roX transgenes that nucleate ectopic bidirectional spreading of the MSL complex over 1 Mb flanking their site of insertion (Kelley et al., 1999). The frequency and the extent of local spreading are highly sensitive to MSL1 and MSL2 levels, to the number of roX loci in the genome and to the level of roX transcription (Park et al., 2002; Demakova et al., 2003; Oh et al., 2003; Kelley et al., 2008).
Fig. 4.
Two-step model for MSL complex targeting. (A) The MSL complex specifically recognizes the X chromosome (X), not autosomes (Au), by co-transcriptional assembly at roX genes (blue) and by recognition of MSL recognition elements (MREs) at chromatin entry sites (CESs, red). Dashed lines indicate the possibility that roX RNAs are assembled into the MSL complex before the complex targets other CESs. (B) Spreading from these sites requires H3K36me3 (stars) and perhaps other features of active genes. It has been demonstrated that the MSL complex can recognize active genes (tan) that lack X chromosome-specific sequences. However, there is evidence that MSL binding might be reinforced in some cases by the presence of additional sequence elements (green bars). Dashed lines indicate hypothetical paths of MSL spreading, although other paths are possible. Spreading might involve a linear scanning mechanism, or it may reflect a capture-and-release mechanism in three-dimensional space. In either case, the MSL complex is able to `skip over' inactive genes (gray).
However, roX RNAs can integrate into the MSL complex and facilitate the recognition of the X chromosome even when encoded on an autosome: autosomal roX transgenes can rescue the viability and MSL targeting defects of males that lack roX RNAs (roX-) (Meller and Rattner, 2002). High-level expression of an autosomal roX transgene favors movement of the MSL complex to the X chromosome, whereas low-level expression favors local spreading flanking the insertion site (Kelley et al., 2008). It has been proposed that co-transcriptional assembly of a complete MSL complex favors local retention of the complex, whereas partial complexes that diffuse away complete their assembly in the nucleoplasm and are then recruited to the X chromosome (Park et al., 2002; Oh et al., 2003; Kelley et al., 2008).
Even though the presence of roX genes on the X chromosome is not necessary for MSL localization, we suspect that their location is not a coincidence. roX genes on the X chromosome may influence the efficiency of MSL targeting by assembling the complex co-transcriptionally (Deng and Meller, 2008). Alternatively, the location of the roX RNAs on the X chromosome might have played a more crucial role early in the evolution of Drosophila dosage compensation, before the acquisition of a sufficient number of DNA targeting sequence elements (Box 3).
Box 3. Evolution of dosage compensation in Drosophila
How do dosage compensation systems evolve to compensate for the progressive loss of genes from the Y chromosome? The targets of the male-specific lethal (MSL) complex on the X chromosome are thought to be acquired gene-by-gene or block-by-block (Bachtrog, 2006). An intermediate stage of this process is observed in Drosophila miranda, in which only some regions along a neo-X-chromosome have acquired MSL binding and dosage compensation (Bone and Kuroda, 1996; Marin et al., 1996). It will be interesting to compare the sequence composition of the neo-X-chromosome in regions that do and do not undergo dosage compensation. Furthermore, identification of MSL targets at high resolution on the neo-X-chromosome should provide key insights into chromatin entry site (CES) function and into the evolution of spreading on the X chromosome.
What about the evolution of MSL proteins? MSL3, Maleless (MLE) and Males absent on the first (MOF), which are required for spreading and chromatin modification in Drosophila, are ancient proteins (Marin and Baker, 2000; Sanjuan and Marin, 2001). However, the key initial targeting subunits, MSL1 and MSL2, are less conserved (Marin, 2003), suggesting that targeting has evolved to serve diverse purposes. Human MSL complexes composed of MSL1, MSL2, MSL3 and MOF (MYST1) have been identified, but their downstream targets are not known (Smith et al., 2005; Mendjan et al., 2006).
Given that dosage compensation is essential for the viability of Drosophila males, one would predict that the MSL complex is subject to purifying selection in Drosophilids. However, two recent studies have demonstrated surprising evidence for adaptive evolution in MSL protein-coding genes in the Drosophila melanogaster lineage and have proposed the co-evolution of MSL binding sites, an idea supported by the recent analysis of three CESs (Levine et al., 2007; Rodriguez et al., 2007; Bachtrog, 2008). Altered specificity of the MSL complex has been hypothesized to play a role in male hybrid inviability in Drosophila (Orr, 1989).
Sequence-dependent MSL targeting to the X chromosome
The ability of the MSL complex to recognize the roX- X chromosome when roX RNAs are encoded on an autosome implies that additional mechanisms exist to identify the X chromosome. Fine mapping of the roX1 and roX2 CESs has identified short fragments (<250 bp) that are sufficient for recruiting partial MSL complexes in an autosomal context (Kageyama et al., 2001; Park et al., 2003). These fragments are thought to function as DNA elements that recruit the MSL complex, although it is unknown whether the MSL complex recognizes these sequences directly or if an accessory factor is required. Conserved sequence elements required for CES function have been identified (Park et al., 2003), and a handful of other CESs have been mapped (Oh et al., 2004; Dahlsveen et al., 2006; Gilfillan et al., 2007), but a genomic approach was necessary to derive unifying principles that characterize CESs (Alekseyenko et al., 2008; Straub et al., 2008). ChIP-on-chip of MSL2 in msl3 mutant embryos identified a set of 150 CESs distributed along the X chromosome (Alekseyenko et al., 2008). ChIP of the intact MSL complex from `male' Clone8 cells followed by Solexa sequencing of the enriched DNA fragments (ChIP-seq) revealed that the tallest peaks of MSL enrichment overlap strikingly with CESs, suggesting that CESs are bona fide high-affinity MSL binding sites. Motif searches revealed a 21 bp GA-rich (or TC-rich) motif, named the MSL recognition element (MRE) (Alekseyenko et al., 2008), which includes sequence motifs found in previously mapped CESs (Park et al., 2003; Gilfillan et al., 2007). At least one copy of the MRE is present in 91% of CESs (Alekseyenko et al., 2008). Importantly, in functional assays, MRE mutations abolish MSL recruitment, whereas scrambling the surrounding sequences has no effect. Therefore, MREs are both necessary and sufficient for MSL recruitment in the contexts that have been tested, demonstrating that they play a key role in MSL recognition of the X chromosome.
Independently, 130 high-affinity sites (HASs) were mapped in the `male' SL2 cell line by ChIP-on-chip of MSL1 and MSL2 following the depletion of msl3, mof or mle by RNAi, or under mild cross-linking conditions (Straub et al., 2008). Strikingly, 69% of HASs overlap with the CES identified in msl3 mutant embryos, and HASs are enriched for sequence motifs, including a GA-rich element highly similar to the MRE. These high-resolution mapping studies collectively identify many more CESs/HASs than have been predicted previously from cytology (Fig. 2B), and these are probably still underestimates. We now predict that as many as 240-300 of these sites are located on the X chromosome (Alekseyenko et al., 2008; Sural et al., 2008), perhaps accounting for the incomplete overlap between the datasets.
