Summary
Mammalian taste buds have properties of both epithelial and neuronal cells, and are thus developmentally intriguing. Taste buds differentiate at birth within epithelial appendages, termed taste papillae, which arise at mid-gestation as epithelial thickenings or placodes. However, the embryonic relationship between placodes, papillae and adult taste buds has not been defined. Here, using an inducible Cre-lox fate mapping approach with the ShhcreERT2 mouse line, we demonstrate that Shh-expressing embryonic taste placodes are taste bud progenitors, which give rise to at least two different adult taste cell types, but do not contribute to taste papillae. Strikingly, placodally descendant taste cells disappear early in adult life. As placodally derived taste cells are lost, we used Wnt1Cre mice to show that the neural crest does not supply cells to taste buds, either embryonically or postnatally, thus ruling out a mesenchymal contribution to taste buds. Finally, using Bdnf null mice, which lose neurons that innervate taste buds, we demonstrate that Shh-expressing taste bud progenitors are specified and produce differentiated taste cells normally, in the absence of gustatory nerve contact. This resolution of a direct relationship between embryonic taste placodes with adult taste buds, which is independent of mesenchymal contribution and nerve contact, allows us to better define the early development of this important sensory system. These studies further suggest that mammalian taste bud development is very distinct from that of other epithelial appendages.
Keywords: Taste bud development, CreER, Shh, Wnt1Cre, Bdnf, Genetic inducible, Fate mapping, Tamoxifen, Mouse
INTRODUCTION
Taste buds are aggregates of receptor cells, which transduce chemical stimuli into neural signals to mediate the sense of taste. In mammals, lingual taste buds reside within three types of epithelial appendages, called taste papillae (Mistretta, 1991): fungiform papillae are arrayed on the anterior tongue, whereas foliate papillae and a single circumvallate papilla in rodents reside posteriorly. Taste receptor cells, like neurons, possess voltage-gated channels, generate action potentials, and release neurotransmitter onto post-synaptic nerves (Finger et al., 2005; Roper, 1992). However, unlike neurons or other receptor cell types, taste cells continually regenerate in adults (Beidler, 1953; Beidler and Smallman, 1965; Farbman, 1980; Delay et al., 1986). Furthermore, taste buds differ from other sensory receptors developmentally, arising from local epithelia rather than from neurogenic ectoderm (Barlow and Northcutt, 1995; Stone et al., 1995). Taste buds are thus unique among sensory receptor cells with characteristics of both epithelia and neurons.
Each taste bud comprises a heterogeneous population of ∼100 cells belonging to three differentiated cell types (I, II and III) that can be identified via expression of marker proteins. Type I cells, assumed to be support cells, express a specific ectoATPase, NTPDase2 (Bartel et al., 2006). Types II and III are taste receptor cells proper; type II cells express the transmembrane receptors and signaling machinery to transduce sweet, bitter and umami tastes (Clapp et al., 2001; Miyoshi et al., 2001), whereas type III cells are sour detectors (Huang et al., 2006; Huang et al., 2008) and function as relay cells (Yang et al., 2000a; Roper, 2007). These three taste cell types are maintained by continuous renewal from the progenitor population, the characteristics of which remain unclear. However, birthdating analyses suggest that taste cells arise from either adjacent basal epithelial cells, intragemmal basal cells within taste buds, or edge cells located laterally outside of taste buds proper (Beidler and Smallman, 1965; Delay et al., 1986; Miura et al., 2006; Nakayama et al., 2008; Okubo et al., 2008).
Taste buds differentiate late in embryogenesis, well after the tongue is innervated. This sequence of innervation followed by differentiation has suggested that taste buds are induced by nerves. In axolotls, an aquatic salamander, the taste periphery is organized simply, with taste buds embedded directly in the epithelium (Takeuchi et al., 1997). In a test of the neural induction model using axolotl embryos, taste buds arise without contact by either nerves (Barlow et al., 1996; Stone, 1940) or cranial mesenchyme (Barlow and Northcutt, 1997); rather, specification and patterning of amphibian taste buds is intrinsic to oral epithelium, and occurs early in development via cell-cell signaling (Parker et al., 2004; Barlow, 2001). Analysis of X-inactivation transgenic female mice also supports an epithelial origin of taste buds in mammals (Stone et al., 1995).
Restriction of mammalian lingual taste buds to papillae adds a level of complexity to the development of these composite taste organs. In mice, taste organ formation begins at mid-gestation, when focal thickenings called taste placodes arise in the lingual epithelium. Placodes evaginate and develop a mesenchymal core, transforming morphologically into taste papillae (Farbman and Mbiene, 1991). Taste buds differentiate within papillae and begin to express taste cell type-specific markers around birth (Krimm and Barlow, 2008). Because placodes transform into papillae, followed by taste bud differentiation, it has been inferred that taste placodes develop into taste papillae, which in turn produce taste buds from a subset of papillary epithelial cells (Mistretta and Liu, 2006). However, the precise relationship between placodes, papillae and buds has not been tackled experimentally.
Discerning how these structures are related to one another is particularly important in the context of the role of innervation in mammalian taste development. Taste placodes, and, at least initially, taste papillae develop independently of innervation (Farbman and Mbiene, 1991; Hall et al., 1999; Hall et al., 2003), whereas taste bud differentiation appears to be nerve dependent (Oakley and Witt, 2004). These findings have been obtained primarily from short-term explant culture, where taste placodes and papillae differentiate morphologically and molecularly, despite a lack of innervation (Farbman, 1972; Hall et al., 2003; Mbiene et al., 1997; Mistretta et al., 2003; Nosrat et al., 2001). In particular, Sonic Hedgehog (Shh) is expressed in early taste placodes, and, consistent with neural independence, Shh is expressed in taste papillae that form in cultured lingual explants. However, the fate of the Shh-expressing cells is unknown, and, thus, Shh has been interpreted to be a marker of taste papillae, rather than of taste buds (Hall et al., 1999; Hall et al., 2003; Liu et al., 2004).
To define the lineage relationship of taste placodes to taste papillae and to taste buds, we crossed mice carrying a drug-sensitive Cre recombinase fusion protein under the Shh promoter (Harfe et al., 2004) with R26RLacZ reporter mice (Soriano, 1999), and tracked the fate of taste placode cells in both embryonic and adult taste organs. We show here that Shh-expressing placodes are taste bud progenitors, which give rise exclusively to cells within taste buds but not to taste papillae. Furthermore, Shh-expressing progenitors give rise to at least two differentiated taste cell types as well as to intragemmal basal and adjacent edge cells in adult mice. However, this contribution is transient; within a few months of birth, placodally derived cells are lost from mature taste buds. We demonstrate that the neural crest does not compensate for this loss, and does not contribute to taste buds at any stage. Finally, we show that the specification, patterning and probable early differentiation of taste bud progenitors are not affected by reduced gustatory innervation caused by a null Bdnf mutation.
MATERIALS AND METHODS
Mice
ShhcreERT2 mice (Harfe et al., 2004) were a gift from Clifford Tabin, Harvard Medical School. R26RLacZ (Soriano, 1999) and Wnt1Cre (Danielian et al., 1998) mice were obtained from Trevor Williams (University of Colorado School of Dentistry, USA). Bdnf+/- mice (Jones et al., 1994) were obtained from Thomas Finger (University of Colorado School of Medicine, USA). ShhcreERT2 mice are maintained on the C57BL/6 background; the background of all other lines is mixed. Mice and embryos were genotyped as described previously (Soriano, 1999; Harfe et al., 2004; Danielian et al., 1998; Jones et al., 1994), and maintained and sacrificed in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver School of Medicine.
Fate mapping of Shh-expressing taste placodes and lingual mesenchyme
ShhcreERT2 males were crossed with homozygous R26RLacZ females, and double transgenic progeny were assayed for β-galactosidase. Embryos and pups with the R26RLacZ allele served as controls. Midday on the day of an observed plug was considered embryonic day (E) 0.5. Pregnant dams were dosed intra-peritoneally once between E12.5-E14.5 with 4-5 mg tamoxifen (T-5648, Sigma), which was prepared and administered as previously described (Nakamura et al., 2006). Embryos were recovered on desired days of gestation, and staged according to Kaufman (Kaufman, 1999). For fate mapping the lingual mesenchyme, Wnt1cre males were crossed with homozygous R26RlacZ females, and embryos (E13.5) and adults (6 weeks, 4 months) with both alleles were assayed for β-galactosidase.
