Abstract
Experience-dependent plasticity is closely linked with the development of sensory function; however, there is also growing evidence for plasticity in the adult visual system. This review re-examines the notion of a sensitive period for the treatment of amblyopia in the light of recent experimental and clinical evidence for neural plasticity. One recently proposed method for improving the effectiveness and efficiency of treatment that has received considerable attention is ‘perceptual learning’. Specifically, both children and adults with amblyopia can improve their perceptual performance through extensive practice on a challenging visual task. The results suggest that perceptual learning may be effective in improving a range of visual performance and, importantly, the improvements may transfer to visual acuity. Recent studies have sought to explore the limits and time course of perceptual learning as an adjunct to occlusion and to investigate the neural mechanisms underlying the visual improvement. These findings, along with the results of new clinical trials, suggest that it might be time to reconsider our notions about neural plasticity in amblyopia.
Keywords: amblyopia, perceptual learning, sensitive periods, plasticity
1. Introduction
Amblyopia (from the Greek, amblyos—blunt; opia—vision) is a developmental abnormality that results from physiological alterations in the visual cortex and impairs form vision (Ciuffreda et al. 1991). It is often successfully treated by patching the sound eye in infants and young children, but has long been widely considered to be untreatable in adults (e.g. Mintz-Hittner & Fernandez 2000). However, a growing number of recent studies have suggested that there is substantial plasticity in the visual system of adults with amblyopia. In this review, we focus on five areas:
sensitive periods in development,
definition, diagnosis and traditional treatment of amblyopia,
perceptual learning in the mature and juvenile amblyopic visual system,
mechanisms of perceptual learning, and
perceptual learning as a clinical tool for treating amblyopia.
(a) Sensitive periods in development
Hubel & Wiesel's Nobel prize-winning work demonstrated the importance of sensory experience in shaping and maintaining neural connections during a sensitive period early in life. This work was inspired, in large measure, by the eighteenth-century notion that early visual deprivation (e.g. congenital cataract at birth) resulted in changes in the brain that, in turn, led to defective visual perception (Wiesel 1982). Based in good measure on the work of Hubel & Wiesel and subsequent anatomical and physiological studies, it is now clear that while the visual cortex is by no means a tabula rasa, there is a good deal of specification at birth (e.g. Held 1984; Horton & Hocking 1996; Chino et al. 1997). However, it is also clear that there is an important role for maturation and experience.
Sensitive periods for experience-dependent plasticity occur in virtually every species, from Drosophila to human (Berardi et al. 2000), and for a wide range of sensory functions. It is now clear that there are different sensitive periods for different functions (even within the same sensory system; e.g. Harwerth et al. 1987, 1990), different sensitive periods for different parts of the brain (even within different layers of the primary visual cortex; Levay et al. 1980) and different sensitive periods for recovery than for induction of sensory deprivation (Berardi et al. 2000).
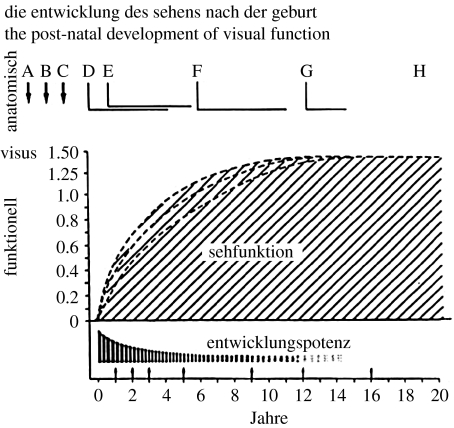
It has long been held that there is a close correspondence between sensory development and the sensitive period, and the idea is illustrated in figure 1 (adapted from Baumgartner by Teller & Movshon 1986). Figure 1 shows visual functions (sehfunktion) developing at different rates, while the developmental potential (entwicklungspotenz) dissipates. The idea that experience-dependent plasticity is closely linked with the development of sensory function is still widely held (Levi & Carkeet 1993; Berardi et al. 2000; Lewis & Maurer 2005). However, as we shall discuss later, there is also growing evidence for plasticity in the adult visual system.
Figure 1.
Cartoon illustrating visual functions (sehfunktion) developing at somewhat different rates, while the developmental potential (entwicklungspotenz) dissipates. Adapted with permission from Teller & Movshon (1986).
Much of the evidence for sensitive periods in the visual system stems from work on the effects of altered sensory input in cat and monkey, in particular monocular visual deprivation, strabismus or unequal refractive error (Wiesel 1982; for a recent review see Mitchell 2004). If the sensory deprivation occurs early, the animal is left with a permanent visual impairment—amblyopia (from the Greek for blunt vision)—and with permanent alterations in the primary visual cortex. Interestingly, brief periods of concordant binocular vision (as little as 30 min per day) may be sufficient to prevent the effects of monocular deprivation (Schwarzkopf et al. 2007).
(b) Definition, diagnosis and traditional treatment of amblyopia
Amblyopia is a developmental disorder of spatial vision usually associated with the presence of strabismus, anisometropia or form deprivation early in life (Ciuffreda et al. 1991). Amblyopia is clinically important because, aside from refractive error, it is the most frequent cause of vision loss in infants and young children (Sachsenweger 1968), and it is of basic interest because it reflects the neural impairment that can occur when normal visual development is disrupted. The damage produced by amblyopia is generally expressed in the clinical setting as a loss of visual acuity in an apparently healthy eye, despite appropriate optical correction; however, there is a great deal of evidence showing that amblyopia results in a broad range of neural, perceptual and clinical abnormalities (for recent reviews see Barrett et al. 2004; Kiorpes 2006; Levi 2006). Currently, there is no positive diagnostic test for amblyopia. Instead, amblyopia is diagnosed by exclusion: in patients with conditions such as strabismus and anisometropia, a diagnosis of amblyopia is made through the exclusion of uncorrected refractive error and the underlying ocular pathology. Amblyopic patients (especially those with strabismic amblyopia) often exhibit crowding problems, meaning they have better visual acuity when letters are presented in isolation (Levi 2008). Clinically, crowding is a useful sign to aid in the diagnosis of amblyopia.