The identification of a functional DNA sequence element at high-affinity MSL binding sites is a significant advance towards understanding how the MSL complex distinguishes the X chromosome from autosomes (Fig. 4A). However, these motifs are only ∼twofold enriched on the X chromosome as compared with autosomes, and many motifs on the X chromosome are not associated with a CES/HAS (Alekseyenko et al., 2008; Straub et al., 2008). Therefore, additional features, such as nucleosome occupancy, are likely to distinguish the MREs that are recognized in vivo. Indeed, CESs/HASs are characterized by histone depletion, in contrast to unoccupied MREs on the X chromosome (Alekseyenko et al., 2008; Straub et al., 2008). Furthermore, CESs/HASs appear to be generally associated with active chromatin domains, thereby recruiting the MSL complex to regions of the X chromosome enriched for its preferred targets - transcribed genes. Restricting MRE searches to active genes, characterized by histone H3 lysine 36 trimethylation (H3K36me3), increases the proportion of MREs found in CESs and enriches MREs on the X chromosome fourfold over autosomes (Alekseyenko et al., 2008). The clustering of MREs in three-dimensional space and the binding of accessory proteins might also influence which MREs are recognized. Further experiments are required to determine the relative contributions of these factors in allowing the MSL complex to utilize MREs to distinguish the X chromosome from autosomes.
Spreading along the X chromosome
High-affinity MSL binding sites probably play a key role not only in distinguishing the X chromosome from autosomes, but also in the spreading of the MSL complex along this chromosome. The idea of spreading is generally associated with the assembly of silencing factors along chromatin to form repressive heterochromatin domains. The term `spreading' is often used to describe the linear propagation of proteins along chromatin, as shown for the Sir proteins in S. cerevisiae, but spreading may also skip regions along the chromatin fiber (Talbert and Henikoff, 2006). In the case of the MSL complex, spreading results in the discontinuous distribution of the complex along the X chromosome, with a bias for the bodies of active genes (Fig. 4B). It is unknown whether the spreading of the MSL complex is a linear process, whereby the complex scans along chromatin and is only stabilized at active genes. Alternatively, the MSL complex may spread via a capture-and-release mechanism, whereby it samples nearby chromatin in three-dimensional space. In either scenario, the recruitment of the MSL complex to CESs generates high local concentrations of this complex along the X chromosome that drive spreading to nearby sites of lower affinity. The identification of at least 150 CESs on the X chromosome and their locations within active gene clusters suggest that spreading might be a local phenomenon (Alekseyenko et al., 2008; Straub et al., 2008). Indeed, the majority of MSL complex bound on the X chromosome is within 5-10 kb of a CES (Sural et al., 2008). The roX genes might be unique in their nucleation of long-range spreading owing to co-transcriptional assembly of the MSL complex at roX loci.
The mechanism for recognizing active genes may involve sequence elements, features of transcription, or both. At one extreme, the strict sequence or `affinities' model postulates that CESs are not qualitatively different from other MSL targets. They represent the highest affinity MSL binding sites, whereas other sites have sequences that bind to the MSL complex at lower affinities (Demakova et al., 2003; Fagegaltier and Baker, 2004; Dahlsveen et al., 2006; Legube et al., 2006). At the other extreme, CESs are considered to be distinct from other MSL binding sites because they contain DNA elements that specify the X chromosome. The transcription model proposes that after the MSL complex identifies the X chromosome, it recognizes active genes by properties of transcription, such as histone modifications or by nascent transcripts themselves (Alekseyenko et al., 2006; Larschan et al., 2007). These two models are explored below.
The affinities model: a continuum of sequences along the X chromosome
The idea that CESs are sites of MSL entry into the X chromosome was originally called into question because several regions of the X chromosome that lack cytologically mapped CESs are competent to recruit the MSL complex in an autosomal context (Fagegaltier and Baker, 2004; Oh et al., 2004; Dahlsveen et al., 2006). To explain this observation, it was proposed that these regions contain multiple low-affinity MSL binding sites that act together to recruit the MSL complex. Such sequences are expected to be degenerate and, therefore, have evaded molecular definition. Given that recent evidence suggests that there are more CESs than previously thought (Alekseyenko et al., 2008; Straub et al., 2008), it is possible that some of these X-derived fragments might actually contain a CES.
However, this is not always the case. Two X chromosome-derived transgenes that do not appear to function as CESs recruit the MSL complex in a transcription-dependent manner when inserted onto an autosome (Kind and Akhtar, 2007). A 339 bp fragment from one of these genes is capable of recruiting the MSL complex in the absence of transcription, but only when multimerized. Therefore, it was proposed that transcription increases the accessibility of X chromosome-specific sequences within genes and is no longer necessary when multiple copies of the sequence are present. This result supports the affinities model, but the nature of the sequence element has not yet been defined.
A requirement for X chromosome-specific sequence motifs in MSL spreading has also been indicated by the observation that the MSL complex binds in a wild-type pattern to X chromosome sequences translocated onto an autosome, but does not spread visibly to adjacent autosomal sequences (Fagegaltier and Baker, 2004; Oh et al., 2004). Furthermore, the MSL complex does not spread visibly to autosomal sequences translocated onto the X chromosome, indicating that autosomes lack sequences that promote MSL complex spreading. As discussed above, spreading is potentially a short-range phenomenon, indicating that high-resolution analyses will be required to assay for local spreading that might have been missed by cytology.
Evidence for a hierarchy of MSL binding sites on the X chromosome was demonstrated by changes in the number of MSL-positive sites on polytene chromosomes in response to alterations in the levels of the MSL complex (Demakova et al., 2003). Given the new estimates of CES density on the X chromosome, perhaps these variations reflect a range of CES affinities. Whereas only a small number of variable, weak autosomal sites are detectable in wild-type males, reproducible sites of autosomal binding are observed when the MSL complex is overexpressed (Demakova et al., 2003). Therefore, MSL complex levels must be carefully modulated to avoid autosomal targeting. It would be interesting to identify the autosomal binding sites molecularly to determine whether they resemble MREs.
Together, these results raise the question of how many sequences with affinity for the MSL complex exist along the X chromosome. Until these elements are better defined, this will remain an open question.
The transcription model: recognition of active genes
The localization of the MSL complex to active genes along the X chromosome suggests that the MSL complex might spread from CESs to sites of active transcription. A comparison of MSL binding in two cell lines revealed rare examples of differential binding (Alekseyenko et al., 2006). In these cases, the genes were also differentially expressed and binding correlated with gene activity, suggesting a causal link between transcription and MSL recruitment.
Additional evidence indicates that X chromosome-specific sequences are not necessary to recruit the MSL complex to active genes. The high-resolution analysis of ectopic spreading of the MSL complex to autosomal sequences that surround a roX transgene has revealed that a strong correlation exists between spreading and gene activity (Larschan et al., 2007). Even though roX genes are unique in their ability to support long-range spreading, CES insertions on an autosome have recently been shown to nucleate short-range spreading to the 3′ end of an active gene ∼10 kb away (Alekseyenko et al., 2008).
The localization of the MSL complex to active genes on the X chromosome, or on autosomes when ectopically recruited, indicates that the MSL complex recognizes features of transcribed genes. Further support for this idea came from the observation that the distribution of the MSL complex along the X chromosome strongly resembles the pattern of H3K36me3, a histone modification associated with active genes on all chromosomes (Larschan et al., 2007). SET2 [also known as K-methyltransferase (KMT) 3] is the enzyme responsible for H3K36me3 and is required for robust MSL recruitment to target genes, but not to CESs, thus distinguishing sequence-dependent from H3K36me3-dependent modes of recruitment (Larschan et al., 2007; Bell et al., 2008). These findings clearly demonstrate that sequence elements are not sufficient to recruit the MSL complex to active genes and that H3K36me3, and perhaps other features of transcription, are also involved (Fig. 4B).
