Abstract
Background
The two closely related species of band-winged grasshoppers, Gastrimargus marmoratus and Oedaleus asiaticus, display significant differences in distribution, biological characteristics and habitat preferences. They are so similar to their respective congeneric species that it is difficult to differentiate them from other species within each genus. Hoppers of the two species have quite similar morphologies to that of Locusta migratoria, hence causing confusion in species identification. Thus we determined and compared the mitochondrial genomes of G. marmoratus and O. asiaticus to address these questions.
Results
The complete mitochondrial genomes of G. marmoratus and O. asiaticus are 15,924 bp and 16,259 bp in size, respectively, with O. asiaticus being the largest among all known mitochondrial genomes in Orthoptera. Both mitochondrial genomes contain a standard set of 13 protein-coding genes, 22 transfer RNA genes, 2 ribosomal RNA genes and an A+T-rich region in the same order as those of the other analysed caeliferan species, but different from those of the ensiferan species by the rearrangement of trnD and trnK. The putative initiation codon for the cox1 gene in the two species is ATC. The presence of different sized tandem repeats in the A+T-rich region leads to size variation between their mitochondrial genomes. Except for nad2, nad4L, and nad6, most of the caeliferan mtDNA genes exhibit low levels of divergence. In phylogenetic analyses, the species from the suborder Caelifera form a monophyletic group, as is the case for the Ensifera. Furthermore, the two suborders cluster as sister groups, supporting the monophyly of Orthoptera.
Conclusion
The mitochondrial genomes of both G. marmoratus and O. asiaticus harbor the typical 37 genes and an A+T-rich region, exhibiting similar characters to those of other grasshopper species. Characterization of the two mitochondrial genomes has enriched our knowledge on mitochondrial genomes of Orthoptera.
Background
The band-winged grasshoppers, species of the subfamily Oedipodinae in Acridiidae, encompass over 900 described species. Indeed, the band-winged grasshoppers include some of the best known and most notorious pests in the world. Two species of band-winged grasshoppers, Gastrimargus marmoratus (Thunberg) and Oedaleus asiaticus Bei-Bienko, are listed as major pest Orthoptera species due to their damage to agriculture [1,2]. G. marmoratus is mainly distributed in the tropical and warm grassland ecosystems in South East Asia, as well as southern and east coastal China. In such areas G. marmoratus is able to move from the wild vegetation to farmlands with rice, maize, sugarcane, or other crops [3]. O. asiaticus is only distributed in the Mongolian Plateau and the Transbaikal region of the southern Russia [4]. It often reaches high population density in overgrazed steppes and xerophytous habitats [5]. O. asiaticus can form swarm bands and exhibit gregarious-like behaviors. In the steppe region of Inner Mongolia, it feeds mainly on grasses, such as Leymus chinensis, Stipa spp. and Cleistogenes squarrosa [6]. Although some biological and ecological research has been done on the two grasshopper species, studies concerning their nucleic and mitochondrial DNA sequences still remain scarce.
There are 23 species in Gastrimargus and 27 in Oedaleus in the world. Many of these are highly localized; however, G. marmoratus and O. asiaticus are widespread and abundant. The morphology of congeneric species in the two genera is so similar that it is difficult to differentiate one from another. Molecular phylogenetic studies have shown that the three genera Gastrimargus, Oedaleus, and Locusta are closely related [7]. The migratory locust, Locusta migratoria, a noxious pest insect in the world, is often found in sympatry with G. marmoratus or O. asiaticus, although the geographical ranges of the two latter species don't overlap. The morphological similarity among hoppers of the three species has sometimes caused confusion in the field. Recent concerns about species confusion raised during estimations of hopper density and dispersal for management practices emphasize the need for accurate nymphal identification. Therefore, the identification of highly polymorphic genetic markers such as mitochondrial sequences or nucleic microsatellites has been eagerly sought.
Mitochondrial genome sequences have been extensively used for inferring phylogenetic relationships. An accumulating body of evidence reveals that analyses based on whole mitochondrial sequence data yield trees with good resolution from higher-level groups down to closely related species [8,9]. Although the Orthoptera encompasses about 22,500 described species in the world [10], merely five complete mitochondrial genome sequences were available in the GenBank when we started this study (currently 12 species have been sequenced). The monophyly of Orthoptera has been widely accepted, and was supported by morphological [11] and molecular data [12]. However, the monophyly was not recovered in previous phylogenetic analyses based on mtDNA sequences [13,14]. Recently, a study using mitochondrial genome sequences confirmed the monophyly of Orthoptera [15]. One possible reason for such contrasting results may be insufficient taxon sampling, with only a single species representing each of the two Orthoptera suborders in earlier studies [13]. Thus, the addition of new complete mitochondrial genomes of orthopteran species will contribute to understanding of phylogenetic relationships in the Orthoptera and Insecta.
In this study, we determined the complete sequences of the mitochondrial genomes of G. marmoratus and O. asiaticus, and compared in detail the full sequences of both species. In addition, we analysed the phylogeny of 14 orthopteran species and 10 other polyneopteran species, as well as 4 outgroup insect species based on a concatenation of 13 mitochondrial protein-coding genes.
Methods
Samples and mitochondria isolation
Samples of G. marmoratus and O. asiaticus were collected in Hainan (109.47°E, 19.02°N) and Inner Mongolia (116.08°E, 43.94°N), China, respectively. They were preserved in 95% ethanol and maintained at 4°C. The mitochondria isolation for both species was performed according to Tamura and Aotsuka [16], with some modifications. A small portion of muscle tissue from a single individual was homogenized in 2 ml of chilled buffer (5 mM Tris, 70 mM sucrose, 220 mM Mannitol and 2 mM EDTA, pH 7.4). The homogenate was centrifuged at 500 g for 10 min at 4°C, and the supernatant was recovered and centrifuged at 800 g for 10 min at 4°C to pellet the nuclei and cellular debris. The resulting supernatant was centrifuged at 12,000 g for 10 min at 4°C to pellet the mitochondria.
Mitochondrial DNA extraction
A modified method of salt-extraction protocol [17] was used to extract mtDNA from the isolated mitochondria. The mitochondria were resuspended in 330 μl of buffer (pH 8.2) containing 100 mM Tris, 40 mM NaCl and 2 mM EDTA. Then, 13 μl of 20% SDS and 6 μl of 20 mg/ml proteinase K were added to the mixture and incubated at 60°C for at least 2 hours or overnight, after which 250 μl of 5.3 M NaCl was added. The mixture was centrifuged at 5,000 rpm for 10 min at 4°C. The supernatant was transferred to a fresh tube, and 480 μl of chilled isopropanol was added and centrifuged at 12,000 g for 15 min at 4°C to pellet mtDNA. The pellet was washed with 75% ethanol, dried and then resuspended in sterile ddH2O.