In situ hybridization, immunofluorescence and β-galactosidase histochemistry
For Shh mRNA expression, wild-type tongues were fixed overnight in 4% PFA, and processed for in situ hybridization as described (Hall et al., 1999) with hybridization of the Shh probe (640 bp, L. Goodrich, Stanford University) and stringency washes at 62°C. For Shh immunodetection, live tongues were cultured in mouse anti-Shh (30 μg/ml, 5E1, DSHB) and processed as described previously (Hall et al., 2003).
To analyze Cre-mediated recombination, embryos were harvested at various times after tamoxifen dose and assayed for β-galactosidase (β-gal) by X-gal staining or processed immunofluorescently with guinea pig anti-β-gal (1:1000) (Yee et al., 2003). For whole-mount X-gal, dissected embryonic tongues were fixed briefly in 0.25% glutaraldehyde and stained in X-gal solution (Harfe et al., 2004). Alternatively, embryonic tongues were processed first as whole mounts for anti-Shh immunofluorescence, followed by sectioning and β-gal immunofluorescence.
Tongues from P0 pups were fixed in 0.2% PFA overnight and stained for X-gal after sectioning. Some P0 sections were double labeled with anti-β-gal and rat anti-cytokeratin 8 (CK8, Troma-1; 1:25, DSHB). Light fixation obviated antigen retrieval required when tongues were fixed more strongly, but required less dilute antibody (see below).
To detect innervation in Bdnf-/- and +/+ embryos, E14.5 tongues immunostained with anti-Shh were fixed in 4% paraformaldehyde overnight, sectioned and immunostained with the neurite marker rabbit anti-Gap43 (1:200; Chemicon; MAB347), followed by goat anti-rabbit Cy3 antibody (1:500; Jackson Immunoresearch; 111-165-006). For detecting CK8 immunoreactivity at E18.5 in wild-type versus Bdnf-/- tongues, embryos were perfused with 4% paraformaldehyde, their tongues embedded in paraffin, sectioned at 8 μm, mounted on slides, dewaxed, rehydrated and treated with proteinase K (antigen retrieval). Sections were incubated with anti-CK8 at 1:100 for 1 hour at 37°C, followed by biotinylated goat anti-rat antiserum and streptoavidin-Cy3.
Lineage tracing in adult mice
Postnatal animals at 2 and 6 weeks, and 4 and 7 months with both Cre and R26RLacZ were transcardially perfused with 4% PFA, post-fixed in 4% PFA overnight at 4°C and cryosectioned (12 μm). Sections were double immunostained with guinea pig anti-β-gal and taste cell type-specific rabbit antisera: (1) anti-NTPDase2 (1:1000, a gift from L. G. Lavoie and J. Se'vigny); (2) anti-PLCβ2 (1:1000, Santa Cruz; sc-206); (3) anti-N-CAM (1:1000, Chemicon; AB5032); or (4) anti-PGP 9.5 (1:1000, Abd Serotec, 7863-0504). Wnt1cre;R26RLacZ adult tongue sections were immunostained with a cocktail of NTPDase2, PLCβ2, and NCAM antisera to label simultaneously all three taste cell types, and guinea pig anti-β-gal antiserum, followed by the appropriate fluorescently conjugated secondaries (1:500, Molecular Probes, Invitrogen).
Image acquisition
Bright-field or multichannel fluorescent images were acquired with an Axiocam CCD camera and Axioplan fluorescence microscope with Axiovision software (Zeiss, Germany). Z-stack confocal images were acquired at 0.75 μm through 12 μm cryosections using a laser-scanning Olympus Fluoview confocal microscope with Fluoview Software. Images were saved as TIFFs, contrast adjusted and cropped, and figures compiled using Adobe Photoshop CS2.
Data analysis and quantitation
Shh-descendent cells in taste buds or epithelial clones at P0 were counted in pups treated with tamoxifen at E12.5 (n=3) or E14.5 (n=3). To avoid double counting of 30 μm diameter taste buds at P0, labeled cells in every third section were counted (3×12 μm=36 μm intervals). Taste buds with β-gal-immunoreactive (IR) cells at 2 weeks (n=3), 6 weeks (n=4) or 4-7 months (n=3) postnatal were tallied from counts made at every 5th section (5×12 μm=60 μm interval) to avoid overcounting of adult taste buds (50 μm diameter). The average number of β-gal-IR cells per labeled taste bud at each postnatal time point was tallied from 20 randomly selected taste buds per stage across animals. The average number of double-labeled cells for each taste cell marker (anti-NTPDase, anti-PLCß2, anti-NCAM or anti-PGP9.5, and anti-β-gal) was tallied at 6 weeks postnatal in 20 taste buds from each of four animals. Means and standard errors were calculated using Microsoft Excel.
For differences between Bdnf+/+ and Bdnf-/- tongues at E14.5, Shh-IR cell clusters were counted from images of whole tongue, which included the entire dorsal surface. Shh-IR cell clusters were also quantified in serial sections, to include the relatively small number at the ventral tongue tip. The average size of Shh-expressing clusters was obtained from clusters in the middle and tip of the left side of the tongue; each cluster was traced and the area measured (NIH image). T-tests were used to compare the number and mean area of Shh-labeled clusters.
To assess the extent of taste placode innervation at E14.5, serial sections immunostained for both Shh and Gap43 were examined and each Shh-labeled papilla was categorized as innervated - anti-Gap43-IR labeled fibers penetrating Shh-labeled epithelial cells or not innervated - Gap43-IR fibers completely absent, or only reaching the papilla core mesenchyme.
The number of differentiating taste buds at E18.5, identified via CK8 immunoreactivity, was quantified in serial sections. Each taste bud was carefully followed through all sections it occupied, such that each taste bud was counted only once. Comparisons between wild-type and Bdnf-/- mice were made using a t-test.
RESULTS
Shh-expressing taste placode cells and their daughters consolidate in the apices of fungiform papillae
The taste placode cells fate mapped in subsequent experiments were targeted by assessing precisely when Shh expression is first restricted to the placodes. As previously reported, Shh mRNA and protein are broadly expressed in the lingual epithelium prior to placode formation, and then focalize to the taste placodes by E12.5 (data not shown) (Hall et al., 1999).
To discern the fate of these Shh-expressing taste placode cells, Cre recombination was activated via tamoxifen at E12.5. When treated embryos were examined at E14.5, β-gal activity was evident in the epithelium of taste placodes (Fig. 1A,D,E, red arrows). Extra-placodal epithelial cells were also labeled (Fig. 1A,D, red arrowheads), and were presumed to be general epithelial cells, which expressed Shh at E12.5 when tamoxifen was injected, but had turned off Shh by E14.5. Consistent with this idea (and see below), in embryos given tamoxifen at E13.5 and examined at E15.5, most labeled cells were limited to taste papillae (Fig. 1B,F, red arrows), with many fewer extra-papillary labeled cells (Fig. 1B,G, red arrowheads). In addition, sectioned material showed that Shh-descendant cells, even at these early stages, contribute only a central population of cells in papillae (Fig. 1F), and that Shh-descendant cells are densely innervated (Fig. 1H); both features suggested that placodes contribute to taste buds, and not papillae. Similar results were obtained when the palatal epithelium of embryos injected 48 hours prior was examined (see Fig. S1 in the supplementary material). Importantly, X-gal reaction product was never observed in embryos lacking Cre (Fig. 1C). Moreover, β-galactosidase activity was routinely observed in positive control tissues where Shh is known to be expressed at E12.5, such as vibrissae and hair follicles (data not shown) (St-Jacques et al., 1998).