In humans, amblyopia occurs naturally in approximately 2–4 per cent of the population (see Ciuffreda et al. 1991), and the presence of amblyopia is almost always associated with an early history of abnormal visual experience: binocular misregistration (strabismus); image degradation (high refractive error and astigmatism, and anisometropia); or form deprivation (congenital cataract and ptosis). The severity of amblyopia appears to be associated with the degree of imbalance between the two eyes (e.g. dense unilateral cataract results in severe loss), and to the age at which the amblyogenic factor occurred. Precisely how these factors interact is as yet unknown, but it is evident that different early visual experiences result in different functional losses in amblyopia (Mckee et al. 2003), and a significant factor that distinguishes performance among amblyopes is the presence or absence of binocular function.
(i) Sensitive periods for the development of amblyopia
Clinicians are well aware that amblyopia does not develop after 6–8 years of age (Worth 1903; von Noorden 1981), suggesting that there is a ‘sensitive period’ for the development of amblyopia; however, in humans with naturally occurring amblyopia, the age of onset of the amblyogenic condition(s) is difficult to ascertain, and the effects of intervention combine to make it difficult to obtain a clear picture of the ‘natural history’ of amblyopia development. Thus, much of our current understanding of the development of amblyopia accrues from animal studies (for a review see Boothe et al. 1985), and from retrospective studies of clinical records (e.g. von Noorden 1981). Technological improvements in infant testing have also provided more direct data on the development of naturally occurring amblyopia in humans (Mohindra et al. 1979; Jacobson et al. 1981; Birch 1983; Maurer et al. 1983, 1999) and monkeys (Kiorpes & Boothe 1981; Kiorpes et al. 1984, 1989). All of these studies provide strong evidence for amblyopia induced by early deprivation.
While the upper limit for susceptibility of binocular interactions (binocular summation and stereopsis) is not yet certain, it appears to be later than that for acuity or contrast sensitivity in monkeys (Harwerth et al. 1987, 1990; Baker et al. 2008), and may extend to at least 7 or 8 years (and possibly more) in humans. Psychophysical studies of interocular transfer in humans with a history of strabismus (Banks et al. 1975; Hohmann & Creutzfeldt 1975) provide an indirect estimate of the period of susceptibility of binocular connections. The results of both studies suggest that binocular connections are highly vulnerable during the first 18 months of life, and remain susceptible to the effects of strabismus until at least 7 years of age.
(ii) Traditional treatment of amblyopia
For centuries, the primary treatment for amblyopia has consisted of patching or penalizing the fellow preferred eye, thus ‘forcing’ the brain to use the weaker amblyopic eye. Typically, patients with mild to moderate amblyopia are prescribed complete occlusion for 2–6 waking hours per day, over several months to more than a year (Pediatric Eye Disease Investigator Group 2003a,b; Repka et al. 2003; Stewart et al. 2004, 2006). Patients with moderate to severe amblyopia are often prescribed 6–10 h or more than a day (Pediatric Eye Disease Investigator Group 2003a,b), and some clinicians recommend more aggressive full-time occlusion for severe amblyopia (Dorey et al. 2001; Bhola et al. 2006; Stankovic & Milenkovic 2007). As reported in a recent large-scale clinical study of children (3–8 years of age), the dose–response rate for occlusion is approximately 0.1 log unit (1 chart line) per 120 h of occlusion, and the treatment efficacy is 3–4 logMAR lines (Stewart et al. 2004). The dose–response appears to plateau only after 100–400 h (Cleary 2000; Stewart et al. 2004, 2005). The treatment outcome is dependent on occlusion dose, the depth of amblyopia, binocular status, fixation pattern, the age at presentation and patient compliance (Loudon et al. 2003; Stewart et al. 2005).
The notion that there is a sensitive period (or periods) for the development of amblyopia has often been taken to indicate that there is also a critical period for the treatment of amblyopia. This concept grew out of the work of Worth (1903). Worth suggested that the presence of a ‘sensory obstacle’ (e.g. unilateral strabismus) arrested the development of visual acuity (‘amblyopia of arrest’), so that the patient's acuity remained at the level achieved at the time of onset of strabismus. In this view, the depth of amblyopia is a direct function of the age of onset of the sensory obstacle. Worth further suggested that if amblyopia of arrest were allowed to persist, that ‘amblyopia of extinction’ could occur as a result of binocular inhibition. In Worth's view, only this ‘extra’ loss of sensory function (i.e. the amblyopia of extinction) could be recovered by treatment. Although this latter notion is open to question in the light of the present knowledge, the ideas of Worth (1903) have had a powerful influence upon both clinicians and basic scientists. Many of our currently held concepts of amblyopia, such as plasticity, sensitive periods and abnormal binocular interaction, were already described more than a century ago, and gained currency with the work of Hubel & Wiesel (1970) and the many anatomical and physiological studies that followed. Consequently, while amblyopia can often be reversed when treated early, treatment is generally not undertaken in older children and adults. Below, we consider both experimental and clinical evidence for plasticity in the adult visual system that calls into question the notion of a sensitive period for treatment.
(iii) Clinical studies
It is often stated that humans with amblyopia cannot be treated beyond a certain age (Mintz-Hittner & Fernandez 2000); however, a review of the literature suggests otherwise. For example, Kupfer (1957) showed marked improvement in acuity, in seven adult strabismic amblyopes, aged 18–22 years. All seven showed improvements ranging from 71 per cent (20/70 to 20/20) to a very dramatic improvement from hand movements only to 20/25 after four weeks. All of these patients had relatively late onset amblyopia (2 years or later), were highly motivated and Kupfer's treatment was aggressive. The patients were hospitalized for four weeks during which time they were continuously patched and given fixation training. However, the very fact that adults with amblyopia can improve suggests that there is no clear upper age limit for recovery of acuity, at least in strabismic amblyopia with an onset later than 2 years or so. Since Kupfer's study, there have been many reports of improvement in acuity of older people with amblyopia (e.g. Birnbaum et al. 1977; Wick et al. 1992). A case report (Simmers & Gray 1999) showed that occlusion therapy appeared to improve not only visual acuity, but also position acuity in an adult strabismic amblyope.