Genome determination and molecular analyses
Nine and seven pairs of PCR primers (See additional file 1: List of primers used for PCR amplification) were designed to amplify overlapping segments of the entire mitochondrial genomes of G. marmoratus and O. asiaticus, respectively. Two fragments of about 4.5 kb were amplified using LA Taq™ (Takara Biomedical, Japan) with an initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 4.5 min, with a final elongation at 72°C for 7 min after the last cycle. The other fragments (~2 kb) were amplified using Takara EX Taq™ (Takara Biomedical, Japan) under the following conditions: 3 min at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s at 50 – 55°C, 2 – 2.5 min at 72°C, and a final extension period of 7 min at 72°C. After purification with AxyPrep™ DNA Gel Extraction Kit, all PCR products were sequenced directly by means of primer walking. Sequencing was performed using BigDye terminator chemistry and ABI 3730x1 DNA Analyzer.
Sequence data were assembled using SeqMan software (DNAStar, Inc.). Transfer RNAs were identified by tRNAscan-SE 1.21 [18], and the other genes were determined by comparison with those of the sequenced orthopterans. Strand skew values were calculated according to the formulae by Perna et al. [19]. Divergences of cox1 sequences were calculated using MEGA 4.1 Beta [20] with the Kimura-2-Parameter model. The formula for calculating the divergence in the sliding-window analysis was as per Proutski et al. [21].
Phylogenetic analyses
A total of 24 polyneopteran mitochondrial genomes available in the GenBank were included in our analyses (Table 1). For outgroups, we excluded the taxa that had been reported to introduce phylogenetic errors, based on the following criteria [14]: extreme compositional bias, inversion or translocation of genes on the opposite strand, inversion of the A+T-rich region, or lack of atp8. As a result, one species from Archaeognatha, one species from Zygentoma, and two species from Hemiptera were selected as outgroups. This selection appears appropriate considering both insect phylogeny and outgroup choice in previous studies [12,22].
Table 1.
List of taxa used in the phylogenetic analysis.
| Order | Family | Species | Accession number | Reference |
| Orthoptera | Acrididae | Locusta migratoria | NC_001712 | [32] |
| Orthoptera | Acrididae | Gastrimargus marmoratus | EU513373 | This study |
| Orthoptera | Acrididae | Oedaleus asiaticus | EU513374 | This study |
| Orthoptera | Acrididae | Oxya chinensis | NC_010219 | [33] |
| Orthoptera | Acrididae | Acrida willemsei | NC_011303 | [15] |
| Orthoptera | Acrididae | Calliptamus italicus | NC_011305 | [15] |
| Orthoptera | Acrididae | Chorthippus chinensis | NC_011095 | [34] |
| Orthoptera | Gryllotalpidae | Gryllotalpa orientalis | NC_006678 | [13] |
| Orthoptera | Gryllotalpidae | Gryllotalpa pluvialis | NC_011302 | [15] |
| Orthoptera | Myrmecophilidae | Myrmecophilus manni | NC_011301 | [15] |
| Orthoptera | Tettigoniidae | Gampsocleis gratiosa | NC_011200 | [39] |
| Orthoptera | Tettigoniidae | Anabrus simplex | NC_009967 | [40] |
| Orthoptera | Tettigoniidae | Ruspolia dubia | NC_009876 | [41] |
| Orthoptera | Rhaphidophoridae | Troglophilus neglectus | NC_011306 | [15] |
| Mantodea | Mantidae | Tamolanica tamolana | NC_007702 | [53] |
| Blattaria | Blattidae | Periplaneta fuliginosa | NC_006076 | [54] |
| Isoptera | Rhinotermitidae | Reticulitermes flavipes | NC_009498 | [55] |
| Isoptera | Rhinotermitidae | Reticulitermes hageni | NC_009501 | [55] |
| Isoptera | Rhinotermitidae | Reticulitermes santonensis | NC_009499 | [55] |
| Isoptera | Rhinotermitidae | Reticulitermes virginicus | NC_009500 | [55] |
| Mantophasmatodea | Mantophasmatidae | Sclerophasma paresisense | NC_007701 | [53] |
| Phasmatodea | Timematidae | Timema californicum | DQ241799 | [53] |
| Grylloblattodea | Grylloblattidae | Grylloblatta sculleni | DQ241796 | [53] |
| Plecoptera | Pteronarcyidae | Pteronarcys princeps | NC_006133 | [56] |
| Hemiptera | Aphrophoridae | Philaenus spumarius | NC_005944 | [49] |
| Hemiptera | Reduviidae | Triatoma dimidiata | NC_002609 | [50] |
| Zygentoma | Lepidotrichidae | Tricholepidion gertschi | NC_005437 | [9] |
| Archaeognatha | Meinertellidae | Nesomachilis australica | NC_006895 | [51] |
The nucleotide and amino acid sequences of the protein-coding genes were retrieved from the Mitome database [23]. The amino acid sequences were individually aligned using BioEdit [24], followed by manual refinements. The corresponding nucleotide sequences were retro-aligned, using the PAL2NAL webserver [25], and then concatenated into a single alignment. With "codons" selected as the type of sequence and other default options, the program Gblocks [26] was applied to remove poorly aligned positions of the nucleotide alignment. Third codon positions were highly saturated as was determined by DAMBE [27]. To investigate the effect of mutational saturation, we employed phylogenetic analyses based on the two data sets: (i) DNA alignment including only first and second codon positions, and (ii) DNA alignment with all codon positions included. The TVM+I+G model was selected as the best-fit one by the program ModelTest (ver. 3.7) [28] based on the Akaike Information Criterion.
Bayesian Inference (BI) and Maximum Likelihood (ML) methods were employed to analyze the two data sets. BI analysis was performed using MrBayes, ver.3.1.2 [29]. Two sets of four chains were allowed to run simultaneously for 1,000,000 generations. Each set was sampled every 100 generations with a burnin of 25%. Stationarity was considered to be reached when the average standard deviation of split frequencies was less than 0.01. Bayesian posterior probabilities (BPP) were estimated on a 50% majority rule consensus tree of the remaining trees. ML analysis was conducted using the program TreeFinder [30] with GTR substitution model. Bootstrap analysis was performed with 500 replicates.
Results and discussion
General features
The complete mtDNA sequences of G. marmoratus and O. asiaticus are 15,924 bp and 16,259 bp in size, respectively (Table 2). The two mitochondrial genomes have been deposited in the GenBank database under the accession numbers EU513373 (for G. marmoratus) and EU513374 (for O. asiaticus). The mitochondrial genome sequence of O. asiaticus is the longest in all orthopteran mitochondrial genomes available in the GenBank. Its relatively large size is mainly owing to the extended A+T-rich region caused by the presence of tandem repeats. Both mitochondrial genomes share the same 37 typical metazoan genes (13 protein-coding genes, 22 transfer RNA genes, and 2 ribosomal RNA genes) and an A+T-rich region [31], and they have identical gene arrangement with L. migratoria [32],Oxya chinensis [33], Chorthippus chinensis [34], Calliptamus italicus [15], and Acrida willemsei [15]. In addition to the A+T-rich region, 72 and 98 noncoding nucleotides are present in the mitochondrial genomes of G. marmoratus and O. asiaticus, respectively. There are overlapping genes in both mitochondrial genomes as in other metazoan mitochondrial genomes. In G. marmoratus, the overlaps occur six times and involve a total of 35 bp, lacking only one 1-bp overlap between trnQ and trnM compared with O. asiaticus. These overlaps exist between atp8 and atp6 on the majority strand, nad4L and nad4 on the minority strand, and between some adjacent genes oriented on opposite strands.