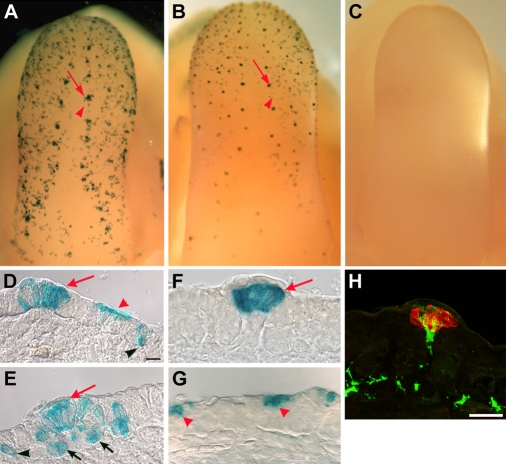
Fig. 1.
Shh-descendent cells restrict to taste placodes. (A) In intact and sectioned tongues from an E14.5 embryo tamoxifen dosed at E12.5, X-gal stained Shh-descendent cells are present in two bilateral rows of placodes (arrow) and numerous epithelial cells (arrowhead). (B) In an E15.5 tongue from an embryo tamoxifen treated at E13.5, labeled cells are predominantly within taste papillae (arrow) and there is less extra-placodal labeling (arrowhead). (C) +/+;R26RLacZ embryos lack β-gal activity. (D,E) E14.5 tongue cryosections from embryos tamoxifen treated at E12.5 reveal placodal (red arrows) and extra-placodal (arrowheads) X-gal-positive cells. Extra-placodal cells were distant from [in the apical (D; red arrowhead) or basal (D,E; black arrowheads) epithelium] or adjacent to (E; black arrows) taste placodes. (F,G) Cryosections of E15.5 embryos given tamoxifen at E13.5 had consolidated placodal labeling (F; arrow); labeled extra-placodal cells were fewer in number and were present in the apical layer (G; arrowheads). (H) Placode-descendent cells at E15.5, from embryos tamoxifen treated at E13.5, stained with anti-β-gal (red) and the pan-neuronal marker PGP9.5 (green) show innervation of Shh-descendent cells. Scale bars: 10 μm in D-H.
Shh-descendent cells within taste placodes continue to express Shh, whereas extra-placodal daughter cells cease Shh expression
Although Shh-descendent cells progressively restrict to placodes, many β-gal-labeled cells were quite close to taste placodes, as well as in extra-placodal epithelium when Cre was induced at E12.5. Labeling of extra-placodal cells suggested that these cells were either: (1) being recruited by and to Shh-expressing placodes, thus contributing to taste organ genesis; or (2) turning off Shh expression and pursuing a lingual epithelial fate. Thus, we examined Shh protein expression in both types of Shh-descendent cells. In placodes, most Shh-descendent cells persisted in expressing Shh (Fig. 2A-C), although some cells at the edge of Shh-descendent clusters (β-gal-IR) ceased to express Shh (Fig. 2A-C, yellow arrowhead). By contrast, all extra-placodal cells indelibly marked with β-galactosidase lacked Shh (Fig. 2D,E). We could not determine, however, whether Shh-descendent cells immediately adjacent to placodes are recruited, or cease Shh expression and become epithelial.
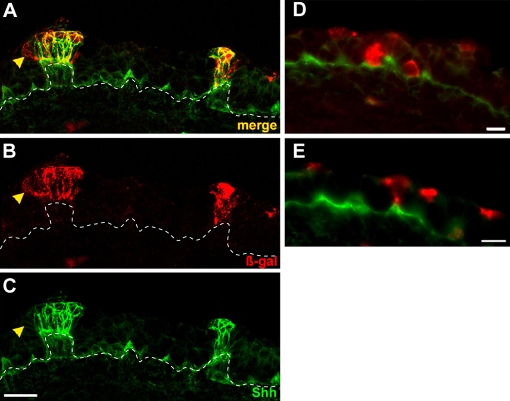
Fig. 2.
Shh-expressing taste placode cells continue to express Shh, whereas extra-placodal descendents cease Shh expression. (A-C) Tongue cryosections from E15.5 embryos tamoxifen treated at E12.5 and immunostained for β-gal (B; red) and Shh (C; green) show co-labeling in taste placodes (yellow in A; merge). Occasional peripheral cells in placodes were β-gal-IR only (yellow arrowheads). Broken lines indicate epithelial basement membrane. (D,E) Double immunostained tongue cryosections from the same embryo as in A-C show that extra-placodal cells labeled at E12.5 but distant from placodes, and in basal (D) and apical (E) epithelial layers, turned off Shh by E14.5. Scale bars: 20 μm in A-C; 10 μm in D,E.
Shh expressing taste placodes contribute to taste buds and not taste papillae at birth
To identify the postnatal fate of Shh-expressing taste placodes, tongues of embryos treated with tamoxifen at E12.5 (n=3) or E14.5 (n=3) were collected at birth (P0). Irrespective of the timing of tamoxifen treatment, β-gal-expressing cells were confined predominantly to taste papilla apices. Furthermore, labeled cells resembled taste buds; they formed onion-shaped clusters, and individual cells had a characteristic fusiform shape (Fig. 3A-C, white asterisks). Anti-cytokeratin 8 (CK8), a marker for taste cells (Zhang et al., 1995; Asano-Miyoshi et al., 2008; Okubo et al., 2008), confirmed that these labeled cells were taste cells. In most taste buds, several β-gal-IR cells were also CK8-IR (Fig. 3D-F), indicating that Shh-expressing taste placodes give rise to taste cells at birth. However, double labeling was not complete; some β-gal-IR cells were not CK8-IR, and vice versa. Cells solely β-gal-IR probably represent taste cells that had not yet differentiated, yet were nonetheless descendant from taste placodes. Conversely, cells immunoreactive only for CK8 could result from mosaic labeling of taste placodes at E12.5, or could correspond to type III taste cells, which we failed to label in this study (see below).
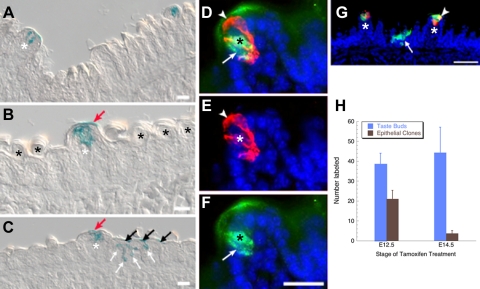
Fig. 3.
At P0, Shh-expressing placodes give rise primarily to immature taste buds, and not to papillae or to general epithelium. (A-C) X-gal-stained P0 tongue sections, tamoxifen treated at E12.5 (A,C-G) or at E14.5 (B), show placode-descendent cells in taste buds (white asterisks), but not papillae. Occasional labeling was detected in the keratinized acellular layer above immature taste buds (red arrows in B,C). (C) In P0 tongues from mice treated at E12.5, X-gal is evident in filiform papillae in basal (white arrows) and keratinized (black arrows) epithelial layers. Black asterisks indicate non-gustatory filiform papillae. (D-G) Cryosectioned P0 tongues from mice tamoxifen treated at E12.5 and immunostained at P0 for β-gal (green) and CK8 (red) reveal that some placodally descendent cells are CK8-IR (D; black and white asterisks indicate double-labeled taste cell; white arrowhead, CK8-IR only cell; white arrow, β-gal-IR only cell). (G) Double immunolabeling for β-gal (green) and CK8 (red) shows that at P0 not all taste buds have CK8-IR taste cells descendent from placodes. One taste bud (white asterisk) has a double-labeled cell (white arrowhead). Arrow indicates an epithelial clone labeled at E12.5. Blue indicates nuclear counterstain. Scale bars: 20 μm in A-F; 50 μm in G. (H) Number of labeled taste buds and epithelial clones at P0 with respect to time of tamoxifen treatment. Labeled taste bud number does not differ with respect to time of treatment (blue bars; n=3), whereas epithelial labeling is diminished with later tamoxifen (brown bars; n=3; *P<0.05).