Recent clinical trials have suggested that in children, 2 h of patching per day may be just as effective as 6 h per day. Moreover, treatment may be just as effective in older (13–17 years) patients who have not been previously treated as in younger (7–12 years) children (Pediatric Eye Disease Investigator Group 2003a,b, 2005a,b).
Plasticity in adults with amblyopia is also dramatically evident in the report of EI Mallah et al. (2000), of amblyopic patients whose visual acuity spontaneously improved in the wake of visual loss due to macular degeneration in the fellow eye. There are also reports suggesting that some adult amblyopes recover vision in their amblyopic eye following loss of vision in their fellow (non-amblyopic) eye (Vereecken & Brabant 1984; Rahi et al. 2002). These studies are consistent with the notion that the connections from the amblyopic eye may be suppressed rather than destroyed. Loss of the fellow eye would allow these existing connections to be unmasked, as occurs in adult cats and monkeys with retinal lesions (Heinen & Skavenski, 1991; Chino et al. 1992; but see Smirnakis et al. 2005).
(c) Perceptual learning in the mature and juvenile amblyopic visual system
Adults are capable of improving performance on sensory tasks, though repeated practice or perceptual learning (yes you can teach old dogs new tricks!; for recent reviews see Fine & Jacobs 2002; Fahle 2005), and this learning is considered to be a form of neural plasticity that also has consequences in the cortex (Buonomano & Merzenich 1998). Specifically, in adults with normal vision, practice can improve performance on a variety of visual tasks, and this learning can be quite specific (to the trained task, orientation, eye, etc.; see Fahle 2005). Interestingly, similar neural plasticity exists in the visual system of adults with naturally occurring amblyopia due to anisometropia and/or strabismus, suggesting that perceptual learning may be a very useful approach for amblyopia treatment. For example, over a decade ago, Levi & Polat (1996) and Levi et al. (1997) showed that practising a vernier task repetitiously can improve visual performance in adults with amblyopia.
(i) Perceptual learning: what works and what does not?
A decade or so on, it is now clear that perceptual learning can remarkably improve visual functions in amblyopia on a wide range of tasks, including: vernier acuity (Levi & Polat 1996; Levi et al. 1997); positional acuity (Li & Levi 2004; Li et al. 2005, 2007); contrast sensitivity (Polat et al. 2004; Zhou et al. 2006; Huang et al. 2008); first-order letter identification (Levi 2005; Chung et al. 2008); and second-order letter identification (Chung et al. 2006). Practising each of these tasks results in improved performance on the practised task.
(ii) Specificity and generalization
The specificity of perceptual learning noted above (Karni & Sagi 1993; Polat & Sagi 1994; Fahle 2005) poses some interesting difficulties. If the improvement following practice was solely limited to the trained stimulus, condition and task, then the type of plasticity documented here would have very limited (if any) therapeutic value for amblyopia, since amblyopia is defined primarily on the basis of reduced Snellen acuity. Importantly, perceptual learning of many tasks (vernier acuity, position discrimination and contrast sensitivity) appears to transfer, at least in part, to improvements in Snellen acuity, as does practising contrast detection (Polat et al. 2004; Zhou et al. 2006; Huang et al. 2008). In addition to visual acuity improvement, other degraded visual functions such as stereoacuity and visual counting improve as well (Li & Levi 2004; Li et al. 2007). One notable exception is learning to identify contrast-defined (second-order) letters. Learning to identify near threshold contrast-defined letters shows very little transfer to improved identification of luminance-defined (first-order) letters (Chung et al. 2006) nor to improved acuity (Chung et al. 2008). Moreover, learning to identify low-contrast large luminance-defined letters (letters approximately eight times larger than the acuity limit) does not transfer to acuity either (Chung et al. 2008).
(iii) Why does perceptual learning transfer (or not)?
A recent study (Huang et al. 2008) has suggested that the bandwidth of learning for contrast sensitivity is very broad in observers with amblyopia (approx. 4 octaves) compared with that of normal observers (approx. 1.4 octaves). The broad bandwidth of learning implies broader generalization in the amblyopic visual system. Given this broad bandwidth, why was there no transfer of improvement to visual acuity after learning to identify large letters? Huang et al. had their observer's practise contrast threshold measurements for a sine-wave grating pattern with a spatial frequency close to the observer's cut-off spatial frequency (their resolution limit). The effect of this training spread to spatial frequencies well below the cut-off (more than 4 octaves below). In the Chung et al. study, observers practised identifying near contrast threshold luminance-defined letters that were considerably larger (approx. 3 octaves or a factor of 8) than their resolution limit but were within the 4 octave range. One possible explanation is that letters are different from gratings. However, we would argue that since letters contain multiple spatial frequencies and orientations, they should generalize to acuity more readily than gratings. A more likely explanation is that the spread of learning may be unidirectional—spreading from near the acuity limit to lower spatial frequencies (larger objects), but not the other way around. Indeed, one of Polat's control groups was trained with detecting low spatial frequency, high-contrast Gabor target and showed no significant acuity improvement. This would explain why practising at a high spatial frequency spreads to a wide range of lower frequencies, but not vice versa. Whether or not this speculation is correct remains to be tested. However, it is critically important if perceptual learning is to be useful for treating amblyopia.