Table 2.
Annotation of the mitochondrial genomes of Gastrimargus marmoratus (Gm) and Oedaleus asiaticus (Oa).
| Feature | Strand | Position | Initiation codon/Stop codon | Anticodon | ||
| Gm | Oa | Gm | Oa | |||
| trnI | J | 1–65 | 1–65 | GAT | ||
| trnQ | N | 63–131 | 63–131 | TTG | ||
| trnM | J | 135–203 | 131–199 | CAT | ||
| nad2 | J | 204–1224 | 200–1220 | ATG/T-- | ATG/T-- | |
| trnW | J | 1225–1290 | 1221–1286 | TCA | ||
| trnC | N | 1283–1351 | 1279–1344 | GCA | ||
| trnY | N | 1362–1429 | 1354–1420 | GTA | ||
| cox1 | J | 1422–2961 | 1413–2952 | ATC/T-- | ATC/T-- | |
| trn L(UUR) | J | 2962–3027 | 2953–3018 | TAA | ||
| cox2 | J | 3031–3712 | 3027–3708 | ATG/T-- | ATG/T-- | |
| trnD | J | 3713–3776 | 3709–3772 | GTC | ||
| trnK | J | 3780–3850 | 3776–3846 | CTT | ||
| atp8 | J | 3868–4026 | 3864–4022 | ATC/TAA | ATC/TAA | |
| atp6 | J | 4020–4697 | 4016–4693 | ATG/TAA | ATG/TAA | |
| cox3 | J | 4702–5493 | 4698–5489 | ATG/TAA | ATG/TAA | |
| trnG | J | 5496–5560 | 5492–5555 | TCC | ||
| nad3 | J | 5561–5912 | 5556–5907 | ATT/T-- | ATT/T-- | |
| trnA | J | 5913–5977 | 5908–5972 | TGC | ||
| trnR | J | 5981–6044 | 5977–6043 | TCG | ||
| trnN | J | 6045–6111 | 6044–6111 | GTT | ||
| trnS (AGN) | J | 6112–6178 | 6112–6178 | GCT | ||
| trnE | J | 6179–6245 | 6179–6244 | TTC | ||
| trnF | N | 6244–6308 | 6243–6308 | GAA | ||
| nad5 | N | 6309–8028 | 6309–8025 | ATT/T-- | ATT/T-- | |
| trnH | N | 8044–8109 | 8041–8108 | GTG | ||
| nad4 | N | 8111–9445 | 8110–9444 | ATG/TAG | ATG/TAA | |
| nad4L | N | 9439–9732 | 9438–9731 | ATG/TAA | ATG/TAA | |
| trnT | J | 9735–9798 | 9734–9797 | TGT | ||
| trnP | N | 9799–9863 | 9798–9862 | TGG | ||
| nad6 | J | 9866–10390 | 9865–10386 | ATG/TAA | ATG/TAA | |
| cob | J | 10395–11533 | 10394–11532 | ATG/TA- | ATG/TA- | |
| trnS (UCN) | J | 11534–11603 | 11533–11603 | TGA | ||
| nad1 | N | 11604–12569 | 11625–12569 | ATG/TAA | ATA/TAA | |
| trnL (CUN) | N | 12573–12638 | 12573–12638 | TAG | ||
| rrnL | N | 12639–13960 | 12639–13956 | |||
| trnV | N | 13961–14032 | 13957–14027 | TAC | ||
| rrnS | N | 14033–14863 | 14028–14858 | |||
| A+T-rich region | J | 14864–15924 | 14859–16259 | |||
J and N refer to the majority and minority strand, respectively. Position numbers refer to positions on the majority strand.
The nucleotide compositions of the entire mtDNA sequences for G. marmoratus and O. asiaticus are significantly biased toward A and T. The A+T content is 75.18% (A = 45.57%, T = 29.62%, C = 15.22%, G = 9.60%) in G. marmoratus and 75.39% in O. asiaticus (A = 45.03%, T = 30.36%, C = 14.57%, G = 10.04%; see Table 3). On the other hand, both of the majority strands of G. marmoratus (AT-skew = 0.212, GC-skew = -0.226) and O. asiaticus (AT-skew = 0.195, GC-skew = -0.184) favor A and C. In mammals, the underlying mechanism for this bias of strand-specific nucleotide composition is the deamination of C and A in the H strand during replication [35].
Table 3.
Nucleotide compositions of Gastrimargus marmoratus (Gm) and Oedaleus asiaticus (Oa).
| Feature | A (%) | C (%) | G (%) | T (%) | A+T (%) | |||||
| Gm | Oa | Gm | Oa | Gm | Oa | Gm | Oa | Gm | Oa | |
| Whole genome | 45.57 | 45.03 | 15.22 | 14.57 | 9.60 | 10.04 | 29.62 | 30.36 | 75.18 | 75.39 |
| Protein-coding genes* | 33.31 | 33.32 | 13.66 | 13.61 | 12.43 | 12.53 | 40.60 | 40.55 | 73.91 | 73.86 |
| 1st codon positions | 34.50 | 34.23 | 12.32 | 12.54 | 18.39 | 18.81 | 34.79 | 34.42 | 69.29 | 68.65 |
| 2nd codon positions | 20.19 | 19.91 | 19.89 | 20.16 | 14.20 | 14.21 | 45.72 | 45.72 | 65.91 | 65.64 |
| 3rd codon positions | 45.23 | 45.80 | 8.78 | 8.13 | 4.70 | 4.57 | 41.29 | 41.50 | 86.52 | 87.30 |
| tRNA genes | 38.50 | 38.21 | 10.95 | 11.01 | 14.22 | 14.34 | 36.33 | 36.44 | 74.83 | 74.64 |
| rrnL genes | 32.45 | 32.09 | 7.87 | 7.74 | 13.99 | 14.11 | 45.69 | 46.05 | 78.14 | 78.15 |
| rrnS genes | 29.96 | 29.48 | 8.78 | 9.51 | 15.52 | 15.04 | 45.73 | 45.97 | 75.69 | 75.45 |
| A+T-rich region | 51.93 | 48.89 | 10.37 | 8.78 | 5.37 | 6.71 | 32.33 | 35.62 | 84.26 | 84.51 |
* Stop codons were excluded.