The efficacy of tamoxifen-induced labeling of Shh-expressing cells throughout the lingual epithelium was also assessed. With either E12.5 or E14.5 tamoxifen injection, the number of labeled taste buds was comparable at birth (Fig. 3H). Tamoxifen treatment at E14.5 versus E12.5 did, however, result in significantly fewer β-gal-IR cells in extra-placodal epithelium. On average, we detected over 20 labeled epithelial clones per tongue when embryos were treated at E12.5 (Fig. 3C,G, white arrows), but fewer than five labeled epithelial clones were present after tamoxifen at E14.5 (Fig. 3H). Again, these data are consistent with more focal labeling of Shh-expressing cells as Shh expression becomes progressively restricted to taste placodes.
Importantly, although most taste buds in papillae were labeled at P0, and epithelial cell clones were also encountered (Fig. 3G), papillary epithelial cells adjacent to labeled taste buds rarely expressed β-galactosidase. Labeling of non-taste bud regions of fungiform papillae was restricted to the acellular keratinized layer above labeled taste buds (Fig. 3B,C, red arrows), probably owing to epithelial differentiation and ultimate loss of these Shh-descendent cells.
Shh-expressing taste placode cells give rise to at least two differentiated taste cell types, as well as to intragemmal basal and perigemmal edge cells
Mature taste buds comprise three differentiated cell types (I, II and III) with discrete functions, and are maintained by continuous renewal from a population of intragemmal basal and/or perigemmal edge cells. Thus, we next asked whether Shh-expressing taste placodes give rise to all cell types, or are restricted in the cell type(s) they produce. Mice were examined at 2 weeks (n=3), 6 weeks (n=4) and 4-7 months (n=3) postnatally, after tamoxifen treatment at E12.5. The number of labeled taste buds per tongue at 2 and 6 weeks was strikingly lower than that encountered at P0, and the number of labeled cells per bud was also reduced as mice aged (Table 1). Compared with 85% labeling of taste buds at P0, only 49% and 41% of taste buds were labeled at 2 and 6 weeks, respectively. The number of labeled cells per taste bud also declined during this period, from six labeled cells per taste bud profile at P0, to only two cells per bud at 6 weeks. By 4 months, Shh-descendent cells were virtually absent from taste buds (Table 1).
Table 1.
Quantitative analysis of Shh-descendent placode cells within postnatal taste buds
| Postnatal day (P) 0 | P14 | P42 | 4 months | |
|---|---|---|---|---|
| Percent taste buds with placode descendent cells±s.e.m.* | 85.5±6.24 (n=6 animals) | 49.7±7.83 (n=3 animals) | 41.7±8.72 (n=4 animals) | 1.75±0.5 (n=3 animals) |
| Placode-descendent cells per taste bud±s.e.m. (n=20 taste buds) | 5.7±0.27 | 2.9±0.41 | 2.2±0.26 | - |
Tamoxifen injection at E12.5.
We next assessed the taste cell type contribution(s) of Shh-expressing placodes. Immunostaining for β-gal and the type I cell marker, anti-NTPDase2, revealed significant double labeling (Fig. 4A-C, asterisks), although not all β-gal-IR cells were NTPDase2-IR. Using anti-PLCβ2 to identify type II cells, double-labeled taste cells were also numerous (Fig. 4D-F). Specifically, 1.5±0.15 β-gal-IR cells per bud were immunopositive for NTPDase, whereas 1.6±0.13 cells per bud were double labeled for β-gal and PLCβ2. As type I cells comprise ∼50% of taste cells per bud (Bartel et al., 2006), and type II cells represent ≤20% of taste cells (Ma et al., 2007), this double labeling frequency would seem to infer that taste placodes give rise predominantly to type II cells. However, we probably undercounted NTPDase-IR cells descendent from taste placodes: because distinguishing type I cell bodies from their extensive processes is difficult, as the protein localizes to the membrane, we only counted double labeled cells when β-gal-IR filled an NTPDase-IR surround. Thus, we conclude here only that embryonic Shh-expressing taste placodes give rise to both type I and II taste cells.
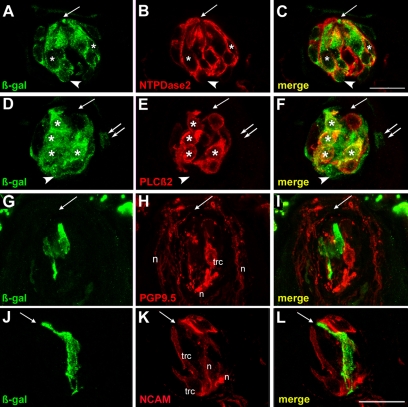
Fig. 4.
Taste placodes give rise to type I and II, but not to type III cells in adult taste buds. Mice treated with tamoxifen at E12.5 were examined for β-gal (green) and differentiated taste cell marker (red) immunoreactivity 6 weeks postnatally. (A-C) Single confocal planes of adult tongue cryosections stained with anti-β-gal (green; cytoplasmic) and anti-NTPDase2 (red; membrane associated) showed many double-labeled cells (white asterisks). Intragemmal basal cells were also labeled by anti-β-gal (white arrowheads). (D-F) Confocal single plane image of a cryosection immunostained for β-gal and PLCβ2 (red; cytoplasm) shows numerous co-expressing cells (white asterisks). An intragemmal basal cell (white arrowheads) and perigemmal edge cell (white double arrows) were also β-gal-IR. (G-I) A taste receptor cells (trc) immunopositive for PGP9.5 (red) was not β-gal-IR. Anti-PGP9.5 also labels nerve fibers (n) in and around buds. (J-L) Staining for β-gal (green) and anti-NCAM (red) did not reveal double-labeled taste receptor cells (trc). Anti-NCAM also labels nerve fibers (n) innervating taste buds. The single white arrow in each panel indicates the taste pore. Scale bars: 20 μm.
Despite the reasonable frequency with which NCAM-IR type III cells and β-gal-IR cells were detected in taste buds (Fig. 4J-L), double-labeled type III cells were never encountered. Using another marker, PGP9.5, which is expressed by a subset of type II and III cells (Yee et al., 2001; Ma et al., 2007), we also did not find double-labeled cells (Fig. 4G-I). This outcome suggests that either: (1) taste placodes are lineage restricted, and do not give rise to type III and type II/III PGP9.5-IR cells; or (2) type III and PGP9.5-IR cells arise from Shh-expressing progenitors, but the reduction in β-gal-IR cells at 6 weeks (Table 1), combined with the relatively sparse distribution of these cell types (<10%) (Ma et al., 2007), results in a low probability of detecting double-labeled cells.
In addition to β-gal-IR fusiform taste cells, we encountered β-gal labeled intragemmal basal cells (Fig. 4A-F, arrowheads) and perigemmal edge cells (Fig. 4D-F, double arrows). These cell types were identified via their shape and location, and their lack of taste cell immunomarker expression. For example, β-gal-IR intragemmal basal cells are polygonal and abut the basal lamina (Fig. 4A-F), whereas the β-gal-IR perigemmal edge cells are located along the lateral margin of the taste bud, and are small and crescent shaped (Fig. 4D-F).
Taste buds derive exclusively from the epithelium with no contribution from neural crest
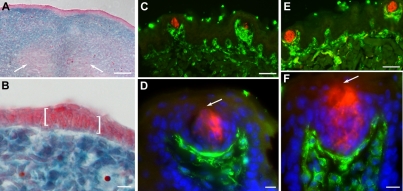
Despite the fact that most taste buds contain Shh-descendent cells at birth, the frequency of labeling in adult taste buds at 6 weeks is dramatically lower, and completely lost by 4 months (Table 1). One explanation for this loss is that taste buds receive a compensatory contribution from the neural crest at a later stage. Although an embryonic neural crest contribution has been ruled out in amphibians (Barlow and Northcutt, 1994), this study did not extend to postnatal stages. To test this idea, we indelibly labeled neural crest cells, including those contributing to tongue mesenchyme (Chai et al., 2000) in Wnt1Cre;R26RLacZ mice, and then searched for labeled neural crest cells in taste buds and papillae at embryonic and adult stages. At E13.5, X-gal-positive neural crest mesenchyme is clearly demarcated from both the X-gal-negative lingual epithelium (Fig. 5A,B) and lingual musculature derived from somitic mesoderm (Fig. 5A, arrows) (Chai et al., 2000). Staining of Wnt1Cre;R26RLacZ adult tongues for anti-β-gal and taste cell immunomarkers at 6 weeks (Fig. 5C,D) and 4 months (Fig. 5E,F) showed that neural crest-derived mesenchyme extends into the taste papilla core but does not contribute to taste buds or to papillary epithelium (Fig. 5C-F). At 4 months, in contrast to the loss of Shh-descendent cells in taste buds (Table 1), there is no loss of β-gal in neural crest-derived cells of the tongue.