The recruitment of the MSL complex to active genes prevents the inappropriate activation of repressed genes, and may explain why autosomal genes can be dosage compensated when inserted onto the X chromosome (Scholnick et al., 1983; Spradling and Rubin, 1983). One advantage of this mechanism is that any transcribed gene on the X chromosome would be dosage compensated without the need to evolve a specific sequence to recruit the complex. However, even though experimental evidence argues against a strict sequence model for MSL spreading, it is possible that genes on the X chromosome have evolved sequence elements that stabilize MSL complex binding after recruitment (Fig. 4B).
Exceptions to the rule
One prediction of a strict transcription model is that the MSL complex binds to all active genes on the X chromosome. However, differences in MSL complex and RNAP II localization have been observed in cytological and high-resolution analyses (Gilfillan et al., 2006; Kotlikova et al., 2006; Legube et al., 2006). One potential explanation for this is that the imperfect correlation between MSL binding and transcription results from the application of somewhat arbitrary thresholds. Using H3K36me3 as a proxy for transcriptional activity, an unbiased computational analysis of the data from a `male' cell line estimates that nearly 80% of active genes on the X chromosome are clearly bound by the MSL complex, whereas less than 1% are clearly unbound (Larschan et al., 2007) (S. Peng, P. J. Park and M.I.K., unpublished). The remaining genes are in an intermediate category that cannot be unambiguously defined.
Although the precise numbers are undefined, a set of transcribed genes does not recruit the MSL complex. Why are these genes skipped? Are they dosage compensated by long-range effects of MSL action, by an MSL-independent mechanism, or do they escape dosage compensation altogether? Nearly all transcribed genes on the X chromosome are dosage compensated (Gupta et al., 2006). The best-studied exception is Lsp1α, a gene expressed in the Drosophila larval fat body (the predominant immune-responsive tissue) that does not appear to recruit the MSL complex in this tissue and that escapes dosage compensation (Weake and Scott, 2007). However, an Lsp1α promoter-lacZ transgene can recruit the MSL-associated H4K16 acetylation mark in other locations on the X chromosome. Why does the endogenous Lsp1α gene fail to recruit the MSL complex? There is no evidence to suggest a repressive chromatin environment or the presence of insulators that block MSL recruitment. It has been proposed that Lsp1α lacks a sequence element necessary to recruit the MSL complex in its endogenous location. Alternatively, it is conceivable that the developmentally regulated expression of the endogenous Lsp1α gene lacks features that are required to recruit the MSL complex. For example, it is possible that some active genes are not associated with H3K36me3. In this scenario, the altered regulation of Lsp1α transgenes might account for their ability to recruit the complex. Genes like Lsp1α will need to be evaluated on a case-by-case basis to investigate the complexities of MSL recruitment.
The nature of spreading: the dynamics of MSL localization
What is the nature of MSL spreading from high-affinity MSL binding sites to the remainder of the X chromosome? As discussed above, recent evidence suggests that spreading might be a short-range phenomenon. It will be interesting to learn whether the loss of an individual CES has a local effect on MSL targeting to nearby genes. Alternatively, other CESs and lower-affinity MSL sequences might compensate for its absence. At the cytological level, removing the roX2 CES has no detectable effect on MSL localization (Meller and Rattner, 2002).
If the MSL complex generally recognizes transcribed genes, how is spreading restricted to the X chromosome? A tight regulation of MSL levels is likely to be involved (Demakova et al., 2003). Once a local concentration of the MSL complex is established on the X chromosome, how is spreading to nearby autosomal regions prevented? One possibility is that the MSL complex is confined to a subnuclear domain that prevents spreading to other chromosomes. The nuclear pore component NUP153 and the associated protein MTOR have been implicated in MSL localization to the discrete subnuclear territory of the X chromosome (Mendjan et al., 2006). An investigation into the spatial organization of the X chromosome, particularly of CESs, might provide insights into the link between the MSL complex and nuclear architecture.
Little is known about the dynamics of MSL binding to the X chromosome. The MSL complex remains associated with the X chromosome throughout mitosis, suggesting that its localization on the X chromosome might need to be established only once during development (Lavender et al., 1994; Straub et al., 2005b). Photobleaching experiments indicate that the association of MSL2 with the X chromosome territory is surprisingly stable, suggesting little MSL turnover on the X chromosome during interphase (Straub et al., 2005b). This experiment is limited to a period of several minutes, but it raises the question of whether the MSL binding pattern can change over time.
The transcription model predicts that the MSL complex can be recruited to a newly activated gene, although this has not yet been demonstrated. The induction of an autosomal roX transgene by the Gal4 activator causes a shift from ectopic local spreading on the autosome to X chromosome targeting within a few hours (Kelley et al., 2008). This result suggests that recognition of the X chromosome might be possible late in development, although a small amount of the MSL complex may preset the pattern on the X chromosome prior to roX induction. In the same experiment, the loss of the MSL complex along the autosome indicates a high turnover at these sites. Does this reflect dynamic MSL binding on the X chromosome? Alternatively, is there a qualitative difference in MSL binding on the X chromosome and on autosomes, whereby sequence elements serve to reinforce MSL binding to genes on the X chromosome and lead to lower turnover?
X chromosome targeting machinery
The results discussed above support a model in which the MSL complex first identifies the X chromosome by co-transcriptional assembly at roX genes and by recognition of DNA elements within CESs (Fig. 4A), and then spreads to active genes by recognition of a transcription-associated histone modification and possibly additional sequence elements (Fig. 4B). How does the MSL complex achieve this multi-step mechanism of targeting along the X chromosome? MSL1 serves as a platform for MSL complex assembly as it independently binds MSL2, MSL3 and MOF (Scott et al., 2000; Morales et al., 2004) (Fig. 3; Table 1). Despite its crucial role in MSL spreading, MLE appears to be only weakly associated with the MSL complex, to the extent that it has been reported to be absent from several biochemical MSL complex purifications (Copps et al., 1998; Smith et al., 2000; Mendjan et al., 2006). MLE may interact weakly with the complex via the C-terminal domain of MSL2 or through roX RNAs, as MLE, MOF and MSL3 are all implicated in RNA binding (Richter et al., 1996; Akhtar et al., 2000; Buscaino et al., 2003; Li et al., 2008). Perhaps chromatin association stabilizes these interactions, as MSL components co-localize along the polytene male X chromosome. In order to fully understand the structural organization of the MSL complex, and in particular how the roX RNAs are incorporated into the complex, biochemical order-of-assembly experiments are required.
The cytological analysis of partial MSL complexes has provided early evidence for the contributions of the different subunits to MSL complex localization. MSL1 and MSL2 are required for the association of the MSL complex with CESs, whereas the addition of MSL3, MLE and MOF is required for its spreading to the full MSL pattern (Palmer et al., 1994; Lyman et al., 1997; Gu et al., 1998). The roX RNAs also play a pivotal role in MSL targeting (Franke and Baker, 1999; Meller and Rattner, 2002). In roX- males, MSL proteins localize to a small number of sites on the X chromosome that may be related to CESs; much of the complex, however, is relocalized to the chromocenter (see Glossary, Box 1), the clustered mass of under-replicated heterochromatin from all chromosomes in Drosophila salivary glands (Meller and Rattner, 2002; Deng et al., 2005). It is intriguing to speculate that, in the absence of roX RNAs, the MSL complex is mistargeted by an aberrant association with RNAs that are derived from repetitive elements in heterochromatin.