(iv) Extended perceptual learning
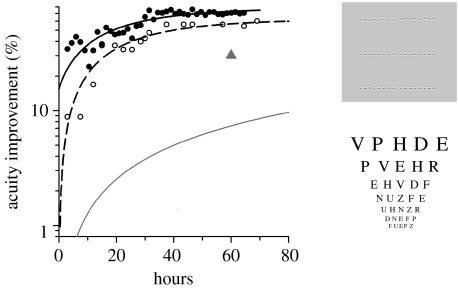
Most perceptual learning studies have used brief periods of practice; however, clinical studies have shown that the time constant for successful patching is long, with acuity improving approximately 26 per cent for every 120 h of occlusion (Stewart et al. 2004). The time constant for perceptual learning in amblyopia is unknown. Our recent results (Li et al. 2007; Li & Levi 2007) have shown that the time constant for perceptual learning in amblyopia may be very much longer than the 10–15 h of practice that is typical of most perceptual learning studies, and that it depends on the degree of amblyopia. Severe amblyopia requires more than 35 000 trials (approx. 50 h) to reach a plateau, resulting in as much as a fivefold improvement in performance. Figure 2 (filled circles) illustrates this ‘slow’ learning in a severe juvenile amblyope (age 8.8 years, from Li et al. 2007), but our work (Li & Levi 2007) has shown that similar (and even longer) extended learning is evident in adults with amblyopia. As is evident from the open circles in figure 2, the improvement transfers to Snellen acuity. Interestingly, the curve for Snellen acuity is approximately parallel to that for the learned position acuity task, just shifted to the right (indicating delayed transfer). Surprisingly, after practising position discrimination, this observer also demonstrated stereopsis of 70 arcsec, whereas he had no measurable stereoacuity (or above 400 arcsec) before the commencement of the experiments.
Figure 2.
Improvement in positional acuity (filled circles) and Snellen acuity (open circles) of a severe juvenile amblyope (observer AL, 8.8 years old, with unilateral strabismus; replotted from Li et al. 2007). The triangle shows the improvement based on occlusion alone (aged 3–8 years; Stewart et al. 2004, 2005). The grey line shows the improvement based on occlusion alone (OT, occlusion therapy) in two amblyopes (aged 6–8 years (n=2)) with acuities similar to that of AL (from Stewart et al. 2007).
It is noteworthy that extended perceptual learning is also highly effective in improving performance in adults with amblyopia (Li & Levi 2007), and, at this point, it is not clear that age (at least up to 30) is an important limitation in the efficacy of perceptual learning in amblyopia (Li & Levi 2004, 2007; Polat et al. 2004).
(v) Perceptual learning: is it long-lasting?
In adults with normal vision, perceptual learning effects are often reported to be long-lasting (Karni & Sagi 1993; Polat & Sagi 1994). The longevity of these effects is clearly of special interest in people with amblyopia. Several studies have addressed this question in adults with amblyopia. In the first study to address this question, Levi et al. (1997) reported on one subject (an adult with anisometropic amblyopia), who was retested approximately 10 months after the conclusion of the study. During this period, he had lost his glasses, and his anisometropia was therefore uncorrected during this period. While his performance on the trained (vernier) task after the hiatus was not as good as when he finished the initial training, he retained approximately 40 per cent of his initial improvement. Similarly, his Snellen acuity (which had improved to 20/20 immediately following the training) regressed over the 10 months, to just slightly better than his entering level (20/42), but, as with his vernier acuity, showed marked improvement after approximately one week of practising vernier acuity. In a later study, we showed that the improvement in visual acuity in the amblyopic eye resulting from position discrimination training was essentially stable for a long time period from 3 to 12 months (Li & Levi 2004). Polat et al. (2004) also reported a very high level of retention of the improved visual acuity as much as 12 months following the cessation of learning in their large group of adult amblyopes, and Zhou et al. (2006) reported that in the few cases tested, improvements in visual acuity showed a retention of approximately 90 per cent for at least one year.
(d) Mechanisms of perceptual learning
Why is perceptual learning so effective? First, during perceptual learning experiments, the preferred eyes of amblyopic observers are patched while they perform the task. Brief periods of occlusion have been shown to result in improvements in young children with amblyopia (Ciuffreda et al. 1991; Repka et al. 2003). Thus, at least some of the improvement may reflect the effects of patching per se. To date, perceptual learning has not been directly compared with patching alone; however, as noted above, patching combined with perceptual learning has a shorter time constant than patching alone. Second, during perceptual learning experiments, observers are engaged in making fine visual discriminations using their amblyopic eyes, under conditions where their visual system is ‘challenged’, thus the learning is ‘intensive’ and ‘active’. Third, observers receive repeated exposure to the same stimuli, and are given feedback. Thus, it is tempting to speculate that perceptual learning in amblyopia reflects the amblyopic brain learning to attend to and use the most salient or reliable information for the task when viewing with the amblyopic eye. This may be akin to strengthening connections that were there in the first place, rather than the development of new connections, perhaps by learning to attend to the information from the (normally suppressed) amblyopic eye. This speculation is consistent with the improvement in efficiency (Li & Levi 2004). It might also explain why learning transfers to some tasks (such as Snellen acuity and visual counting) but not to others. It should be noted that during normal everyday life, an amblyopic patient wearing a patch may engage in fine visual discriminations and challenges, without undertaking specific perceptual learning, and that may at least in part account for the success of patching alone, since there is evidence showing that performing near visual activities during patching may be beneficial in treating children with amblyopia (Pediatric Eye Disease Investigator Group 2005a,b). Moreover, our own work shows that playing action video games with the amblyopic eye results in a range of improved spatial and temporal visual functions including visual acuity (Li et al. 2008). However, our speculation is that perceptual learning provides intensive, active, supervised visual experience with feedback, and thus may be more efficient than simply relying on everyday experiences.
(i) Psychophysical mechanisms
Much of the focus of recent work is on the question of whether perceptual learning operates via a reduction of internal neural noise or through more efficient use of the stimulus information by retuning the weighting of the information (referred to as template retuning; e.g. Dosher & Lu 1998, 1999, 2004; Gold et al. 1999; Li et al. 2004; Lu & Dosher 2004). Most amblyopes suffer from abnormally elevated spatial uncertainty, with the neural representation of the visual image being somewhat distorted at the cortical level (Lagreze & Sireteanu 1991; Wang et al. 1998). Using positional noise, our earlier findings showed that practising position discrimination can indeed reduce spatial distortion (internal positional noise) and enhance sampling efficiency (the ability to extract stimulus information) in amblyopic vision (Li & Levi 2004). In another study, Levi (2005) reported that the improved contrast threshold of letter recognition against a luminance noise background is primarily a consequence of increased efficiency. Our work (Li & Levi 2007) has further quantified the retuning dynamics of perceptual receptive fields (decision template) during the course of visual training, and showed that the amblyopic brain is able to recalibrate neuronal connections with response feedback to use the spatial information from lower level visual mechanisms more effectively and appropriately.