Fig. 5.
Neural crest does not contribute to taste placodes or adult taste buds. (A,B) Sections of E13.5 Wnt1cre;R26LacZ tongues stained with X-gal and counterstained with Neutral Red confirm the absence of neural crest cells in lingual epithelium. White arrows in A indicate X-gal-negative tongue muscle. In B, an X-gal-negative taste placode is bracketed. (C,D) Sections of a 6-week-old Wnt-1cre;R26LacZ tongue stained for anti-β-gal (green) and anti-PLC β2 (red) reveal neural crest cells in taste papilla mesenchyme. (E,F) In 4-month-old Wnt-1cre;R26LacZ tongue sections immunostained for a cocktail of type I, II and III taste cell markers (red) and β-gal (green), neural crest cells are found only in lingual mesenchyme. (D,F) Blue is Hoechst nuclear counterstain. Arrows indicate the taste pore. Scale bars: 50 μm in A,C,E; 10 μm in B,D,F.
Development of taste bud progenitors in embryos which lose gustatory innervation is indistinguishable from that of controls
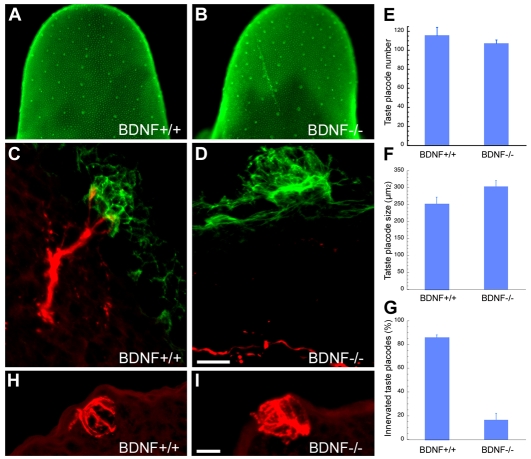
Several groups have shown that development of taste placodes is initially nerve independent; tongue explants develop taste placodes that evaginate as taste papillae and express Shh (Hall et al., 2003; Liu et al., 2004). However, these cultures do not survive sufficiently long to monitor taste bud differentiation. Thus, we examined Shh-expressing taste progenitors within emerging papillae at E14.5 in embryos homozygous null for the gene encoding the neurotrophin BDNF (Fig. 6), which lose gustatory innervation early on (Nosrat et al., 1997; Krimm, 2007). The distribution, total number, and size of Shh-expressing taste placodes did not differ between Bdnf+/+ and Bdnf-/- embryos at E14.5 (Fig. 6A-F), yet the number of innervated placodes was dramatically reduced in the Bdnf-/- embryos (Fig. 6D,G).
Fig. 6.
Embryonic development of taste bud progenitors is normal without Bdnf-dependent gustatory innervation. (A,B) At E14.5, Shh-expressing taste bud progenitors do not differ between Bdnf+/+ (A) and Bdnf-/- (B) littermates. (C,D) In transverse sections of E14.5 tongues immunostained with anti-Shh (green) and anti-Gap43 (red), Shh-IR taste progenitors are comparable regardless of genotype, although Gap-43-IR fiber contact occurs in wild types (C), but is absent in Bdnf-/- mice (D). (E-G) The number (E) and size (F) of taste progenitors in Bdnf+/+ versus Bdnf-/- embryos are not affected by loss of Bdnf-supported innervation (G). (H,I) CK8-IR immature taste buds in sections of E18.5 tongues do not differ between (H) wild-type and (I) Bdnf-/- embryos. Scale bars: 10 μm in C,D,H,I.
To determine whether removal of Bdnf-dependent innervation impacts embryonic taste bud differentiation, we counted developing taste buds in wild type and Bdnf-/- mice using anti-CK8 to mark fusiform taste cells. At E18.5, immature taste buds with numerous CK8-IR cells were evident in both wild-type and mutant tongues (Fig. 6H,I), and their number did not differ significantly between wild-type (59±9.3, n=7) and Bdnf-/- (40±8.4, n=8) mice. However, this last result must be viewed with caution, as the number of CK8-IR taste buds was very variable at this stage even in control embryos. Thus, our data indicate that patterning and specification of taste bud progenitors, and perhaps their early differentiation, is not dependent on either Bdnf or the neural innervation it supports.
DISCUSSION
Shh expressing taste placodes give rise to taste buds and not taste papillae
The postnatal fate of embryonic taste placodes has been unclear, obscuring the relationship of these earliest lingual taste structures to adult taste buds and their surrounding papillae (Farbman and Mbiene, 1991). Immature taste buds only become evident in papillary epithelium late in gestation, with fully differentiated taste buds present within 1 week of birth (Mistretta, 1991). This developmental sequence of placodes transforming into papillae, followed by differentiation of taste buds, has suggested that placodes are papilla precursors, which subsequently organize taste bud progenitors within the apical epithelium (Mistretta and Liu, 2006).
We show here, using inducible Cre-lox lineage tracing, that taste placodes do not give rise to papillae, but rather contribute almost exclusively to taste buds. At postnatal stages, embryonic Shh-expressing taste placodes are progenitors for the majority of taste cells, giving rise to both type I and II cells, as well as to intragemmal basal and perigemmal edge cells, although we have yet to identify type III cells as being descended from taste placodes. Unexpectedly, Shh-expressing progenitor cells and their descendents, although evident at birth, begin to disappear prior to weaning, and are completely absent in adults. This loss of placodal cells is not compensated for by a neural crest contribution, either embryonically or postnatally. Finally, we show that taste bud progenitors develop in vivo independently of nerve contact, in that specification, patterning and likely initial differentiation of Shh-expressing progenitors cells remains constant, even when BDNF-dependent taste innervation is lost genetically.
Taste bud progenitors are specified early, and may organize subsequent papilla morphogenesis
Taste placodes express several signaling factors, including Shh, Bmp and Wnt/β-catenin, and their expression continues as taste papillae undergo morphogenesis (Hall et al., 1999; Iwatsuki et al., 2007; Jung et al., 1999; Liu et al., 2007). Alteration of each of these pathways during placode formation and papillary morphogenesis in vitro affects the patterning of taste placodes and resultant papillae. For example, both Shh and Bmp inhibit (Hall et al., 2003; Mistretta et al., 2003; Zhou et al., 2006), whereas Wnt/β-catenin promotes placode development, including expression of Shh, in cultured tongue explants (Iwatsuki et al., 2007; Liu et al., 2007). However, as lingual explants do not survive until taste buds differentiate, this in vitro approach has lead only to the conclusion that these pathways regulate papilla development (Mbiene and Roberts, 2003).
Our results shift this interpretation; early alteration of Shh, Bmp and Wnt/β-catenin signaling in vitro impacts taste bud progenitors directly. In support of this view, in Wnt/β-catenin gain-of-function experiments, mutant embryos examined at E14.5 have many more Shh-expressing taste bud progenitors distributed throughout the lingual epithelium compared with controls. At birth, mutant tongues are completely covered by immature taste buds that express both Shh and some taste cell markers, embedded in enlarged and often fused taste papillae not expressing Shh (Liu et al., 2007). Thus, the increased number of Shh-expressing progenitors in embryonic tongues correlates well with increased taste buds in greatly expanded fungiform papillae observed at birth. Given our new interpretation of early events in taste bud patterning, we speculate that Shh-expressing taste bud progenitors may function as transient signaling centers within the epithelium, which in turn regulate subsequent development of taste buds and papillae. Intriguingly, these taste progenitor cells share a number of similarities with known signaling centers, such as the tooth enamel knot (Obara and Lesot, 2007), and the apical ectodermal ridge of the developing limb (Antalikova et al., 1989). Specifically, taste placodes express a number of secreted signaling factors (see above), are mitotically quiescent during embryogenesis (Farbman and Mbiene, 1991; Mbiene and Roberts, 2003; Zhou et al., 2006) and contribute only transiently to postnatal taste buds (this study).