The role of MSL proteins in X chromosome recognition and spreading
MSL1 and MSL2 are expected to be the only MSL proteins required to recognize CESs and the associated MREs. However, they lack any obvious DNA-binding domains, suggesting that MRE recognition might be mediated by other factors. The GA-rich core of the MRE suggests that the GAGA factor (GAF, encoded by Trl) might be involved (Alekseyenko et al., 2008). To date, there is little evidence to support a general role for GAF in MSL recruitment (Sun et al., 2003; van Steensel et al., 2003), although Trl mutations subtly affect MSL binding on polytene chromosomes (Greenberg et al., 2004). Perhaps GAF and other GAGA-binding proteins function redundantly to recruit the MSL complex to MREs. Additional experiments are required to determine whether partial MSL complexes are capable of sequence-specific binding to MREs or to identify factors that mediate this interaction.
An N-terminal basic region of MSL1 (amino acids 1-26) is implicated in CES recognition, but is dispensable for interactions with other MSL proteins (Li et al., 2005). This same region is necessary for the chromocenter localization of MSL complexes that form in the absence of the C-terminal domain of MSL2, which is required for the efficient incorporation of roX RNAs into the complex (Li et al., 2008). Therefore, the MSL1 N-terminal region is likely to mediate common interactions that underlie localization to these distinct targets. The adjacent region (amino acids 26-84) is required for the self-association of MSL1, which might promote the cooperative binding of the MSL complex to clustered binding sites (Li et al., 2005).
How do MSL3, MOF and MLE promote spreading along the X chromosome? MSL3 contains an N-terminal chromodomain, which is required for preferential interaction with H3K36-trimethylated nucleosomes in vitro (Sural et al., 2008). Mutations in the chromodomain disrupt spreading of the MSL complex beyond ∼1 kb from CESs in vivo, suggesting that MSL3 promotes spreading through the recognition of H3K36me3. Defects in spreading associated with chromodomain mutants were not detectable by cytology, demonstrating the importance of high-resolution approaches.
The enzymatic activities of MOF and MLE are also required for spreading (Lee et al., 1997; Gu et al., 2000). One attractive model is that H4K16 acetylation by MOF serves as a docking site that facilitates spreading along the chromatin fiber. Recently, an MSL-independent mechanism was shown to recruit MOF to the 5′ ends of active genes on all chromosomes in `male' and `female' cell lines (Kind et al., 2008). It was proposed that H4K16 acetylation at the 5′ ends of genes promotes MSL spreading to the 3′ ends of genes, although this model still requires direct testing. Even though there is no known module of the MSL complex that recognizes H4K16 acetylation, MOF activity might facilitate spreading by increasing the accessibility of MSL targets. Furthermore, MOF at the 5′ and 3′ ends of genes may facilitate the formation of a loop that promotes dosage compensation by enhancing the recycling of RNAP II to the promoter (Kind et al., 2008).
MLE binds to nucleic acids and is a double-stranded RNA and RNA/DNA helicase (Lee et al., 1997; Izzo et al., 2008). Hence, MLE might regulate the incorporation and/or conformation of roX RNAs in the MSL complex. Mutations that specifically impair the helicase activity of MLE give rise to spreading defects (Morra et al., 2008). The helicase activity of MLE might be required to unwind secondary structures associated with roX RNAs or nascent transcripts, or to displace RNA-binding proteins. Alternatively, the homology of MLE to ATPases from the SNF2 superfamily involved in chromatin remodeling indicates that MLE might act as a DNA translocase to promote spreading. Although the precise mechanisms by which MLE and MOF contribute to spreading are currently unknown, it is likely that a high-resolution analysis of the localization defects associated with mle and mof mutants will generate new insights.
The role of non-coding roX RNAs in X chromosome targeting and spreading
The non-coding roX RNAs clearly play a crucial role in targeting because the MSL complex is mislocalized to the chromocenter in their absence (Meller and Rattner, 2002). A few MSL binding sites on the X chromosome remain, but it is unclear how these sites are related to CESs (Deng et al., 2005). When MSL2 levels are severely limited, the MSL complex is only observed at four non-roX CESs, suggesting that the roX loci are not the first sites targeted by the MSL complex (Demakova et al., 2003). However, it is not known whether the roX RNAs are incorporated into the complex at this stage. In the future, the molecular identification of the roX-independent sites on the X chromosome will be important to understand the role of roX RNAs in MSL targeting.
The functions of the roX RNAs have been difficult to determine owing to internal redundancy within each RNA. However, recent advances have been made through sequence comparisons with roX RNAs from other Drosophila genomes. These studies have identified putative stem-loop structures that form in the roX RNAs and multiple short repeat elements, known as roX boxes, at the 3′ ends of roX RNAs that are important for MSL complex assembly, localization and function (Fig. 3F,G) (Stuckenholz et al., 2003; Park et al., 2007; Kelley et al., 2008; Park et al., 2008). Alternative splicing of roX2 has also been implicated in efficient incorporation into the MSL complex (Park et al., 2005).
Accessory factors involved in MSL targeting
The MSL complex requires additional factors for its targeting to the X chromosome (Table 2). As discussed above, SET2 is required for the MSL complex to efficiently target active genes on the X chromosome (Larschan et al., 2007; Bell et al., 2008), and mutations in the gene that encodes GAF, Trl, subtly alter MSL localization along the polytene X chromosome (Greenberg et al., 2004). In addition, Upstream of N-ras (UNR), an RNA-binding protein, is required for MSL localization, potentially through its association with the roX RNAs (Patalano et al., 2009). Several other proteins have been linked to the MSL complex through biochemical purifications, including the JIL-1 histone H3 serine 10 kinase, nuclear pore components, interband-associated proteins, and components of the nuclear exosome (Jin et al., 2000; Mendjan et al., 2006). Of these, only the nuclear pore protein NUP153 and the associated protein MTOR have been implicated in MSL targeting thus far (Mendjan et al., 2006). These interactions suggest that the compartmentalization of the X chromosome might promote the targeting and/or spreading of the MSL complex.
Table 2.
Accessory factors implicated in MSL targeting
| Factor | Protein type | Relevant mutant/RNAi phenotype | References |
|---|---|---|---|
| SET2 |
H3K36 methyltransferase |
Mutation/RNAi reduces MSL binding at active genes on X chromosome, not CES (ChIP of third instar larvae, SL2 cells) |
(Larschan et al., 2007; Bell et al., 2008) |
| GAF (TRL) |
GAGA-binding factor |
Mutation is associated with an increased number of MSL sites on autosomes and the loss of a single CES (immunostaining of polytenes) |
(Greenberg et al., 2004) |
| UNR |
RNA-binding protein |
Mutation/RNAi decreases MSL staining on X chromosome (immunostaining of polytenes, SL2 cells) |
(Patalano et al., 2009) |
| NUP153 | Nuclear pore protein | ||
| MTOR | Nuclear pore-associated protein | RNAi depletion disrupts MSL localization to the X chromosome (immunostaining of SL2 cells) | (Mendjan et al., 2006) |
CES, chromatin entry site; ChIP, chromatin immunoprecipitation; RNAi, RNA interference.
Other genes, including Jil-1 and supercoiling factor (scf), have been implicated in dosage compensation based on their mutant phenotypes of preferential male lethality and of abnormal X chromosome morphology (Deuring et al., 2000; Wang et al., 2001; Ebert et al., 2004; Liu et al., 2005; Spierer et al., 2005; Furuhashi et al., 2006; Zhang et al., 2006). Although these factors are not known to affect MSL targeting, their interplay with the MSL complex remains to be determined and might shed further light onto the mechanism of dosage compensation.