(ii) The locus of learning
Where does perceptual learning take place? The question of whether perceptual learning reflects alterations in neural responses in the early visual cortex or alterations in decision processes at a higher level has been much debated (for reviews see Ahissar & Hochstein 1993; Fahle 2004), and is beyond the scope of the present review. However, it is crucial for understanding the recovery of visual function in amblyopia. Our own work (Li & Levi 2004, 2007) suggests that learning in amblyopia occurs via template retuning and internal noise reduction. Whether this learning takes place at a higher ‘decision stage’ of visual processing, at a lower level (e.g. cortical area V1) or both (e.g. via feedback or at a low level but under top-down control) remains a very important open question.
(e) Perceptual learning as a clinical tool for treating amblyopia
Occlusion therapy is the ‘gold standard’ method for treating amblyopia. In all previous perceptual learning studies, the subjects are occluded while performing the visual task, so it is reasonable to ask whether active perceptual learning actually provides an added benefit over occlusion alone.
We have argued that perceptual learning does indeed provide an added benefit for the following reasons. First, in one study (Li et al. 2005), we found that perceptual learning improved both position discrimination and letter acuity in amblyopes who are no longer responsive, or are non-responsive, to occlusion, and demonstrated that even after occlusion therapy is terminated, room remains for visual improvement with perceptual learning. This reveals neural plasticity that might not be ‘taken up’ completely by occlusion therapy. Second, a previous study showed that the dose–response rate for occlusion (in patients aged 3–8 years) is approximately 0.1 log unit per 120 h of occlusion (Stewart et al. 2004, 2005). The triangle in figure 2 shows the improvement predicted from Stewart et al.'s study in younger children (aged 3–8 years with a broad range of acuities). The grey line shows the improvement based on occlusion alone in two amblyopes (aged 6–8 years, taken from Stewart et al. 2007) with acuities similar to that of a severe 8.8-year-old amblyope who undertook our perceptual learning treatment (black symbols). To the extent that we can use these data as a basis for comparison, it seems clear that occlusion plus perceptual learning is more effective than occlusion alone, approximately a factor of 8 after 50 h of treatment. It is important to note that there are individual differences in responding to occlusion therapy. Finally, the effects of occlusion alone on position acuity have been shown to be modest (Simmers et al. 1999), while the effects noted here are substantial.
We suggest that this new approach, combining occlusion with perceptual learning, may be a useful method for obtaining the optimal treatment outcome in the shortest possible time. Eliminating or reducing the need to wear an eyepatch in public would eliminate, or at the very least reduce, the emotional stress that often accompanies occlusion therapy (Koklanis et al. 2006). We note that the in-house training itself is labour-intensive and requires considerable parental dedication. Ultimately, a home-based training version using the Internet, under close monitoring, may help to lessen the time commitment and the financial burden on parents. Significant acuity improvements may lead to diplopia (in strabismic patients) or necessitate a spectacle prescription change. Therefore, regular eye examinations are indicated during the course of treatment and corresponding action can be taken as needed (e.g. prismatic correction). However, before this perceptual learning approach is used to treat amblyopia clinically, there are still many questions to be addressed. Only two amblyopic patients participated in the Li et al. (2007) study, and the response to treatment is likely to vary among individuals. Therefore, a large-scale clinical study is needed to determine the dose–response function and compare that with the dose–response function of occlusion alone, as well as to evaluate the prognosis for different types and depths of amblyopia.
Over the centuries, there have been numerous attempts to increase the effectiveness of treatment. These attempts have a long and chequered history, ranging from the sublime to the ridiculous, and include: subcutaneous injection of strychnine; electrical stimulation of the retina and optic nerve, flashing lights, red filters and rotating gratings (reviewed by Revell 1971); and, most recently, administration of levodopa (Leguire et al. 1993, and see Levi 1994) and shocks to the brain via transcranial magnetic stimulation (Thompson et al. 2008). Few were subjected to rigorous scrutiny, and those that were often failed to stand up to it (e.g. Tytla & Labow-Daily 1981). Thus, any ‘promising’ new method should be examined critically and there is a clear need for careful controlled studies.
2. Summary and conclusions
Treatment for amblyopia is generally only undertaken in children; however, as discussed above, there is now considerable evidence that treatment of amblyopia can be effective in adults. Our hypothesis is that perceptual learning accounts for at least some of the improvement that occurs in the clinical treatment of amblyopia. Indeed, perceptual learning may be thought of as a form of active treatment. Observers are engaged in making fine judgements near the limit of their performance, using their amblyopic eyes (with their preferred eye occluded), and they receive feedback. The results reviewed above show that perceptual learning is effective in improving visual performance and that the effects may transfer to visual acuity. These findings, along with the results of new clinical trials, suggest that it might be time to reconsider our notions about neural plasticity in amblyopia, in much the same way as we had to change our concepts about stroke in the last century.
Acknowledgments
This work was supported by National Eye Institute grants R01EY01728 from the National Eye Institute and a James S. McDonnell Foundation grant—collaborative network for Critical Period Re-Examination (Brain CPR).
Footnotes
One contribution of 12 to a Theme Issue ‘Sensory learning: from neural mechanisms to rehabilitation’.