Transfer and ribosomal RNA genes
The complete set of 22 tRNA genes typical of metazoan mitochondrial genomes is present in the two mitochondrial genomes: two for serine and leucine, and one for the other amino acids. All tRNA genes were determined by tRNAscan-SE 1.21 [18], except for trnH in G. marmoratus and trnS(AGN) in both mitochondrial genomes, which were determined through sequence comparison with previously published orthopteran mitochondrial genomes. Twenty-one tRNA genes can be folded into the typical cloverleaf structure, whereas trnS(AGN) in both mitochondrial genomes has an unpaired stretch of 11 nucleotides instead of the DHU arm, as is often found in arthropod mitochondrial genomes. Both mitochondrial genomes have the identical nucleotide sequence for only trnL(CUN). Unmatched base pairs have been observed in stems of tRNA secondary structures. In G. marmoratus, there are 22 unmatched base pairs, consisting of 19 G-U pairs, 1 A-A and 2 U-U mismatches; whereas in the case of O. asiaticus, 16 G-U pairs, 1 A-A and 3 U-U mismatches have been identified. Their anticodons are identical to those of all the other available orthopteran mitochondrial genomes. Compared with the published ensiferan mitochondrial gene order cox2-trnK-trnD-atp8, there is a rearrangement of trnD and trnK in both G. marmoratus and O. asiaticus mitochondrial genomes, as well as in those of all the other caeliferans determined so far. This is consistent with the previous finding that in the Orthoptera such rearrangement only occurs within the suborder Caelifera [36].
The two genes encoding the large and small ribosomal RNA subunits (rrnL and rrnS) are located between trnL(CUN) and trnV, and between trnV and the A+T-rich region, respectively. The length of rrnL is 1,322 bp in G. marmoratus and 1,318 bp in O. asiaticus, with an A+T content of 78.14% and 78.15%, respectively. The rrnS is 831 bp in both mitochondrial genomes, and the A+T content is 75.69% for G. marmoratus and 75.45% for O. asiaticus (Table 3).
Protein-coding genes
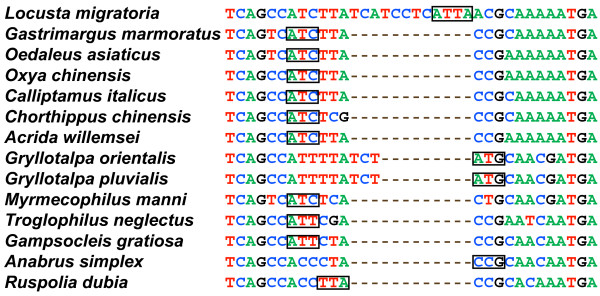
All of the 13 G. marmoratus protein-coding genes start with a typical ATN codon: two (nad3, nad5) with ATT, two (cox1, atp8) with ATC and the other nine with ATG (Table 2). In comparison with G. marmoratus, merely nad1 in O. asiaticus possesses a different initiation codon ATA. Previous studies reported no typical ATN initiation codon for cox1 in mitochondrial genomes of many species. As a consequence, many other irregular initiation codons, such as ATTA [37] and ATTTAA [38], were postulated. In G. marmoratus and O. asiaticus, however, the putative initiation codon for cox1 is ATC, which is located 8-bp upstream of the adjacent trnY. In the cox1 start region, L. migratoria has an insert of 9 or 12 nucleotides compared with the other orthopterans (Figure 1). The initiation codon for cox1 remains ambiguous in L. migratoria, where a 4-bp start sequence, ATTA, has been proposed [32]. The counterparts for O. chinensis [33], C. italicus [15], and C. chinensis [34] have been presumed to be ATC. Based on the cox1 sequence in A. willemsei (GenBank: NC_011303), ATC could be regarded as the potential initiation codon. Therefore, the caeliferan species share the same initiation codon ATC except for L. migratoria. In both mole crickets Gryllotalpa orientalis [13] and Gryllotalpa pluvialis [15], cox1 has a canonical initiation codon ATG. The initiation codon is ATC in the tettigoniid species like Myrmecophilus manni [15] and ATT in both Troglophilus neglectus [15] and Gampsocleis gratiosa [39]. The triplet CCG has been identified as the cox1 initiation codon for the cricket Anabrus simplex [40]. Although the corresponding triplet is also CCG for the katydid Ruspolia dubia, TTA has been proposed to initiate cox1 [41]. Annotation of a new mitochondrial genome is commonly carried out by comparison with closely related mitochondrial genomes already determined. This approach, therefore, has limitations to this extent. The precise initiation codon for cox1 can be finally determined by protein sequencing.
Figure 1.
The alignment of the cox1 start region of orthopteran mitochondrial genomes currently available. Hyphens indicate inferred gaps. Boxed nucleotides have been proposed to act as initiation codons, except for ATC in G. marmoratus, O. asiaticus and A. willemsei, which was proposed in our analysis.
Seven of the 13 protein-coding genes terminate with the conventional stop codons TAG or TAA, and the remaining ones have incomplete stop codons T or TA adjacent to a downstream tRNA gene (Table 2). The only difference in stop codons between the two mitochondrial genomes lies in that nad4 gene in G. marmoratus uses TAG, while the stop codon is TAA in O. asiaticus. The secondary structure of tRNA genes facilitates correct processing of the polycistronic transcript into mature RNA molecules [42]. The presence of incomplete stop codon is common in metazoan mitochondrial genomes, and these truncated stop codons are presumed to be completed via post-transcriptional polyadenylation [42]. In accordance with other arthropods, overlapping protein-coding genes are present in both G. marmoratus and O. asiaticus mitochondrial genomes; a 7-bp overlap exists not only between atp8 and atp6 but also between nad4L and nad4. In this case, hairpin structures forming at the 3' end of the upstream protein's mRNA, rather than secondary structures of tRNA genes, may act as a signal for the cleavage of the polycistronic primary transcript [40].
A DNA barcoding approach based on cox1 sequence diversity has been utilized for identification of closely allied species [43]. We calculated pairwise divergences of a 650-bp sequence (corresponding to nucleotide positions 1428–2077 in G. marmoratus mitochondrial genome) from the cox1 5' terminus in the fourteen known orthopteran mitochondrial genomes (See additional file 2: Pairwise divergences (%) of a 650-bp sequence from cox1 5' terminus). Pairwise between the caeliferans and the ensiferans exhibits overall high divergences. The pairwise divergences range from 11.04% to 31.65%, indicating that this fragment of cox1 gene is effective enough to discriminate these species. However, whether the cox1 barcode sequence could be applied to the whole Orthoptera requires broad taxon sampling.
The nucleotide sequence identities of G. marmoratus and O. asiaticus protein-coding genes range from 84.3% (nad6) to 92.1% (nad4L; see Table 4). Based on identity of inferred amino acid sequences, cox1 (97.0%) is the most conserved protein-coding gene, while atp8 (80.7%) is the least conserved with a variable domain at the C-terminus.
Table 4.