We must also formally consider, however, that postnatal loss of embryonic taste progenitors may be an artifact of our fate-mapping technique. The R26RLacZ mouse is widely used in developmental studies, owing to ubiquitous expression from the R26 promoter throughout life (Friedrich and Soriano, 1991; Zambrowicz et al., 1997). In studies of thymic development using the Wnt1Cre;R26LacZ mice, neural crest-derived cells were detected in embryonic but not postnatal thymus (Jiang et al., 2000; Yamazaki et al., 2005). In a recent report, however, Wnt1Cre;R26YFP reporter mice were employed, and neural crest-derived mesenchyme persisted in adult thymus (Foster et al., 2008). These authors suggested that the lacZ gene itself was silenced, owing to heavy methylation of the lacZ-coding sequence (Chevalier-Mariette et al., 2003). To date, a loss of lacZ transcription has not been reported in epithelial lineage studies (e.g. Berton et al., 2000). Our results, moreover, tend to reject an artifactual loss of embryonic taste progenitors postnatally. First, we see no loss of β-gal in neural crest-derived cells of the tongue mesenchyme up to 4 months of age, suggesting that, in lingual tissue immediately adjacent to taste buds, the lacZ reporter is not silenced. Second, the loss of labeled taste cells in adults follows a comparable time course across animals, already evident at P14 and complete by 4 months, implying a regulated, rather than a stochastic, process.
Are Shh-expressing taste bud progenitors lineage restricted?
Taste buds are a heterogeneous population of three differentiated cells types (I, II and III), which can be identified via expression of distinct proteins: type I glial-like cells; type II sweet, bitter and umami detectors; and type III sour detectors and putative relay cells (Clapp et al., 2001; Clapp et al., 2004; Yang et al., 2000b; Huang et al., 2006; Roper, 2006). The entire taste bud cell population has been estimated to arise embryonically from 7-13 progenitor cells, and then is continually renewed throughout adult life from a proliferative progenitor pool within the papillary epithelium (Stone et al., 2002; Okubo et al., 2008). However, the cell lineage for taste cells generated at any stage is completely unknown. One view is that taste cell types represent separate lineages, which remain distinct throughout the life of each cell (Farbman, 1965). Alternatively, taste cells may have a common lineage, with different cell types representing different stages in the lifespan of a single cell (Delay et al., 1986). More recently, type I cells have been proposed to arise from a dedicated lineage, whereas types II and III have an intermingled relationship, with a subset of type III cells giving rise to type II cells (Miura et al., 2006).
Our fate-mapping studies indicate that Shh-expressing embryonic taste bud progenitors generate both type I and II cells, suggesting that they share a common embryonic lineage. This leaves the issue of type III cell lineage unresolved. We cannot rule out the possibility that type III cells may descend from this same progenitor pool, as we may be looking for a very rare event. Embryonically labeled progenitor cells are steadily lost postnatally, and combined with the low frequency of type III cells within taste buds (Ma et al., 2007), the chance that double-labeled type III taste cells would be detected is very low (<1 double labeled type III cell per animal). We must therefore look at many more experimental animals in order to encounter an example of this lineage relationship. Alternatively, type III cells may have a distinct embryonic origin.
To address this possibility, we asked whether taste buds receive a cellular contribution from the neural crest, either embryonically or postnatally as the taste placode-descendant cells are lost; perhaps type III cells arise uniquely from neural crest? Although an embryonic neural crest contribution has been excluded in mouse circumvallate papilla epithelium in early embryos (Jitpukdeebodintra et al., 2002), as well as in early development of amphibian taste buds (Barlow and Northcutt, 1995), our studies extend these results. Using the Wnt1Cre line, we confirm extensive neural crest in the lingual mesenchyme (Chai et al., 2000), including that of taste papillae; however, in no case and at no time did we observe neural crest-derived cells within taste buds, let alone in lingual epithelium.
Although not derived from neural crest, type III cells may develop via processes that differs from that of types I and II, perhaps because of their distinct morphology and life history within taste buds. Type III cells are the only taste cell type to form conventional synapses with sensory neurons conveying taste information to the CNS (Roper, 2006; Yang et al., 2000a), and may also be the most long-lived cell within buds. Although the average lifespan of rodent taste cells is 10 days, 4-week-old cells with a type III morphology have been documented in adults (Hamamichi et al., 2006). One final explanation for our failure to label type III cells as placodal descendents is that they may arise from a non-Shh expressing population, which is induced by signals from the taste placodes. This recruitment of type III cells or their progenitors could occur embryonically, or early on in postnatal life. However, they must be recruited before postnatal day 4, when we first observe differentiated type III cells (T. Glover, H. Nguyen and L.A.B., unpublished). In any case, the identity of Type III taste cell progenitors remains unknown.
Neural dependence of taste bud development
In amphibians, development of taste buds can occur without nerves (Barlow et al., 1996). Similarly, cultured lingual explants from rodents form taste placodes and papillae, which express crucial signaling molecules, despite the lack of innervation (Hall et al., 2003; Farbman and Mbiene, 1991; Nosrat et al., 2001; Mistretta et al., 2003). A number of groups have also used an in vivo genetic approach to address this issue. Brain-derived neurotrophic factor (BDNF) is expressed in developing taste papillae (Nosrat and Olson, 1995; Nosrat et al., 1996), and loss of BDNF results in embryonic loss of taste sensory neurons (Jones et al., 1994; Liebl et al., 1997). In tongues of these mutant mice examined 1-2 weeks postnatally, lingual innervation to taste buds is indeed lost, and taste buds and papillae are dramatically reduced (Nosrat et al., 1997). However, we show here that this reported postnatal effect is probably due to a postnatal role of either Bdnf or neural maintenance of taste progenitor cells, rather than to an absolute requirement for either BDNF or nerve contact for taste progenitor induction and initial taste bud differentiation. Specifically, specification and patterning of taste bud progenitors does not differ between wild type and Bdnf-/- mice, despite our finding that most of these progenitors are not successfully innervated during development. Furthermore, 4 days later at birth, taste cells, as defined by CK8 expression, do not differ significantly between control and mutant embryos. However, because of the high variability in CK8-IR taste bud numbers at this later stage in both mutants and controls, it is possible that gustatory innervation may impact taste bud progenitor differentiation at this time. Nonetheless, specification and patterning of mammalian taste bud progenitors, and probably the initial differentiation of taste buds, are independent of both BDNF and gustatory innervation.
The development of mammalian taste buds and papillae is typically considered to be similar to that of other epithelial appendages, such as teeth, hair follicles and feathers, which require extensive interactions between epithelium and mesenchyme (Chuong et al., 2000; Pispa and Thesleff, 2003). The observation that mammalian taste placodes give rise to taste buds only, and not to papillae, is reminiscent of taste organ formation in axolotls, where taste buds lack papillae and are embedded directly in the lingual epithelium (Takeuchi et al., 1997). Development of axolotl taste organs occurs independently of mesenchyme (Barlow and Northcutt, 1997), and, moreover, has been shown to be an early epithelium-intrinsic process (Parker et al., 2004). Thus, we speculate that mammalian taste progenitors are also specified via an epithelial event, which is probably independent of mesenchyme.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/9/1519/DC1
Supplementary Material
Acknowledgments
We thank Elizabeth Harvey and Brooke Baxter for technical assistance, Dr Kristin Artinger for comments on the manuscript, and the Rocky Mountain Taste and Smell Center for animal and imaging support (NIDCD DC004657 to Drs D. Restrepo and T. Finger).
This work was supported by NIDCD DC008373 to L.A.B. and DC007176 to R.F.K. Deposited in PMC for release after 12 months.