References
- Ahissar M., Hochstein S. Attentional control of early perceptual learning. Proc. Natl Acad. Sci. USA. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. doi:10.1073/pnas.90.12.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.H., Meese T.S., Hess R.F. Contrast masking in strabismic amblyopia: attenuation, noise, interocular suppression and binocular summation. Vision Res. 2008;48:1625–1640. doi: 10.1016/j.visres.2008.04.017. doi:10.1016/j.visres.2008.04.017 [DOI] [PubMed] [Google Scholar]
- Banks M.S., Aslin R.N., Letson R.D. Sensitive period for the development of human binocular vision. Science. 1975;190:675–677. doi: 10.1126/science.1188363. doi:10.1126/science.1188363 [DOI] [PubMed] [Google Scholar]
- Barrett B.T., Bradley A., McGraw P.V. Understanding the neural basis of amblyopia. Neuroscientist. 2004;10:106–117. doi: 10.1177/1073858403262153. doi:10.1177/1073858403262153 [DOI] [PubMed] [Google Scholar]
- Berardi N., Pizzorusso T., Maffei L. Critical periods during sensory development. Curr. Opin. Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. doi:10.1016/S0959-4388(99)00047-1 [DOI] [PubMed] [Google Scholar]
- Bhola R., Keech R.V., Kutschke P., Pfeifer W., Scott W.E. Recurrence of amblyopia after occlusion therapy. Ophthalmology. 2006;113:2097–2100. doi: 10.1016/j.ophtha.2006.04.034. doi:10.1016/j.ophtha.2006.04.034 [DOI] [PubMed] [Google Scholar]
- Birch E.E. Assessment of binocular function during infancy. Ophthalmic Pediatr. Genet. 1983;2:43–50. [Google Scholar]
- Birnbaum M.H., Koslowe K., Sanet R. Success in amblyopia therapy as a function of age: a literature survey. Am. J. Optom. Physiol. Opt. 1977;54:269–275. [PubMed] [Google Scholar]
- Boothe R.G., Dobson V., Teller D.Y. Postnatal development of vision in human and non-human primates. Annu. Rev. Neurosci. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. doi:10.1146/annurev.ne.08.030185.002431 [DOI] [PubMed] [Google Scholar]
- Buonomano D.V., Merzenich M.M. Cortical plasticity: from synapses to maps. Annu. Rev. Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. doi:10.1146/annurev.neuro.21.1.149 [DOI] [PubMed] [Google Scholar]
- Chino Y.M., Kaas J.H., Smith E.L., Langston A.L., Cheng H. Rapid reorganization of cortical maps in adult cats following restricted deafferation in retina. Vision Res. 1992;32:789–796. doi: 10.1016/0042-6989(92)90021-a. doi:10.1016/0042-6989(92)90021-A [DOI] [PubMed] [Google Scholar]
- Chino Y.M., Smith E.L., III, Hatta S., Cheng H. Postnatal development of binocular disparity sensitivity in neurons of the primate visual cortex. J. Neurosci. 1997;17:296–307. doi: 10.1523/JNEUROSCI.17-01-00296.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.T.L., Li R.W., Levi D.M. Identification of contrast-defined letters in adults with amblyopia benefits from perceptual learning. Vision Res. 2006;46:3853–3861. doi: 10.1016/j.visres.2006.06.014. doi:10.1016/j.visres.2006.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.T.L., Li R.W., Levi D.M. Learning to identify near-threshold luminance-defined and contrast-defined letters in observers with amblyopia. Vision Res. 2008;48:2739–2750. doi: 10.1016/j.visres.2008.09.009. doi:10.1016/j.visres.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda K.J., Levi D.M., Selenow A. Butterworth-Heinemann; Stoneham, MA: 1991. Amblyopia: basic and clinical aspects. [Google Scholar]
- Cleary M. Efficacy of occlusion for strabismic amblyopia: can an optimal duration be identified? Br. J. Ophthalmol. 2000;84:572–578. doi: 10.1136/bjo.84.6.572. doi:10.1136/bjo.84.6.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey S.E., Adams G.G., Lee J.P., Sloper J.J. Intensive occlusion therapy for amblyopia. Br. J. Ophthalmol. 2001;85:310–313. doi: 10.1136/bjo.85.3.310. doi:10.1136/bjo.85.3.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher B.A., Lu Z.L. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc. Natl Acad. Sci. USA. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. doi:10.1073/pnas.95.23.13988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher B.A., Lu Z.L. Mechanisms of perceptual learning. Vision Res. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. doi:10.1016/S0042-6989(99)00059-0 [DOI] [PubMed] [Google Scholar]
- Dosher B.A., Lu Z.L. Perceptual learning in first- and second- order letter identification. J. Vis. 2004;4:296a. doi: 10.1167/4.1.5. [DOI] [PubMed] [Google Scholar]
- EI Mallah M.K., Chakravarthy U., Hart P.M. Amblyopia: is visual loss permanent? Br. J. Ophthalmol. 2000;84:952–956. doi: 10.1136/bjo.84.9.952. doi:10.1136/bjo.84.9.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: a case for early selection. J. Vis. 2004;4:879–890. doi: 10.1167/4.10.4. doi:10.1167/4.10.4 http://journalofvision.org/4/10/4/ [DOI] [PubMed] [Google Scholar]
- Fahle M. Learning to tell apples from oranges. Trends Cogn. Sci. 2005;9:455–457. doi: 10.1016/j.tics.2005.07.005. doi:10.1016/j.tics.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Fine I., Jacobs R.A. Comparing perceptual learning tasks: a review. J. Vis. 2002;2:190–203. doi: 10.1167/2.2.5. doi:10.1167/2.2.5 [DOI] [PubMed] [Google Scholar]
- Gold J., Bennett P.J., Sekuler A.B. Signal but not noise changes with perceptual learning. Nature. 1999;402:176–178. doi: 10.1038/46027. doi:10.1038/46027 [DOI] [PubMed] [Google Scholar]