Sequence identity of G. marmoratus and O. asiaticus protein-coding genes.
| gene | number of codons | % sequence identity | ||
| G. marmoratus | O. asiaticus | nucleotide | Amino acid | |
| atp6 | 225 | 225 | 88.9 | 92.8 |
| atp8 | 52 | 52 | 86.1 | 80.7 |
| cox1 | 513 | 513 | 90.1 | 97.0 |
| cox2 | 227 | 227 | 91.2 | 95.1 |
| cox3 | 263 | 263 | 89.3 | 93.1 |
| cob | 379 | 379 | 87.5 | 91.5 |
| nad1 | 321 | 314 | 90.4 | 92.8 |
| nad2 | 340 | 340 | 86.0 | 84.4 |
| nad3 | 117 | 117 | 89.4 | 89.7 |
| nad4 | 444 | 444 | 89.8 | 91.2 |
| nad4L | 97 | 97 | 92.1 | 95.8 |
| nad5 | 573 | 572 | 91.3 | 92.4 |
| nad6 | 174 | 173 | 84.3 | 83.9 |
The A+T content of protein-coding genes, excluding stop codons, is 73.91% and 73.86% in G. marmoratus and O. asiaticus, respectively (Table 3). This significant AT-bias affects codon usage in proteins, with ATT (encoding isoleucine) being the most frequently used codon and GC-rich codons being least frequently used (e.g., CGC is absent in both mitochondrial genomes). In G. marmoratus and O. asiaticus, when first, second and third codon positions are considered separately, the highest A+T content is in third codon positions (86.52% and 87.30%, respectively), the strongest bias toward T is in second codon positions (both 45.72%), and the lowest content of G is in third codon positions (4.70% and 4.57%, respectively; see Table 3).
The A+T-rich region
This region has an A+T content of 84.26% in G. marmoratus and 84.51% in O. asiaticus (Table 3). The A+T-rich regions of two grasshopper species, Schistocerca gregaria and Chorthippus parallelus, have been sequenced [44]. The A+T-rich region, usually the largest noncoding part of the metazoan mitochondrial DNA molecule, evolves relatively fast due to few selective constraints. For the orthopterans, a high mutation rate of this region results in significant size variation ranging from 70 bp in the katydid R. dubia [41] to 1,512 bp in the grasshopper C. parallelus [44]. This variation is predominantly due to both length variation within tandem repeats and differences in their copy numbers.
In G. marmoratus, there are three tandem repeat units. The first one begins in the rrnS gene and extends into the A+T-rich region. The first two repeat units are 166 bp long and identical in sequence, slightly different from the third one (155 bp). The A+T content is 79.88% for these repeat sequences and 87.68% for the A+T-rich region excluding these repeat sequences. The longest open reading frame (255 bp in length, encoding 85 amino acids) detected in the A+T-rich region is located in the minority strand of these tandem repeats, but a tblastn research found no significant similarity with sequences in the GenBank database, suggesting it is a non-functional ORF. In O. asiaticus, the A+T-rich region contains two repeat regions. The first one (75.83% A+T) consists of two complete repeat units (155 bp) and one truncated repeat unit (141 bp), with 5 to 7 nucleotide substitutions between them. Of these repeat units, the first one is partially located in the rrnS gene. The minority strand of this repeat region has the longest open reading frame (237 bp) of the A+T-rich region, but we got negative results using the tblastn research. The other repeat region (90.35% A+T), situated near the trnI gene, comprises two repeat units (345 and 339 bp, respectively) with the shorter one truncated at 3' end. Similar large tandem repeats are also present in the A+T-rich region of L. migratoria, C. parallelus, T. neglectus and G. gratiosa but they are absent in the other orthopteran mitochondrial genomes determined so far. The fact that tandem repeats are non-conserved among these orthopteran mitochondrial genomes indicates a lack of a functional role for these tandem repeats. Replication slippage is regarded as a dominant mechanism to account for the existence of tandem repeats [45,46].
The A+T-rich region contains control elements for replication and transcription of animal mitochondrial genomes [31]. Of them, the stem-loop secondary structure is potentially involved in initiation of a second-strand replication [44]. The possible stem-loop structure located immediately upstream of the origin of the minority strand has been detected in L. migratoria [47]. Such stem-loop structures also exist in all the other previously determined caeliferan mitochondrial genomes. For G. marmoratus and O. asiaticus, using the program mfold [48], we have predicted the possible stem-loop structures, both of which have the same secondary structure and nucleotide sequence as that of L. migratoria. The conserved stem-loop structures in these mitochondrial genomes suggest their functional importance and may provide clues for understanding the initiation process of mtDNA replication.
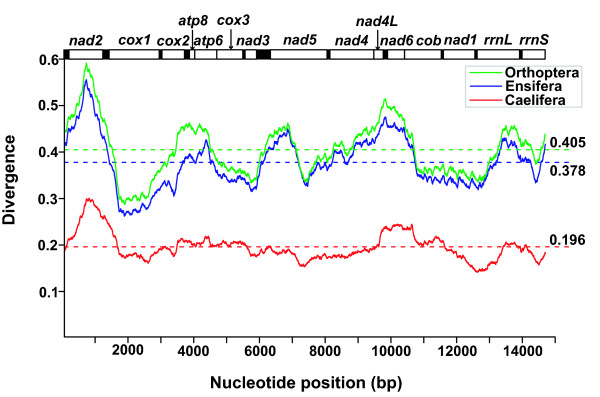
Divergence of mtDNA sequences
A sliding-window analysis was performed to compute divergence of the fourteen orthopteran mtDNA sequences excluding the A+T-rich region (Figure 2). The mean divergence of the fourteen orthopteran mtDNA sequences is 0.405. The nad2 gene has undergone accelerated evolution, as evidenced by the highest level of divergence. The cox1 gene is the most conserved protein-coding gene, and is therefore a useful marker for investigating phylogenetic relationships at higher taxonomic levels. Divergence of mtDNA sequences of the seven caeliferans and the seven ensiferans was also calculated, respectively (Figure 2). The seven ensiferans has not only overall similar sequence divergence pattern to that of the fourteen orthopterans but much higher divergence (mean divergence = 0.378) than the seven caeliferans (mean divergence = 0.196), indicating that the high divergence of the fourteen orthopterans attributes to the ensiferan mtDNA sequence divergence. By contrast, mtDNA sequences of the caeliferans are more conserved, except for sequences of nad2, nad4L, and nad6. Due to the highest divergence, the nad2 nucleotide sequence can be used as an effective molecular marker to analyse intraspecific relationships and to distinguish closely related grasshopper species.
Figure 2.
Plot of divergences among the Orthoptera mtDNA sequences excluding the A+T-rich region. The bar at the top illustrates the position of protein-coding genes and rRNAs, and the tRNAs are represented as black boxes. Dashed lines indicate mean divergence. The window is 1,000 bp in length and slides 1 bp at a time. The sliding-window analysis calculates the divergence of the 14 orthopterans, the 7 caeliferans, and the 7 ensiferans, respectively.
Phylogenetic relationships
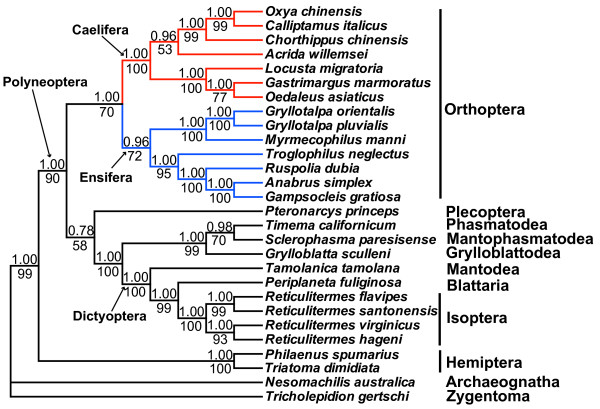
We performed phylogenetic analysis with nucleotide sequences of 13 mitochondrial protein-coding genes from 24 polyneopteran species and 4 outgroup insect species (Philaenus spumarius [49],Triatoma dimidiate [50],Tricholepidion gertschi [9] and Nesomachilis australica [51]). BI and ML analyses using only first and second codon positions of the 13 protein-coding genes generate identical tree topologies (Figure 3).