References
- Antalikova, L., Kren, V., Kasparek, R. and Bila, V. (1989). Patterns of physiological cell death and mitoses in the apical ectodermal ridge in normodactylous and polydactylous rat limb buds. A quantitative evaluation. Folia Biol. (Praha) 35, 339-346. [PubMed] [Google Scholar]
- Asano-Miyoshi, M., Hamamichi, R. and Emori, Y. (2008). Cytokeratin 14 is expressed in immature cells in rat taste buds. J. Mol. Histol. 39, 193-199. [DOI] [PubMed] [Google Scholar]
- Barlow, L. A. (2001). Specification of pharyngeal endoderm is dependent on early signals from axial mesoderm. Development 128, 4573-4583. [DOI] [PubMed] [Google Scholar]
- Barlow, L. A. and Northcutt, R. G. (1994). Analysis of the embryonic lineage of vertebrate taste buds. Chem. Senses 19, 715-724. [DOI] [PubMed] [Google Scholar]
- Barlow, L. A. and Northcutt, R. G. (1995). Embryonic origin of amphibian taste buds. Dev. Biol. 169, 273-285. [DOI] [PubMed] [Google Scholar]
- Barlow, L. A. and Northcutt, R. G. (1997). Taste buds develop autonomously from endoderm without induction by cephalic neural crest or paraxial mesoderm. Development 124, 949-957. [DOI] [PubMed] [Google Scholar]
- Barlow, L. A., Chien, C. B. and Northcutt, R. G. (1996). Embryonic taste buds develop in the absence of innervation. Development 122, 1103-1111. [DOI] [PubMed] [Google Scholar]
- Bartel, D. L., Sullivan, S. L., Lavoie, E. G., Sevigny, J. and Finger, T. E. (2006). Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J. Comp. Neurol. 497, 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler, L. M. (1953). Properties of chemoreceptors of tongue of rat. J. Neurophysiol. 16, 595-607. [DOI] [PubMed] [Google Scholar]
- Beidler, L. M. and Smallman, R. L. (1965). Renewal of cells within taste buds. J. Cell Biol. 27, 263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton, T. R., Wang, X. J., Zhou, Z., Kellendonk, C., Schutz, G., Tsai, S. and Roop, D. R. (2000). Characterization of an inducible, epidermal-specific knockout system: differential expression of lacZ in different Cre reporter mouse strains. Genesis 26, 160-161. [PubMed] [Google Scholar]
- Chai, Y., Jiang, X., Ito, Y., Bringas, P., Jr, Han, J., Rowitch, D. H., Soriano, P., McMahon, A. P. and Sucov, H. M. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671-1679. [DOI] [PubMed] [Google Scholar]
- Chevalier-Mariette, C., Henry, I., Montfort, L., Capgras, S., Forlani, S., Muschler, J. and Nicolas, J. F. (2003). CpG content affects gene silencing in mice: evidence from novel transgenes. Genome Biol. 4, R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong, C. M., Patel, N., Lin, J., Jung, H. S. and Widelitz, R. B. (2000). Sonic hedgehog signaling pathway in vertebrate epithelial appendage morphogenesis: perspectives in development and evolution. Cell Mol. Life Sci. 57, 1672-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp, T. R., Stone, L. M., Margolskee, R. F. and Kinnamon, S. C. (2001). Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp, T. R., Yang, R., Stoick, C. L., Kinnamon, S. C. and Kinnamon, J. C. (2004). Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J. Comp. Neurol. 468, 311-321. [DOI] [PubMed] [Google Scholar]
- Danielian, P. S., Muccino, D., Rowitch, D. H., Michael, S. K. and McMahon, A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323-1326. [DOI] [PubMed] [Google Scholar]
- Delay, R. J., Kinnamon, J. C. and Roper, S. D. (1986). Ultrastructure of mouse vallate taste buds: II. Cell types and cell lineage. J. Comp. Neurol. 253, 242-252. [DOI] [PubMed] [Google Scholar]
- Farbman, A. I. (1965). Electron microscope study of the developing taste bud in rat fungiform papilla. Dev. Biol. 11, 110-135. [DOI] [PubMed] [Google Scholar]
- Farbman, A. I. (1972). Differentiation of taste buds in organ culture. J. Cell Biol. 52, 489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbman, A. I. (1980). Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 13, 349-357. [DOI] [PubMed] [Google Scholar]
- Farbman, A. I. and Mbiene, J. P. (1991). Early development and innervation of taste bud-bearing papillae on the rat tongue. J. Comp. Neurol. 304, 172-186. [DOI] [PubMed] [Google Scholar]
- Finger, T. E., Danilova, V., Barrows, J., Bartel, D. L., Vigers, A. J., Stone, L., Hellekant, G. and Kinnamon, S. C. (2005). ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310, 1495-1499. [DOI] [PubMed] [Google Scholar]
- Foster, K., Sheridan, J., Veiga-Fernandes, H., Roderick, K., Pachnis, V., Adams, R., Blackburn, C., Kioussis, D. and Coles, M. (2008). Contribution of neural crest-derived cells in the embryonic and adult thymus. J. Immunol. 180, 3183-3189. [DOI] [PubMed] [Google Scholar]
- Friedrich, G. and Soriano, P. (1991). Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513-1523. [DOI] [PubMed] [Google Scholar]
- Hall, J. M., Hooper, J. E. and Finger, T. E. (1999). Expression of sonic hedgehog, patched, and Gli1 in developing taste papillae of the mouse. J. Comp. Neurol. 406, 143-155. [DOI] [PubMed] [Google Scholar]
- Hall, J. M., Bell, M. L. and Finger, T. E. (2003). Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev. Biol. 255, 263-277. [DOI] [PubMed] [Google Scholar]
- Hamamichi, R., Asano-Miyoshi, M. And Emori, Y. (2006). Taste bud contains both short-lived and long-lived cell populations. Neuroscience 141, 2129-2138. [DOI] [PubMed] [Google Scholar]
- Harfe, B. D., Scherz, P. J., Nissim, S., Tian, H., McMahon, A. P. and Tabin, C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528. [DOI] [PubMed] [Google Scholar]
- Huang, A. L., Chen, X., Hoon, M. A., Chandrashekar, J., Guo, W., Trankner, D., Ryba, N. J. and Zuker, C. S. (2006). The cells and logic for mammalian sour taste detection. Nature 442, 934-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. A., Maruyama, Y., Stimac, R. and Roper, S. D. (2008). Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J. Physiol. 586, 2903-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki, K., Liu, H. X., Gronder, A., Singer, M. A., Lane, T. F., Grosschedl, R., Mistretta, C. M. and Margolskee, R. F. (2007). Wnt signaling interacts with Shh to regulate taste papilla development. Proc. Natl. Acad. Sci. USA 104, 2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., Rowitch, D. H., Soriano, P., McMahon, A. P. and Sucov, H. M. (2000). Fate of the mammalian cardiac neural crest. Development 127, 1607-1616. [DOI] [PubMed] [Google Scholar]
- Jitpukdeebodintra, S., Chai, Y. and Snead, M. L. (2002). Developmental patterning of the circumvallate papilla. Int. J. Dev. Biol. 46, 755-763. [PubMed] [Google Scholar]
- Jones, K. R., Farinas, I., Backus, C. and Reichardt, L. F. (1994). Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76, 989-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, H. S., Oropeza, V. and Thesleff, I. (1999). Shh, Bmp-2, Bmp-4 and Fgf-8 are associated with initiation and patterning of mouse tongue papillae. Mech. Dev. 81, 179-182. [DOI] [PubMed] [Google Scholar]
- Kaufman, M. (1999). The Atlas of Mouse Development. London: Academic Press.
- Krimm, R. F. (2007). Factors that regulate embryonic gustatory development. BMC Neurosci. 8, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm, R. F.,and Barlow, L. A. (2008). Development of the taste system. In The Senses: A Comprehensive Reference, Vol. 4, Olfaction and Taste (ed. S. Firestein and G. K. Beauchamp), pp. 157-182. San Diego, CA: Academic Press.