- Harwerth R.S., Smith E.L., III, Duncan G.C., Crawford M.L.J., von Noorden G.K. Multiple sensitive periods in the development of the primate visual system. Science. 1987;232:235–238. doi: 10.1126/science.3952507. doi:10.1126/science.3952507 [DOI] [PubMed] [Google Scholar]
- Harwerth R.S., Smith E.L., III, Duncan G.C., Crawford M.L.J., von Noorden G.K. Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behav. Brain Res. 1990;41:179–198. doi: 10.1016/0166-4328(90)90107-p. doi:10.1016/0166-4328(90)90107-P [DOI] [PubMed] [Google Scholar]
- Heinen S.J., Skavenski A.A. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp. Brain Res. 1991;83:670–674. doi: 10.1007/BF00229845. doi:10.1007/BF00229845 [DOI] [PubMed] [Google Scholar]
- Held R. Binocular vision-behavioral and neuronal development. In: Mehler J., Fox R., editors. Neonate cognition: beyond the blooming, buzzing confusion. Lawrence Erlbaum Press; Hillsdale, NJ: 1984. [Google Scholar]
- Hohmann A., Creutzfeldt O.D. Squint and the development of binocularity in humans. Nature. 1975;254:613–614. doi: 10.1038/254613a0. doi:10.1038/254613a0 [DOI] [PubMed] [Google Scholar]
- Horton J.C., Hocking D.R. An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. J. Neurosci. 1996;16:1791–1807. doi: 10.1523/JNEUROSCI.16-05-01791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.B., Zhou Y., Lu Z.L. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc. Natl Acad. Sci. USA. 2008;105:4068–4073. doi: 10.1073/pnas.0800824105. doi:10.1073/pnas.0800824105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. (Lond.) 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.G., Mohindra I., Held R. Age of onset of amblyopia in infants with esotropia. Ophthalmol. Proc. Ser. 1981;30:210–216. [Google Scholar]
- Karni A., Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. doi:10.1038/365250a0 [DOI] [PubMed] [Google Scholar]
- Kiorpes L. Visual processing in amblyopia: animal studies. Strabismus. 2006;14:3–10. doi: 10.1080/09273970500536193. doi:10.1080/09273970500536193 [DOI] [PubMed] [Google Scholar]
- Kiorpes L., Boothe R.G. Naturally occurring strabismus in monkeys (Macaca nemestrina) Invest. Ophthalmol. Vis. Sci. 1981;20:257–263. [PubMed] [Google Scholar]
- Kiorpes L., Boothe R.G., Carlson M.R. Acuity development in surgically strabismic monkeys. Invest. Ophthalmol. Vis. Sci. 1984;25(Suppl.):216. [Google Scholar]
- Kiorpes L., Carlson M.R., Alfi D., Boothe R.G. Development of visual acuity in experimentally strabismic monkeys. Clin. Vision Sci. 1989;4:95–106. [Google Scholar]
- Koklanis K., Abel L.A., Aroni R. Psychosocial impact of amblyopia and its treatment: a multidisciplinary study. Clin. Exp. Ophthalmol. 2006;34:743–750. doi: 10.1111/j.1442-9071.2006.01317.x. doi:10.1111/j.1442-9071.2006.01317.x [DOI] [PubMed] [Google Scholar]
- Kupfer C. Treatment of amblyopia: ex anopsia in adults. Am. J. Ophthalmol. 1957;43:918–922. doi: 10.1016/0002-9394(57)91795-6. [DOI] [PubMed] [Google Scholar]
- Lagreze W.D., Sireteanu R. Two-dimensional spatial distortions in human strabismic amblyopia. Vision Res. 1991;31:1271–1288. doi: 10.1016/0042-6989(91)90051-6. doi:10.1016/0042-6989(91)90051-6 [DOI] [PubMed] [Google Scholar]
- Leguire L.E., Rogers G.L., Bremer D.L., Walson P.D., McGregor M.L. Levodopa/carbidopa for childhood amblyopia. Invest. Ophthalmol. Vis. Sci. 1993;34:3090–3095. [PubMed] [Google Scholar]
- Levay S., Wiesel T.N., Hubel D.H. The development of ocular dominance columns in normal and visually deprived monkeys. J. Comp. Neurol. 1980;191:1–5. doi: 10.1002/cne.901910102. doi:10.1002/cne.901910102 [DOI] [PubMed] [Google Scholar]
- Levi D.M. Pathophysiology of binocular vision and amblyopia. Curr. Opin. Ophthalmol. 1994;5:3–10. doi:10.1097/00055735-199410000-00002 [PubMed] [Google Scholar]
- Levi D.M. Perceptual learning in adults with amblyopia: a reevaluation of critical periods in human vision. Dev. Psychobiol. 2005;46:222–232. doi: 10.1002/dev.20050. doi:10.1002/dev.20050 [DOI] [PubMed] [Google Scholar]
- Levi D.M. Visual processing in amblyopia: human studies. Strabismus. 2006;14:11–19. doi: 10.1080/09273970500536243. doi:10.1080/09273970500536243 [DOI] [PubMed] [Google Scholar]
- Levi D.M. Crowding—an essential bottleneck for object recognition: a mini-review. Vision Res. 2008;48:635–654. doi: 10.1016/j.visres.2007.12.009. doi:10.1016/j.visres.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D.M., Carkeet A. Amblyopia: a consequence of abnormal visual development. In: Simons K., editor. Early visual development, normal and abnormal. Oxford University Press; Oxford, UK: 1993. pp. 391–408. [Google Scholar]
- Levi D.M., Polat U. Neural plasticity in adults with amblyopia. Proc. Natl Acad. Sci. USA. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. doi:10.1073/pnas.93.13.6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D.M., Polat U., Hu Y.S. Improvement in vernier acuity in adults with amblyopia. Practice makes better. Invest. Ophthalmol. Vis. Sci. 1997;38:1493–1510. [PubMed] [Google Scholar]
- Lewis T.L., Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev. Psychobiol. 2005;46:163–183. doi: 10.1002/dev.20055. doi:10.1002/dev.20055 [DOI] [PubMed] [Google Scholar]
- Li R.W., Levi D.M. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. J. Vis. 2004;6:476–487. doi: 10.1167/4.6.7. doi:10.1167/4.6.7 [DOI] [PubMed] [Google Scholar]