Figure 3.
Phylogenetic tree of 24 polyneopterans. Phylogenetic analysis was based on first and second codon positions of 13 protein-coding genes. The tree was rooted by Archaeognatha, Zygentoma, and Hemiptera. Numbers refer to Bayesian posterior probabilities (BPP; above nodes) and bootstrap support values (BS; below nodes).
Polyneoptera refers to an assemblage of eleven insect orders, and its monophyly remains contentious [22]. Using both morphological and molecular data, Wheeler et al. [52] recovered monophyletic Polyneoptera. However, Terry & Whiting [22] supported the paraphyly of Polyneoptera based on an extensive data set (18S rDNA, 28S rDNA, Histone 3 DNA sequences, and 125 morphological characters). In the present study, eight orders of the polyneopteran lineages are included and they cluster as a monophyletic clade (Figure 3). Our result may provide evidence for resolving phylogenetic relationships of Polyneoptera, although the currently limited taxon sampling highlights the preliminary nature of this analysis. The unsampled Dermaptera, Embiidina, and Zoraptera, should be included in further studies to provide a more accurate phylogenetic estimate.
Dictyoptera (Isoptera, Blattaria, and Mantodea) is recovered as monophyletic (Figure 3), with Tamolanica tamolana [53] (Mantodea) as sister taxon to Periplaneta fuliginosa [54] (Blattaria) and four Reticulitermes species [55] (Isoptera). The relationships within Dictyoptera are in agreement with previous analyses [22]. The sister relationship between Mantophasmatodea and Phasmatodea is supported and this clade is sister to Grylloblattodea (Figure 3). Our result is consistent with previous analyses using mitochondrial protein-coding genes [14,53], but different from the study by Terry & Whiting [22] who suggested a sister-group relationship between Mantophasmatodea and Grylloblattodea.
Plecoptera, here represented by Pteronarcys princeps [56], is sister group to the assemblage (((Mantophasmatodea + Phasmatodea) + Grylloblattodea) + Dictyoptera), but the low support values (BPP = 0.78, BS = 58%; see Figure 3) suggest that the position of Plecoptera is not well resolved in the phylogenetic analysis. Plecoptera has been proposed as the sister taxon to Dermaptera and Zoraptera [22], whose mitochondrial genomes are not available. Carapelli et al. [14] clustered P. princeps with Diptera (flies) rather than polyneopterans, and detected no problematic characteristics within its mitochondrial genome. Later, when examining the phylogenetic signal from mitochondrial genome data, Fenn et al. [15] found that P. princeps introduced instability in phylogenetic analysis possibly due to base composition heterogeneity, and thus they excluded it. To test the effect caused by P. princeps, in the present study we have reconstructed the phylogeny by removing P. princeps and compared it with the phylogeny including this taxon. We find that exclusion of P. princeps makes no difference in placement of all other taxa but results in a more stable tree topology, as is evident from higher nodal supports (See additional file 3: Phylogenetic tree of Polyneoptera without Pteronarcys princeps). Although P. princeps has not considerably affected phylogenetic inference in our study, sampling other closely related plecopterans might be a strategy for further progress in the reconstruction of Polyneoptera phylogeny.
The two Orthoptera suborders, Caelifera and Ensifera, are both recovered as monophyletic (the latter taxon with a lower statistical support: BPP = 0.96, BS = 72%; see Figure 3). This result is consistent with traditional morphological taxonomics and previous studies [57,58]. The sister taxon relationship (BPP = 1.00, BS = 70%) between Caelifera and Ensifera supports the monophyly of Orthoptera [10-12,15]. Within Caelifera, five subfamilies of Acrididae are represented and Oedipodinae occupies the basal position. The ensiferan species split into two clades, (Tettigoniidae + Rhaphidophoridae) and (Gryllotalpidae + Myrmecophilidae), concordant with the study of Fenn et al. [15]. In contrast with the failure to recover the monophyletic Orthoptera in previous mtDNA-based phylogenetic analyses [13,14], our study demonstrates that analyses with only two species representing the Orthoptera may lead to false phylogenetic inferences. Nevertheless, given the more than 20,000 species of the Orthoptera, the present taxon sampling is still far from enough. To further clarify the phylogeny of the Orthoptera, more extensive sequencing of orthopteran mitochondrial genomes is required.
Our initial analysis using all codon positions of protein-coding genes leads to quite different tree topologies, compared with the tree based on only first and second codon positions. Neither the ML nor the BI tree recovers monophyletic Orthoptera (See additional file 4: Phylogenetic trees of Polyneoptera using all codon positions of 13 protein-coding genes). Furthermore, the assemblage (((Mantophasmatodea + Phasmatodea) + Grylloblattodea) + Dictyoptera) is sister either to Caelifera (BS = 53%) in the ML tree or to the clade of Ensifera and Plecoptera (BPP = 0.54) in the BI tree. It is likely due to the mutational saturation of third codon positions plaguing the phylogenetic analysis and subsequently decreasing support values. Therefore, the analysis using all codon positions is not suitable in the context of our taxon sampling, although Fenn et al. [15] found that inclusion of third codon positions did not negatively affect phylogenetic inference. Here, we regard the analysis using only first and second positions as our best estimate.
Conclusion
The mitochondrial genomes of G. marmoratus and O. asiaticus have overall similarities. Both species, with other grasshopper species, share the same mitochondrial genome organization, which differs from that of the available ensiferan species by the translocation between trnD and trnK. The potential initiation codon for cox1 gene in G. marmoratus and O. asiaticus is ATC. In addition to stem-loop structures in the A+T-rich region, another common feature of both mitochondrial genomes is the existence of tandem repeats, but the kinds of repeats and the copy number of each repeat unit are variable. The sliding-window analysis reveals that mtDNA sequences of the analysed caeliferans have lower divergence than those of the ensiferans. The nad2 nucleotide sequence may serve as an effective marker to determine phylogenetic relationships of intraspecies and closely related grasshopper species. The phylogenetic analysis based on mtDNA sequences of 13 protein-coding genes confirms the monophyly of Orthoptera. The analyses of G. marmoratus and O. asiaticus mitochondrial genomes have added to our knowledge on mitochondrial genomes of Orthoptera.
Abbreviations
atp6 and atp8: ATP synthase subunits 6 and 8; cob: cytochrome b; cox1-3: cytochrome c oxidase subunits 1–3; nad1–6 and nad4L: NADH dehydrogenase subunits 1–6 and 4L; rrnS and rrnL: small and large ribosomal RNA (rRNA) subunits; trnX: transfer RNA (tRNA) genes, where X is the one-letter abbreviation of the corresponding amino acid; BS: bootstrap support.