In the future, the identification of novel factors in MSL targeting might require additional screening approaches. An RNAi screen for factors required for MSL targeting to the X chromosome territory in `male' SL2 cells has revealed known MSL proteins and a regulator of mle splicing (Worringer and Panning, 2007). Recently, a roX2 reporter that exhibits MSL-dependent transcriptional enhancement was utilized to test for factors required for CES recruitment and dosage compensation in cell culture (Yokoyama et al., 2007). This type of strategy should be useful in order to identify novel components involved in MSL targeting and function.
Conclusions
Significant progress has been made in recent years towards understanding the mechanism by which the MSL complex recognizes the X chromosome. The utilization of high-resolution approaches has contributed greatly to these advances. The MSL complex first identifies the X chromosome by co-transcriptional assembly on roX RNAs and by sequence-specific recognition of CESs. We favor the idea that subsequent spreading of the MSL complex to active genes is largely mediated by the recognition of transcriptional marks. However, many questions still remain about how the MSL complex identifies the X chromosome and how it spreads to its active gene targets. How does the MSL complex specifically recognize MREs on the X chromosome? Are there other sequence elements that facilitate MSL recruitment or perhaps even repel the complex? Aside from H3K36me3, are there additional features of transcribed genes that the MSL complex recognizes? How do the components of the MSL complex, particularly the roX RNAs, contribute to sequence-specific binding and to the identification of active genes? What are the accessory factors that facilitate these events? How dynamic is MSL binding on the X chromosome? Can MSL recruitment be induced in response to changes in the transcriptional state of a gene and, if so, is MSL loading restricted to specific time-points, such as DNA replication?
It is critically important to continue to apply recently developed technologies to address these problems. For example, high-resolution localization analysis has enabled the detection of short-range MSL spreading along an autosome (Alekseyenko et al., 2008). Coupling high-resolution approaches to the analysis of MSL mutants can provide novel insights into the contributions of individual MSL components to the targeting of the complex. Furthermore, site-specific integration methodologies enable the comparison of mutant MSL components expressed from the same locus (Kelley et al., 2008; Sural et al., 2008). Eliminating the confounding effects of the chromatin environment is also useful for comparing the recruitment potential of X chromosome-derived fragments on the autosomes (Alekseyenko et al., 2008). In this type of experiment, it is important to remember the caveat that pairing between the autosomal insertion and the endogenous locus on the X chromosome may facilitate MSL transmission to the transgene (Kelley et al., 1999). Even though the ectopic recruitment of the MSL complex to autosomes is a stringent test of the recruitment ability of X chromosome-derived DNA fragments, high-resolution analyses are also important to investigate the behavior of autosomal or heterologous sequences in the X-chromosomal environment. Perhaps we can utilize these tools not only to describe the steady-state MSL pattern, but also to observe the establishment of MSL binding along the X chromosome over time at the molecular level. These types of experiments will trigger the next wave of discoveries regarding how the ribonucleoprotein MSL complex recognizes a complex landscape of DNA sequence and epigenetic marks to target and regulate a chromosome.
Acknowledgments
We apologize for any omissions of primary literature citation owing to space constraints. We thank members of the Kuroda laboratory, Michele Markstein and Rick Kelley for critical reading of the manuscript, Art Alekseyenko and Andrey Gortchakov for the images in Fig. 2, and Peter Becker for communications prior to publication.
The work in our laboratory is supported by the National Institutes of Health. M.E.G. is a Fellow supported by the Damon Runyon Cancer Research Foundation. Deposited in PMC for release after 12 months.
References
- Akhtar, A. and Becker, P. B. (2000). Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell 5, 367-375. [DOI] [PubMed] [Google Scholar]
- Akhtar, A. and Becker, P. B. (2001). The histone H4 acetyltransferase MOF uses a C2HC zinc finger for substrate recognition. EMBO Rep. 2, 113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar, A., Zink, D. and Becker, P. B. (2000). Chromodomains are protein-RNA interaction modules. Nature 407, 405-409. [DOI] [PubMed] [Google Scholar]
- Alekseyenko, A. A., Larschan, E., Lai, W. R., Park, P. J. and Kuroda, M. I. (2006). High-resolution ChIP-chip analysis reveals that the Drosophila MSL complex selectively identifies active genes on the male X chromosome. Genes Dev. 20, 848-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko, A. A., Peng, S., Larschan, E., Gorchakov, A. A., Lee, O. K., Kharchenko, P., McGrath, S. D., Wang, C. I., Mardis, E. R., Park, P. J. et al. (2008). A sequence motif within chromatin entry sites directs MSL establishment on the Drosophila X chromosome. Cell 134, 599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein, H. and Axel, R. (1997). Genes expressed in neurons of adult male Drosophila. Cell 88, 459-469. [DOI] [PubMed] [Google Scholar]
- Bachtrog, D. (2006). A dynamic view of sex chromosome evolution. Curr. Opin. Genet. Dev. 16, 578-585. [DOI] [PubMed] [Google Scholar]
- Bachtrog, D. (2008). Positive selection at the binding sites of the male-specific lethal complex involved in dosage compensation in Drosophila. Genetics 180, 1123-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, O., Conrad, T., Kind, J., Wirbelauer, C., Akhtar, A. and Schubeler, D. (2008). Transcription-coupled methylation of histone H3 at lysine 36 regulates dosage compensation by enhancing recruitment of the MSL complex in Drosophila melanogaster. Mol. Cell. Biol. 28, 3401-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra, U., Pal-Bhadra, M. and Birchler, J. A. (1999). Role of the male specific lethal (msl) genes in modifying the effects of sex chromosomal dosage in Drosophila. Genetics 152, 249-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., Pal-Bhadra, M. and Bhadra, U. (2003). Dosage dependent gene regulation and the compensation of the X chromosome in Drosophila males. Genetica 117, 179-190. [DOI] [PubMed] [Google Scholar]
- Blauwkamp, T. A. and Csankovszki, G. (2009). Two classes of DCC binding elements facilitate binding and spreading of the DCC along C. elegans X-chromosomes. Mol. Cell. Biol. (in press). [DOI] [PMC free article] [PubMed]
- Bone, J. R. and Kuroda, M. I. (1996). Dosage compensation regulatory proteins and the evolution of sex chromosomes in Drosophila. Genetics 144, 705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaino, A., Kocher, T., Kind, J. H., Holz, H., Taipale, M., Wagner, K., Wilm, M. and Akhtar, A. (2003). MOF-regulated acetylation of MSL-3 in the Drosophila dosage compensation complex. Mol. Cell 11, 1265-1277. [DOI] [PubMed] [Google Scholar]