- Liebl, D. J., Tessarollo, L., Palko, M. E. and Parada, L. F. (1997). Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J. Neurosci. 17, 9113-9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F., Thirumangalathu, S., Gallant, N. M., Yang, S. H., Stoick-Cooper, C. L., Reddy, S. T., Andl, T., Taketo, M. M., Dlugosz, A. A., Moon, R. T. et al. (2007). Wnt-beta-catenin signaling initiates taste papilla development. Nat. Genet. 39, 106-112. [DOI] [PubMed] [Google Scholar]
- Liu, H. X., Maccallum, D. K., Edwards, C., Gaffield, W. and Mistretta, C. M. (2004). Sonic hedgehog exerts distinct, stage-specific effects on tongue and taste papilla development. Dev. Biol. 276, 280-300. [DOI] [PubMed] [Google Scholar]
- Ma, H., Yang, R., Thomas, S. M. and Kinnamon, J. C. (2007). Qualitative and quantitative differences between taste buds of rat and mouse. BMC Neurosci. 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbiene, J. P. and Roberts, J. D. (2003). Distribution of keratin 8-containing cell clusters in mouse embryonic tongue: evidence for a prepattern for taste bud development. J. Comp. Neurol. 457, 111-122. [DOI] [PubMed] [Google Scholar]
- Mbiene, J. P., Maccallum, D. K. and Mistretta, C. M. (1997). Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J. Comp. Neurol. 377, 324-340. [DOI] [PubMed] [Google Scholar]
- Mistretta, C. M. (1991). Developmental neurobiology of taste. In Smell and Taste in Health and Disease (ed. T. V. Getchell, L. M. Bartoshuk, R. L. Doty and J. B. Snow), pp. 35-64. New York: Raven Press.
- Mistretta, C. M. and Liu, H. X. (2006). Development of fungiform papillae: patterned lingual gustatory organs. Arch. Histol. Cytol. 69, 199-208. [DOI] [PubMed] [Google Scholar]
- Mistretta, C. M., Liu, H. X., Gaffield, W. and MacCallum, D. K. (2003). Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev. Biol. 254, 1-18. [DOI] [PubMed] [Google Scholar]
- Miura, H., Kusakabe, Y. and Harada, S. (2006). Cell lineage and differentiation in taste buds. Arch. Histol. Cytol. 69, 209-225. [DOI] [PubMed] [Google Scholar]
- Miyoshi, M. A., Abe, K. and Emori, Y. (2001). IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem. Senses 26, 259-265. [DOI] [PubMed] [Google Scholar]
- Nakamura, E., Nguyen, M. T. and Mackem, S. (2006). Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev. Dyn. 235, 2603-2612. [DOI] [PubMed] [Google Scholar]
- Nakayama, A., Miura, H., Shindo, Y., Kusakabe, Y., Tomonari, H. and Harada, S. (2008). Expression of the basal cell markers of taste buds in the anterior tongue and soft palate of the mouse embryo. J. Comp. Neurol. 509, 211-224. [DOI] [PubMed] [Google Scholar]
- Nosrat, C. A. and Olson, L. (1995). Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. J. Comp. Neurol. 360, 698-704. [DOI] [PubMed] [Google Scholar]
- Nosrat, C. A., Ebendal, T. and Olson, L. (1996). Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. J. Comp. Neurol. 376, 587-602. [DOI] [PubMed] [Google Scholar]
- Nosrat, C. A., Blomlof, J., El Shamy, W. M., Ernfors, P. and Olson, L. (1997). Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development 124, 1333-1342. [DOI] [PubMed] [Google Scholar]
- Nosrat, C. A., MacCallum, D. K. and Mistretta, C. M. (2001). Distinctive spatiotemporal expression patterns for neurotrophins develop in gustatory papillae and lingual tissues in embryonic tongue organ cultures. Cell Tissue Res. 303, 35-45. [DOI] [PubMed] [Google Scholar]
- Oakley, B. and Witt, M. (2004). Building sensory receptors on the tongue. J. Neurocytol. 33, 631-646. [DOI] [PubMed] [Google Scholar]
- Obara, N. and Lesot, H. (2007). Asymmetrical growth, differential cell proliferation, and dynamic cell rearrangement underlie epithelial morphogenesis in mouse molar development. Cell Tissue Res. 330, 461-473. [DOI] [PubMed] [Google Scholar]
- Okubo, T., Clark, C. and Hogan, B. L. (2008). Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells 26, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, M. A., Bell, M. L. and Barlow, L. A. (2004). Cell contact-dependent mechanisms specify taste bud pattern during a critical period early in embryonic development. Dev. Dyn. 230, 630-642. [DOI] [PubMed] [Google Scholar]
- Pispa, J. and Thesleff, I. (2003). Mechanisms of ectodermal organogenesis. Dev. Biol. 262, 195-205. [DOI] [PubMed] [Google Scholar]
- Roper, S. D. (1992). The microphysiology of peripheral taste organs. J. Neurosci. 12, 1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper, S. D. (2006). Cell communication in taste buds. Cell Mol. Life Sci. 63, 1494-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper, S. D. (2007). Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 454, 759-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. V. and Davis, B. J. (2000). Neural representation of taste. In The Neurobiology of Taste and Smell (ed. T. E. Finger, W. L. Silver and D. Restrepo), pp. 353-394. New York: Wiley-Liss.
- Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71. [DOI] [PubMed] [Google Scholar]
- St-Jacques, B., Dassule, H. R., Karavanova, I., Botchkarev, V. A., Li, J., Danielian, P. S., McMahon, J. A., Lewis, P. M., Paus, R. and McMahon, A. P. (1998). Sonic hedgehog signaling is essential for hair development. Curr. Biol. 8, 1058-1068. [DOI] [PubMed] [Google Scholar]
- Stone, L. M., Finger, T. E., Tam, P. P. and Tan, S. S. (1995). Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc. Natl. Acad. Sci. USA 92, 1916-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, L. M., Tan, S. S., Tam, P. P. and Finger, T. E. (2002). Analysis of cell lineage relationships in taste buds. J. Neurosci. 22, 4522-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, L. S. (1940). The origin and development of taste organs in salamanders observed in the living condition. J. Exp. Zool. 83, 481-506. [Google Scholar]
- Takeuchi, H., Ido, S., Kaigawa, Y. and Nagai, T. (1997). Taste disks are induced in the lingual epithelium of salamanders during metamorphosis. Chem. Senses 22, 535-545. [DOI] [PubMed] [Google Scholar]
- Yamazaki, H., Sakata, E., Yamane, T., Yanagisawa, A., Abe, K., Yamamura, K., Hayashi, S. and Kunisada, T. (2005). Presence and distribution of neural crest-derived cells in the murine developing thymus and their potential for differentiation. Int. Immunol. 17, 549-558. [DOI] [PubMed] [Google Scholar]
- Yang, R., Crowley, H. H., Rock, M. E. and Kinnamon, J. C. (2000a). Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J. Comp. Neurol. 424, 205-215. [DOI] [PubMed] [Google Scholar]
- Yang, R., Tabata, S., Crowley, H. H., Margolskee, R. F. and Kinnamon, J. C. (2000b). Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J. Comp. Neurol. 425, 139-151. [DOI] [PubMed] [Google Scholar]
- Yee, C. L., Yang, R., Bottger, B., Finger, T. E. and Kinnamon, J. C. (2001). `Type III' cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J. Comp. Neurol. 440, 97-108. [DOI] [PubMed] [Google Scholar]
- Yee, C. L., Jones, K. R. and Finger, T. E. (2003). Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J. Comp. Neurol. 459, 15-24. [DOI] [PubMed] [Google Scholar]
- Zambrowicz, B. P., Imamoto, A., Fiering, S., Herzenberg, L. A., Kerr, W. G. and Soriano, P. (1997). Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA 94, 3789-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C., Cotter, M., Lawton, A., Oakley, B., Wong, L. and Zeng, Q. (1995). Keratin 18 is associated with a subset of older taste cells in the rat. Differentiation 59, 155-162. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., Liu, H. X. and Mistretta, C. M. (2006). Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev. Biol. 297, 198-213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.