Authors' contributions
LK was primarily responsible for the design, coordination and conduction of this study. CM was responsible for determining and assembling the mtDNA sequences, and drafted the manuscript, tables, and figures. CM and CL performed phylogenetic analyses. PY calculated divergence of mtDNA sequences. LK extensively revised the manuscript. All authors read and approved the final manuscript.
Supplementary Material
List of primers used for PCR amplification. * Numbers refer to the nucleotide positions of primers' 5 prime ends. Primers in bold were used to amplify ~4.5 Kb fragments with LA Taq™ polymerase.
Pairwise divergences (%) of a 650-bp sequence from cox1 5' terminus. Divergences were calculated using MEGA 4.1 Beta [20] with the Kimura 2-Parameter model.
Phylogenetic tree of Polyneoptera without Pteronarcys princeps. Phylogenetic analysis was based on first and second codon positions of 13 protein-coding genes. To avoid the potential effect introduced by P. princeps, we excluded it in this analysis. Numbers refer to Bayesian posterior probabilities (above nodes) and bootstrap support values (below nodes).
Phylogenetic trees of Polyneoptera using all codon positions of 13 protein-coding genes. Numbers at nodes refer to Bayesian posterior probabilities (left tree) and ML bootstrap support values (right tree).
Acknowledgments
Acknowledgements
We thank Xiaoyan Jiang for her assistance in mtDNA extraction. We also appreciate the valuable comments by anonymous reviewers on the earlier version of this manuscript. The study is supported by the grants from the Natural Science Foundation of China (No. 30770307) and the National Basic Research Program of China (2007CB411604).
Contributor Information
Chuan Ma, Email: machuan@ioz.ac.cn.
Chunxiang Liu, Email: liucx@ioz.ac.cn.
Pengcheng Yang, Email: yangpc@ioz.ac.cn.
Le Kang, Email: lkang@ioz.ac.cn.
References
- COPR . The Locust and Grasshopper Agricultural Manual. London: Centre for Overseas Pest Research; 1982. [Google Scholar]
- Samways MJ, Lockwood JA. Orthoptera conservation: pests and paradoxes. J Insect Conserv. 1998;2:143–149. doi: 10.1023/A:1009652016332. [DOI] [Google Scholar]
- Ritchie JM. A taxonomic revision of the genus Gastrimargus Saussure (Orthoptera: Acrididae) Bull Br Mus Nat Hist (Ent) 1982;44:239–329. [Google Scholar]
- Ritchie JM. A taxonomic revision of the genus Oedaleus Fieber (Orthoptera: Acrididae) Bull Br Mus Nat Hist (Ent) 1981;42:83–183. [Google Scholar]
- Kang L, Han X, Zhang Z, Sun O. Grassland ecosystems in China: review of current knowledge and research advancement. Phil Trans R Soc B. 2007;362:997–1008. doi: 10.1098/rstb.2007.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Chen Y. Trophic niche of steppe grasshoppers. Acta Ent Sin. 1994;37:178–189. [Google Scholar]
- Fries M, Chapco W, Contreras D. A molecular phylogenetic analysis of the Oedipodinae and their intercontinental relationships. J Orthoptera Res. 2007;16:115–125. doi: 10.1665/1082-6467(2007)16[115:AMPAOT]2.0.CO;2. [DOI] [Google Scholar]
- Ingman M, Kaessmann H, Pääbo S, Gyllensten U. Mitochondrial genome variation and the origin of modern humans. Nature. 2000;408:708–713. doi: 10.1038/35047064. [DOI] [PubMed] [Google Scholar]
- Nardi F, Spinsanti G, Boore JL, Carapelli A, Dallai R, Frati F. Hexapod origins: monophyletic or paraphyletic? Science. 2003;299:1887–1889. doi: 10.1126/science.1078607. [DOI] [PubMed] [Google Scholar]
- Grimaldi D, Engel MS. Evolution of the Insects. Cambridge University Press; 2005. [Google Scholar]
- Hennig W. Insect Phylogeny. Chichester: John Wiley and Sons; 1981. [Google Scholar]
- Flook PK, Rowell CH. Inferences about orthopteroid phylogeny and molecular evolution from small subunit nuclear ribosomal DNA sequences. Insect Mol Biol. 1998;7:163–178. doi: 10.1046/j.1365-2583.1998.72060.x. [DOI] [PubMed] [Google Scholar]
- Kim I, Cha SY, Yoon MH, Hwang JS, Lee SM, Sohn HD, Jin BR. The complete nucleotide sequence and gene organization of the mitochondrial genome of the oriental mole cricket, Gryllotalpa orientalis (Orthoptera: Gryllotalpidae) Gene. 2005;353:155–168. doi: 10.1016/j.gene.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Carapelli A, Liò P, Nardi F, Wath E van der, Frati F. Phylogenetic analysis of mitochondrial protein coding genes confirms the reciprocal paraphyly of Hexapoda and Crustacea. BMC Evol Biol. 2007;7:S8. doi: 10.1186/1471-2148-7-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn JD, Song H, Cameron SL, Whiting MF. A preliminary mitochondrial genome phylogeny of Orthoptera (Insecta) and approaches to maximizing phylogenetic signal found within mitochondrial genome data. Mol Phylogenet Evol. 2008;49:59–68. doi: 10.1016/j.ympev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Tamura K, Aotsuka T. Rapid isolation method of animal mitochondrial DNA by the alkaline lysis procedure. Biochem Genet. 1988;26:815–819. doi: 10.1007/BF02395525. [DOI] [PubMed] [Google Scholar]
- Aljanabi SM, Martinez I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997;25:4692–4693. doi: 10.1093/nar/25.22.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna NT, Kocher TD. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J Mol Evol. 1995;41:353–358. doi: 10.1007/BF01215182. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Proutski V, Holmes E. SWAN: sliding window analysis of nucleotide sequence variability. Bioinformatics. 1998;14:467–468. doi: 10.1093/bioinformatics/14.5.467. [DOI] [PubMed] [Google Scholar]
- Terry MD, Whiting MF. Mantophasmatodea and phylogeny of the lower neopterous insects. Cladistics. 2005;21:240–257. doi: 10.1111/j.1096-0031.2005.00062.x. [DOI] [Google Scholar]
- Lee YS, Oh J, Kim YU, Kim N, Yang S, Hwang UW. Mitome: dynamic and interactive database for comparative mitochondrial genomics in metazoan animals. Nucleic Acids Res. 36:D938–942. doi: 10.1093/nar/gkm763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34:W609–612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Xia X, Xie Z. DAMBE: software package for data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jobb G, von Haeseler A, Strimmer K. TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evol Biol. 2004;4:18. doi: 10.1186/1471-2148-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Boore JL. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flook PK, Rowell CH, Gellissen G. The sequence, organization, and evolution of the Locusta migratoria mitochondrial genome. J Mol Evol. 1995;41:928–941. doi: 10.1007/BF00173173. [DOI] [PubMed] [Google Scholar]