- Buscaino, A., Legube, G. and Akhtar, A. (2006). X-chromosome targeting and dosage compensation are mediated by distinct domains in MSL-3. EMBO Rep. 7, 531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, K. A. and Kuroda, M. I. (1998). Modulation of MSL1 abundance in female Drosophila contributes to the sex specificity of dosage compensation. Genetics 150, 699-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, B. (1996). The evolution of chromosomal sex determination and dosage compensation. Curr. Biol. 6, 149-162. [DOI] [PubMed] [Google Scholar]
- Chaumeil, J., Le Baccon, P., Wutz, A. and Heard, E. (2006). A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev. 20, 2223-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson, C. M., McNeil, J. A., Willard, H. F. and Lawrence, J. B. (1996). XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 132, 259-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copps, K., Richman, R., Lyman, L. M., Chang, K. A., Rampersad-Ammons, J. and Kuroda, M. I. (1998). Complex formation by the Drosophila MSL proteins: role of the MSL2 RING finger in protein complex assembly. EMBO J. 17, 5409-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona, D. F., Clapier, C. R., Becker, P. B. and Tamkun, J. W. (2002). Modulation of ISWI function by site-specific histone acetylation. EMBO Rep. 3, 242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csankovszki, G., McDonel, P. and Meyer, B. J. (2004). Recruitment and spreading of the C. elegans dosage compensation complex along X chromosomes. Science 303, 1182-1185. [DOI] [PubMed] [Google Scholar]
- Dahlsveen, I. K., Gilfillan, G. D., Shelest, V. I., Lamm, R. and Becker, P. B. (2006). Targeting determinants of dosage compensation in Drosophila. PLoS Genet. 2, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakova, O. V., Kotlikova, I. V., Gordadze, P. R., Alekseyenko, A. A., Kuroda, M. I. and Zhimulev, I. F. (2003). The MSL complex levels are critical for its correct targeting to the chromosomes in Drosophila melanogaster. Chromosoma 112, 103-115. [DOI] [PubMed] [Google Scholar]
- Deng, X. and Meller, V. H. (2008). Molecularly severe roX1 mutations contribute to dosage compensation in Drosophila. Genesis 47, 49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X., Rattner, B. P., Souter, S. and Meller, V. H. (2005). The severity of roX1 mutations is predicted by MSL localization on the X chromosome. Mech. Dev. 122, 1094-1105. [DOI] [PubMed] [Google Scholar]
- Deuring, R., Fanti, L., Armstrong, J. A., Sarte, M., Papoulas, O., Prestel, M., Daubresse, G., Verardo, M., Moseley, S. L., Berloco, M. et al. (2000). The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol. Cell 5, 355-365. [DOI] [PubMed] [Google Scholar]
- Ebert, A., Schotta, G., Lein, S., Kubicek, S., Krauss, V., Jenuwein, T. and Reuter, G. (2004). Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 18, 2973-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan, S., Giresi, P. G., Whittle, C. M., Zhang, X., Green, R. D. and Lieb, J. D. (2007). X chromosome repression by localization of the C. elegans dosage compensation machinery to sites of transcription initiation. Nat. Genet. 39, 403-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagegaltier, D. and Baker, B. S. (2004). X chromosome sites autonomously recruit the dosage compensation complex in Drosophila males. PLoS Biol. 2, e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, A. and Baker, B. S. (1999). The rox1 and rox2 RNAs are essential components of the compensasome, which mediates dosage compensation in Drosophila. Mol. Cell 4, 117-122. [DOI] [PubMed] [Google Scholar]
- Furuhashi, H., Nakajima, M. and Hirose, S. (2006). DNA supercoiling factor contributes to dosage compensation in Drosophila. Development 133, 4475-4483. [DOI] [PubMed] [Google Scholar]
- Gallach, M., Arnau, V. and Marin, I. (2007). Global patterns of sequence evolution in Drosophila. BMC Genomics 8, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan, G. D., Straub, T., de Wit, E., Greil, F., Lamm, R., van Steensel, B. and Becker, P. B. (2006). Chromosome-wide gene-specific targeting of the Drosophila dosage compensation complex. Genes Dev. 20, 858-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan, G. D., Konig, C., Dahlsveen, I. K., Prakoura, N., Straub, T., Lamm, R., Fauth, T. and Becker, P. B. (2007). Cumulative contributions of weak DNA determinants to targeting the Drosophila dosage compensation complex. Nucleic Acids Res. 35, 3561-3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, A. J., Yanowitz, J. L. and Schedl, P. (2004). The Drosophila GAGA factor is required for dosage compensation in males and for the formation of the male-specific-lethal complex chromatin entry site at 12DE. Genetics 166, 279-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, W., Szauter, P. and Lucchesi, J. C. (1998). Targeting of MOF, a putative histone acetyl transferase, to the X chromosome of Drosophila melanogaster. Dev. Genet. 22, 56-64. [DOI] [PubMed] [Google Scholar]
- Gu, W., Wei, X., Pannuti, A. and Lucchesi, J. C. (2000). Targeting the chromatin-remodeling MSL complex of Drosophila to its sites of action on the X chromosome requires both acetyl transferase and ATPase activities. EMBO J. 19, 5202-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, V., Parisi, M., Sturgill, D., Nuttall, R., Doctolero, M., Dudko, O. K., Malley, J. D., Eastman, P. S. and Oliver, B. (2006). Global analysis of X-chromosome dosage compensation. J. Biol. 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, F. N., Park, P. J., Gordadze, P. R. and Kuroda, M. I. (2005). Global regulation of X chromosomal genes by the MSL complex in Drosophila melanogaster. Genes Dev. 19, 2289-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzing, L. B., Romer, J. T., Horn, J. M. and Ashworth, A. (1997). Xist has properties of the X-chromosome inactivation centre. Nature 386, 272-275. [DOI] [PubMed] [Google Scholar]
- Hilfiker, A., Hilfiker-Kleiner, D., Pannuti, A. and Lucchesi, J. C. (1997). mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 16, 2054-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo, A., Regnard, C., Morales, V., Kremmer, E. and Becker, P. B. (2008). Structure-function analysis of the RNA helicase maleless. Nucleic Acids Res. 36, 950-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Y., Wang, Y., Johansen, J. and Johansen, K. M. (2000). JIL-1, a chromosomal kinase implicated in regulation of chromatin structure, associates with the male specific lethal (MSL) dosage compensation complex. J. Cell Biol. 149, 1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama, Y., Mengus, G., Gilfillan, G., Kennedy, H. G., Stuckenholz, C., Kelley, R. L., Becker, P. B. and Kuroda, M. I. (2001). Association and spreading of the Drosophila dosage compensation complex from a discrete roX1 chromatin entry site. EMBO J. 20, 2236-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, R. L., Solovyeva, I., Lyman, L. M., Richman, R., Solovyev, V. and Kuroda, M. I. (1995). Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell 81, 867-877. [DOI] [PubMed] [Google Scholar]
- Kelley, R. L., Wang, J., Bell, L. and Kuroda, M. I. (1997). Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature 387, 195-199. [DOI] [PubMed] [Google Scholar]
- Kelley, R. L., Meller, V. H., Gordadze, P. R., Roman, G., Davis, R. L. and Kuroda, M. I. (1999). Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell 98, 513-522. [DOI] [PubMed] [Google Scholar]