- Zhang C, Huang Y. Complete mitochondrial genome of Oxya chinensis (Orthoptera, Acridoidea) Acta Biochim Biophys Sin (Shanghai) 2008;40:7–18. doi: 10.1111/j.1745-7270.2008.00375.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang Y. Sequencing and Analysis of Complete Mitochondrial Genome of Chorthippus chinensis Tarb. Chin J Biochem Mol Biol. 2008;24:329–335. [Google Scholar]
- Reyes A, Gissi C, Pesole G, Saccone C. Asymmetrical directional mutation pressure in the mitochondrial genome of mammals. Mol Biol Evol. 1998;15:957–966. doi: 10.1093/oxfordjournals.molbev.a026011. [DOI] [PubMed] [Google Scholar]
- Flook P, Rowell H, Gellissen G. Homoplastic Rearrangements of Insect Mitochondrial tRNA Genes. Naturwissenschaften. 1995;82:336–337. doi: 10.1007/BF01131531. [DOI] [Google Scholar]
- Van Raay TJ, Crease TJ. Partial mitochondrial DNA sequence of the crustacean Daphnia pulex. Curr Genet. 1994;25:66–72. doi: 10.1007/BF00712970. [DOI] [PubMed] [Google Scholar]
- Nardi F, Carapelli A, Fanciulli PP, Dallai R, Frati F. The complete mitochondrial DNA sequence of the basal hexapod Tetrodontophora bielanensis : evidence for heteroplasmy and tRNA translocations. Mol Biol Evol. 2001;18:1293–1304. doi: 10.1093/oxfordjournals.molbev.a003914. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Shi F, Huang Y. The complete mitogenome of the Chinese bush cricket, Gampsocleis gratiosa (Orthoptera: Tettigonioidea) J Genet Genomics. 2008;35:341–348. doi: 10.1016/S1673-8527(08)60050-8. [DOI] [PubMed] [Google Scholar]
- Fenn JD, Cameron SL, Whiting MF. The complete mitochondrial genome sequence of the Mormon cricket (Anabrus simplex : Tettigoniidae: Orthoptera) and an analysis of control region variability. Insect Mol Biol. 2007;16:239–252. doi: 10.1111/j.1365-2583.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Huang Y, Shi F. The mitochondrial genome of Ruspolia dubia (Orthoptera: Conocephalidae) contains a short A+T-rich region of 70 bp in length. Genome. 2007;50:855–866. doi: 10.1139/G07-057. [DOI] [PubMed] [Google Scholar]
- Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Hebert PD, Ratnasingham S, deWaard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond B. 2003;270:S96–99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Szymura JM, Hewitt GM. Evolution and structural conservation of the control region of insect mitochondrial DNA. J Mol Evol. 1995;40:382–391. doi: 10.1007/BF00164024. [DOI] [PubMed] [Google Scholar]
- Levinson G, Gutman GA. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987;4:203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Fumagalli L, Taberlet P, Favre L, Hausser J. Origin and evolution of homologous repeated sequences in the mitochondrial DNA control region of shrews. Mol Biol Evol. 1996;13:31–46. doi: 10.1093/oxfordjournals.molbev.a025568. [DOI] [PubMed] [Google Scholar]
- Saito S, Tamura K, Aotsuka T. Replication origin of mitochondrial DNA in insects. Genetics. 2005;171:1695–1705. doi: 10.1534/genetics.105.046243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M, Mathews DH, Turner DH. Algorithms and Thermodynamics for RNA Secondary Structure Prediction: A Practical Guide in RNA Biochemistry and Biotechnology. Kluwer Academic Publishers; 1999. [Google Scholar]
- Stewart JB, Beckenbach AT. Insect mitochondrial genomics: the complete mitochondrial genome sequence of the meadow spittlebug Philaenus spumarius (Hemiptera: Auchenorrhyncha: Cercopoidae) Genome. 2005;48:46–54. doi: 10.1139/g04-090. [DOI] [PubMed] [Google Scholar]
- Dotson EM, Beard CB. Sequence and organization of the mitochondrial genome of the Chagas disease vector, Triatoma dimidiata. Insect Mol Biol. 2001;10:205–215. doi: 10.1046/j.1365-2583.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- Cameron SL, Miller KB, D'Haese CA, Whiting MF, Barker SC. Mitochondrial genome data alone are not enough to unambiguously resolve the relationships of Entognatha, Insecta and Crustacea sensu lato (Arthropoda) Cladistics. 2004;20:534–557. doi: 10.1111/j.1096-0031.2004.00040.x. [DOI] [PubMed] [Google Scholar]
- Wheeler WC, Whiting M, Wheeler QD, Carpenter JM. The Phylogeny of the Extant Hexapod Orders. Cladistics. 2001;17:113–169. doi: 10.1111/j.1096-0031.2001.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Cameron SL, Barker SC, Whiting MF. Mitochondrial genomics and the new insect order Mantophasmatodea. Mol Phylogenet Evol. 2006;38:274–279. doi: 10.1016/j.ympev.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Yamauchi MM, Miya MU, Nishida M. Use of a PCR-based approach for sequencing whole mitochondrial genomes of insects: two examples (cockroach and dragonfly) based on the method developed for decapod crustaceans. Insect Mol Biol. 2004;13:435–442. doi: 10.1111/j.0962-1075.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- Cameron SL, Whiting MF. Mitochondrial genomic comparisons of the subterranean termites from the Genus Reticulitermes (Insecta: Isoptera: Rhinotermitidae) Genome. 2007;50:188–202. doi: 10.1139/G06-148. [DOI] [PubMed] [Google Scholar]
- Stewart JB, Beckenbach AT. Insect mitochondrial genomics 2: The complete mitochondrial genome sequence of a giant stonefly, Pteronarcys princeps, asymmetric directional mutation bias, and conserved plecopteran A+T-region elements. Genome. 2006;49:815–824. doi: 10.1139/G06-037. [DOI] [PubMed] [Google Scholar]
- Flook PK, Rowell CH. The phylogeny of the Caelifera (Insecta, Orthoptera) as deduced from mtrRNA gene sequences. Mol Phylogenet Evol. 1997;8:89–103. doi: 10.1006/mpev.1997.0412. [DOI] [PubMed] [Google Scholar]
- Jost MC, Shaw KL. Phylogeny of Ensifera (Hexapoda: Orthoptera) using three ribosomal loci, with implications for the evolution of acoustic communication. Mol Phylogenet Evol. 2006;38:510–530. doi: 10.1016/j.ympev.2005.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of primers used for PCR amplification. * Numbers refer to the nucleotide positions of primers' 5 prime ends. Primers in bold were used to amplify ~4.5 Kb fragments with LA Taq™ polymerase.
Pairwise divergences (%) of a 650-bp sequence from cox1 5' terminus. Divergences were calculated using MEGA 4.1 Beta [20] with the Kimura 2-Parameter model.
Phylogenetic tree of Polyneoptera without Pteronarcys princeps. Phylogenetic analysis was based on first and second codon positions of 13 protein-coding genes. To avoid the potential effect introduced by P. princeps, we excluded it in this analysis. Numbers refer to Bayesian posterior probabilities (above nodes) and bootstrap support values (below nodes).
Phylogenetic trees of Polyneoptera using all codon positions of 13 protein-coding genes. Numbers at nodes refer to Bayesian posterior probabilities (left tree) and ML bootstrap support values (right tree).