- Kelley, R. L., Lee, O. K. and Shim, Y. K. (2008). Transcription rate of noncoding roX1 RNA controls local spreading of the Drosophila MSL chromatin remodeling complex. Mech. Dev. 125, 1009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind, J. and Akhtar, A. (2007). Cotranscriptional recruitment of the dosage compensation complex to X-linked target genes. Genes Dev. 21, 2030-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind, J., Vaquerizas, J. M., Gebhardt, P., Gentzel, M., Luscombe, N. M., Bertone, P. and Akhtar, A. (2008). Genome-wide analysis reveals MOF as a key regulator of dosage compensation and gene expression in Drosophila. Cell 133, 813-828. [DOI] [PubMed] [Google Scholar]
- Kotlikova, I. V., Demakova, O. V., Semeshin, V. F., Shloma, V. V., Boldyreva, L. V., Kuroda, M. I. and Zhimulev, I. F. (2006). The Drosophila dosage compensation complex binds to polytene chromosomes independently of developmental changes in transcription. Genetics 172, 963-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larschan, E., Alekseyenko, A. A., Gortchakov, A. A., Peng, S., Li, B., Yang, P., Workman, J. L., Park, P. J. and Kuroda, M. I. (2007). MSL complex is attracted to genes marked by H3K36 trimethylation using a sequence-independent mechanism. Mol. Cell 28, 121-133. [DOI] [PubMed] [Google Scholar]
- Lavender, J. S., Birley, A. J., Palmer, M. J., Kuroda, M. I. and Turner, B. M. (1994). Histone H4 acetylated at lysine 16 and proteins of the Drosophila dosage compensation pathway co-localize on the male X chromosome through mitosis. Chromosome Res. 2, 398-404. [DOI] [PubMed] [Google Scholar]
- Lee, C. G., Chang, K. A., Kuroda, M. I. and Hurwitz, J. (1997). The NTPase/helicase activities of Drosophila maleless, an essential factor in dosage compensation. EMBO J. 16, 2671-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. T., Strauss, W. M., Dausman, J. A. and Jaenisch, R. (1996). A 450 kb transgene displays properties of the mammalian X-inactivation center. Cell 86, 83-94. [DOI] [PubMed] [Google Scholar]
- Legube, G., McWeeney, S. K., Lercher, M. J. and Akhtar, A. (2006). X-chromosome-wide profiling of MSL-1 distribution and dosage compensation in Drosophila. Genes Dev. 20, 871-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, M. T., Holloway, A. K., Arshad, U. and Begun, D. J. (2007). Pervasive and largely lineage-specific adaptive protein evolution in the dosage compensation complex of Drosophila melanogaster. Genetics 177, 1959-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., Parry, D. A. and Scott, M. J. (2005). The amino-terminal region of Drosophila MSL1 contains basic, glycine-rich, and leucine zipper-like motifs that promote X chromosome binding, self-association, and MSL2 binding, respectively. Mol. Cell. Biol. 25, 8913-8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., Schiemann, A. H. and Scott, M. J. (2008). Incorporation of the noncoding roX RNAs alters the chromatin-binding specificity of the Drosophila MSL1/MSL2 complex. Mol. Cell. Biol. 28, 1252-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. P., Ni, J. Q., Shi, Y. D., Oakeley, E. J. and Sun, F. L. (2005). Sex-specific role of Drosophila melanogaster HP1 in regulating chromatin structure and gene transcription. Nat. Genet. 37, 1361-1366. [DOI] [PubMed] [Google Scholar]
- Lucchesi, J. C., Kelly, W. G. and Panning, B. (2005). Chromatin remodeling in dosage compensation. Annu. Rev. Genet. 39, 615-651. [DOI] [PubMed] [Google Scholar]
- Lyman, L. M., Copps, K., Rastelli, L., Kelley, R. L. and Kuroda, M. I. (1997). Drosophila male-specific lethal-2 protein: structure/function analysis and dependence on MSL-1 for chromosome association. Genetics 147, 1743-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon, M. F. (2003). The Lyon and the LINE hypothesis. Semin. Cell Dev. Biol. 14, 313-318. [DOI] [PubMed] [Google Scholar]
- Marin, I. (2003). Evolution of chromatin-remodeling complexes: comparative genomics reveals the ancient origin of `novel' compensasome genes. J. Mol. Evol. 56, 527-539. [DOI] [PubMed] [Google Scholar]
- Marin, I. and Baker, B. S. (2000). Origin and evolution of the regulatory gene male-specific lethal-3. Mol. Biol. Evol. 17, 1240-1250. [DOI] [PubMed] [Google Scholar]
- Marin, I., Franke, A., Bashaw, G. J. and Baker, B. S. (1996). The dosage compensation system of Drosophila is co-opted by newly evolved X chromosomes. Nature 383, 160-163. [DOI] [PubMed] [Google Scholar]
- McDonel, P., Jans, J., Peterson, B. K. and Meyer, B. J. (2006). Clustered DNA motifs mark X chromosomes for repression by a dosage compensation complex. Nature 444, 614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, V. H. and Rattner, B. P. (2002). The roX genes encode redundant male-specific lethal transcripts required for targeting of the MSL complex. EMBO J. 21, 1084-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, V. H., Wu, K. H., Roman, G., Kuroda, M. I. and Davis, R. L. (1997). roX1 RNA paints the X chromosome of male Drosophila and is regulated by the dosage compensation system. Cell 88, 445-457. [DOI] [PubMed] [Google Scholar]
- Mendjan, S., Taipale, M., Kind, J., Holz, H., Gebhardt, P., Schelder, M., Vermeulen, M., Buscaino, A., Duncan, K., Mueller, J. et al. (2006). Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol. Cell 21, 811-823. [DOI] [PubMed] [Google Scholar]
- Meyer, B. J. (2005). X-Chromosome dosage compensation. WormBook, 1-14: www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Morales, V., Straub, T., Neumann, M. F., Mengus, G., Akhtar, A. and Becker, P. B. (2004). Functional integration of the histone acetyltransferase MOF into the dosage compensation complex. EMBO J. 23, 2258-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, V., Regnard, C., Izzo, A., Vetter, I. and Becker, P. B. (2005). The MRG domain mediates the functional integration of MSL3 into the dosage compensation complex. Mol. Cell. Biol. 25, 5947-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra, R., Smith, E. R., Yokoyama, R. and Lucchesi, J. C. (2008). The MLE subunit of the Drosophila MSL complex uses its ATPase activity for dosage compensation and its helicase activity for targeting. Mol. Cell. Biol. 28, 958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, D. K. and Disteche, C. M. (2006). Dosage compensation of the active X chromosome in mammals. Nat. Genet. 38, 47-53. [DOI] [PubMed] [Google Scholar]
- Oh, H., Park, Y. and Kuroda, M. I. (2003). Local spreading of MSL complexes from roX genes on the Drosophila X chromosome. Genes Dev. 17, 1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, H., Bone, J. R. and Kuroda, M. I. (2004). Multiple classes of MSL binding sites target dosage compensation to the X chromosome of Drosophila. Curr. Biol. 14, 481-487. [DOI] [PubMed] [Google Scholar]
- Orr, H. A. (1989). Does postzygotic isolation result from improper dosage compensation? Genetics 122, 891-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, M. J., Richman, R., Richter, L. and Kuroda, M. I. (1994). Sex-specific regulation of the male-specific lethal-1 dosage compensation gene in Drosophila. Genes Dev. 8, 698-706. [DOI] [PubMed] [Google Scholar]
- Park, S. W., Kang, Y., Sypula, J. G., Choi, J., Oh, H. and Park, Y. (2007). An evolutionarily conserved domain of roX2 RNA is sufficient for induction of H4-Lys16 acetylation on the Drosophila X chromosome. Genetics 177, 1429-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. W., Kuroda, M. I. and Park, Y. (2008). Regulation of histone H4 Lys16 acetylation by predicted alternative secondary structures in roX noncoding RNAs. Mol. Cell. Biol. 28, 4952-4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y., Kelley, R. L., Oh, H., Kuroda, M. I. and Meller, V. H. (2002). Extent of chromatin spreading determined by roX RNA recruitment of MSL proteins. Science 298, 1620-1623. [DOI] [PubMed] [Google Scholar]
- Park, Y., Mengus, G., Bai, X., Kageyama, Y., Meller, V. H., Becker, P. B. and Kuroda, M. I. (2003). Sequence-specific targeting of Drosophila roX genes by the MSL dosage compensation complex. Mol. Cell 11, 977-986. [DOI] [PubMed] [Google Scholar]
- Park, Y., Oh, H., Meller, V. H. and Kuroda, M. I. (2005). Variable splicing of non-coding roX2 RNAs influences targeting of MSL dosage compensation complexes in Drosophila. RNA Biol. 2, 157-164. [DOI] [PubMed] [Google Scholar]
- Patalano, S., Mihailovich, M., Belacortu, Y., Paricio, N. and Gebauer, F. (2009). Dual sex-specific functions of Drosophila Upstream of N-ras in the control of X chromosome dosage compensation. Development 136, 689-698. [DOI] [PubMed] [Google Scholar]
- Richter, L., Bone, J. R. and Kuroda, M. I. (1996). RNA-dependent association of the Drosophila maleless protein with the male X chromosome. Genes Cells 1, 325-336. [DOI] [PubMed] [Google Scholar]
- Robinson, P. J., An, W., Routh, A., Martino, F., Chapman, L., Roeder, R. G. and Rhodes, D. (2008). 30 nm chromatin fibre decompaction requires both H4-K16 acetylation and linker histone eviction. J. Mol. Biol. 381, 816-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, M. A., Vermaak, D., Bayes, J. J. and Malik, H. S. (2007). Species-specific positive selection of the male-specific lethal complex that participates in dosage compensation in Drosophila. Proc. Natl. Acad. Sci. USA 104, 15412-15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan, R. and Marin, I. (2001). Tracing the origin of the compensasome: evolutionary history of DEAH helicase and MYST acetyltransferase gene families. Mol. Biol. Evol. 18, 330-343. [DOI] [PubMed] [Google Scholar]
- Sass, G. L., Pannuti, A. and Lucchesi, J. C. (2003). Male-specific lethal complex of Drosophila targets activated regions of the X chromosome for chromatin remodeling. Proc. Natl. Acad. Sci. USA 100, 8287-8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholnick, S. B., Morgan, B. A. and Hirsh, J. (1983). The cloned dopa decarboxylase gene is developmentally regulated when reintegrated into the Drosophila genome. Cell 34, 37-45. [DOI] [PubMed] [Google Scholar]
- Scott, M. J., Pan, L. L., Cleland, S. B., Knox, A. L. and Heinrich, J. (2000). MSL1 plays a central role in assembly of the MSL complex, essential for dosage compensation in Drosophila. EMBO J. 19, 144-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogren-Knaak, M., Ishii, H., Sun, J. M., Pazin, M. J., Davie, J. R. and Peterson, C. L. (2006). Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311, 844-847. [DOI] [PubMed] [Google Scholar]
- Smith, E. R., Pannuti, A., Gu, W., Steurnagel, A., Cook, R. G., Allis, C. D. and Lucchesi, J. C. (2000). The Drosophila MSL complex acetylates histone H4 at lysine 16, a chromatin modification linked to dosage compensation. Mol. Cell. Biol. 20, 312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E. R., Allis, C. D. and Lucchesi, J. C. (2001). Linking global histone acetylation to the transcription enhancement of X-chromosomal genes in Drosophila males. J. Biol. Chem. 276, 31483-31486. [DOI] [PubMed] [Google Scholar]
- Smith, E. R., Cayrou, C., Huang, R., Lane, W. S., Cote, J. and Lucchesi, J. C. (2005). A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol. Cell. Biol. 25, 9175-9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer, A., Seum, C., Delattre, M. and Spierer, P. (2005). Loss of the modifiers of variegation Su(var)3-7 or HP1 impacts male X polytene chromosome morphology and dosage compensation. J. Cell Sci. 118, 5047-5057. [DOI] [PubMed] [Google Scholar]
- Spradling, A. C. and Rubin, G. M. (1983). The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell 34, 47-57. [DOI] [PubMed] [Google Scholar]
- Stenberg, P., Pettersson, F., Saura, A. O., Berglund, A. and Larsson, J. (2005). Sequence signature analysis of chromosome identity in three Drosophila species. BMC Bioinformatics 6, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, T., Gilfillan, G. D., Maier, V. K. and Becker, P. B. (2005a). The Drosophila MSL complex activates the transcription of target genes. Genes Dev. 19, 2284-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, T., Neumann, M. F., Prestel, M., Kremmer, E., Kaether, C., Haass, C. and Becker, P. B. (2005b). Stable chromosomal association of MSL2 defines a dosage-compensated nuclear compartment. Chromosoma 114, 352-364. [DOI] [PubMed] [Google Scholar]
- Straub, T., Grimaud, C., Gilfillan, G. D., Mitterweger, A. and Becker, P. B. (2008). The chromosomal high-affinity binding sites for the Drosophila dosage compensation complex. PLoS Genet. 4, e1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckenholz, C., Meller, V. H. and Kuroda, M. I. (2003). Functional redundancy within roX1, a noncoding RNA involved in dosage compensation in Drosophila melanogaster. Genetics 164, 1003-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L. V., Chen, L., Greil, F., Negre, N., Li, T. R., Cavalli, G., Zhao, H., Van Steensel, B. and White, K. P. (2003). Protein-DNA interaction mapping using genomic tiling path microarrays in Drosophila. Proc. Natl. Acad. Sci. USA 100, 9428-9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sural, T. H., Peng, S., Li, B., Workman, J. L., Park, P. J. and Kuroda, M. I. (2008). The MSL3 chromodomain directs a key targeting step for dosage compensation of the Drosophila melanogaster X chromosome. Nat. Struct. Mol. Biol. 15, 1318-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert, P. B. and Henikoff, S. (2006). Spreading of silent chromatin: inaction at a distance. Nat. Rev. Genet. 7, 793-803. [DOI] [PubMed] [Google Scholar]
- van Steensel, B., Delrow, J. and Bussemaker, H. J. (2003). Genomewide analysis of Drosophila GAGA factor target genes reveals context-dependent DNA binding. Proc. Natl. Acad. Sci. USA 100, 2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Zhang, W., Jin, Y., Johansen, J. and Johansen, K. M. (2001). The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105, 433-443. [DOI] [PubMed] [Google Scholar]
- Weake, V. M. and Scott, M. J. (2007). The non-dosage compensated Lsp1alpha gene of Drosophila melanogaster escapes acetylation by MOF in larval fat body nuclei, but is flanked by two dosage compensated genes. BMC Mol. Biol. 8, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worringer, K. A. and Panning, B. (2007). Zinc finger protein Zn72D promotes productive splicing of the maleless transcript. Mol. Cell. Biol. 27, 8760-8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz, A. and Jaenisch, R. (2000). A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol. Cell 5, 695-705. [DOI] [PubMed] [Google Scholar]
- Yokoyama, R., Pannuti, A., Ling, H., Smith, E. R. and Lucchesi, J. C. (2007). A plasmid model system shows that Drosophila dosage compensation depends on the global acetylation of histone H4 at lysine 16 and is not affected by depletion of common transcription elongation chromatin marks. Mol. Cell. Biol. 27, 7865-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., Deng, H., Bao, X., Lerach, S., Girton, J., Johansen, J. and Johansen, K. M. (2006). The JIL-1 histone H3S10 kinase regulates dimethyl H3K9 modifications and heterochromatic spreading in Drosophila. Development 133, 229-235. [DOI] [PubMed] [Google Scholar]
- Zhao, J., Sun, B. K., Erwin, J. A., Song, J. J. and Lee, J. T. (2008). Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322, 750-756. [DOI] [PMC free article] [PubMed] [Google Scholar]