- Li R.W., Levi D.M. ‘Slow’ perceptual learning of vernier acuity in adult amblyopia: an intensive amblyopia treatment study. Invest. Ophthalmol. Vis. Sci. 2007;48:4889. [Google Scholar]
- Li R.W., Levi D.M., Klein S. A perceptual learning improves efficiency by re-tuning the ‘template’ for position discrimination. Nat. Neurosci. 2004;7:178–183. doi: 10.1038/nn1183. doi:10.1038/nn1183 [DOI] [PubMed] [Google Scholar]
- Li R.W., Young K.G., Hoenig P., Levi D.M. Perceptual learning improves visual perception in juvenile amblyopia. Invest. Ophthalmol. Vis. Sci. 2005;46:3161–3168. doi: 10.1167/iovs.05-0286. doi:10.1167/iovs.05-0286 [DOI] [PubMed] [Google Scholar]
- Li R.W., Provost A., Levi D.M. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Invest. Ophthalmol. Vis. Sci. 2007;48:5046–5051. doi: 10.1167/iovs.07-0324. doi:10.1167/iovs.07-0324 [DOI] [PubMed] [Google Scholar]
- Li R.W., Ngo C., Nguyen J., Lam J., Nia B., Ren D., Levi D.M. Playing video game improves visual acuity and visual attention in adult amblyopia—a potentially useful tool for amblyopia treatment. Invest. Ophthalmol. Vis. Sci. 2008;49:2832. [Google Scholar]
- Loudon S.E., Polling J.R., Simonsz H.J. Electronically measured compliance with occlusion therapy for amblyopia is related to visual acuity increase. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241:176–178. doi: 10.1007/s00417-002-0570-z. doi:10.1007/s00417-002-0570-z [DOI] [PubMed] [Google Scholar]
- Lu Z.L., Dosher B.A. Perceptual learning retunes the perceptual template in foveal orientation identification. J. Vis. 2004;4:44–56. doi: 10.1167/4.1.5. doi:10.1167/4.1.5 [DOI] [PubMed] [Google Scholar]
- Maurer D., Lewis T.L., Tytla M.E. Contrast sensitivity in cases of unilateral congenital cataract. Invest. Ophthalmol. Vis. Sci. 1983;24(Suppl.):21. [Google Scholar]
- Maurer D., Lewis T.L., Brent H.P., Levin A.V. Rapid improvement in the acuity of infants after visual input. Science. 1999;286:108–110. doi: 10.1126/science.286.5437.108. doi:10.1126/science.286.5437.108 [DOI] [PubMed] [Google Scholar]
- Mckee S.P., Levi D.M., Movshon J.A. The pattern of visual deficits in amblyopia. J. Vis. 2003;3:380–405. doi: 10.1167/3.5.5. doi:10.1167/3.5.5 [DOI] [PubMed] [Google Scholar]
- Mintz-Hittner H.A., Fernandez K.M. Successful amblyopia therapy initiated after age 7 years: compliance cures. Arch. Ophthalmol. 2000;118:1535–1541. doi: 10.1001/archopht.118.11.1535. [DOI] [PubMed] [Google Scholar]
- Mitchell D.E. The effects of early forms of visual deprivation on perception. In: Chalupa L.M., Werner J.S., editors. The visual neurosciences. vol. 1. The MIT Press; Cambridge, MA: 2004. pp. 189–204. [Google Scholar]
- Mohindra I., Jacobson S.G., Thomas J., Held R. Development of amblyopia in infants. Trans. Ophthal. Soc. UK. 1979;99:344–346. [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A comparison of atropine and patching treatments for moderate amblyopia by patient age, cause of amblyopia, depth of amblyopia, and other factors. Ophthalmology. 2003;110:1632–1638. doi: 10.1016/S0161-6420(03)00500-1. doi:10.1016/S0161-6420(03)00500-1 [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A randomized trial of patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2057–2087. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A randomized pilot study of near activities versus non-near activities during patching therapy for amblyopia. J. AAPOS. 2005a;9:129–136. doi: 10.1016/j.jaapos.2004.12.014. doi:10.1016/j.jaapos.2004.12.014 [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch. Ophthalmol. 2005b;123:437–447. doi: 10.1001/archopht.123.4.437. doi:10.1001/archopht.123.4.437 [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A randomized trial to evaluate 2 h of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113:904–912. doi: 10.1016/j.ophtha.2006.01.069. doi:10.1016/j.ophtha.2006.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U., Sagi D. Spatial Interactions in human vision: from near to far via experience-dependent cascades of connections. Proc. Natl Acad. Sci. USA. 1994;91:1206–1209. doi: 10.1073/pnas.91.4.1206. doi:10.1073/pnas.91.4.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U., Ma-Naim T., Belkin M., Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc. Natl Acad. Sci. USA. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. doi:10.1073/pnas.0401200101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahi J.S., Logan S., Borja M.C., Timms C., Russell-Eggitt I., Taylor D. Prediction of improved vision in the amblyopic eye after visual loss in the non-amblyopic eye. Lancet. 2002;360:621–622. doi: 10.1016/S0140-6736(02)09775-1. doi:10.1016/S0140-6736(02)09775-1 [DOI] [PubMed] [Google Scholar]
- Repka M.X., et al. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch. Ophthalmol. 2003;121:603–611. doi: 10.1001/archopht.121.5.603. doi:10.1001/archopht.121.5.603 [DOI] [PubMed] [Google Scholar]
- Revell M.J. Barrie and Jenkins; London, UK: 1971. Strabismus: a history orthoptic techniques. [Google Scholar]
- Sachsenweger R. Problems of organic lesions in functional amblyopia. In: Arruga H., editor. International strabismus symposium. S. Karger A.G; Basel, Switzerland; New York, NY: 1968. p. 63. [Google Scholar]
- Schwarzkopf D.S., Vorobyov V., Mitchell D.E., Sengpiel F. Brief daily binocular vision prevents monocular deprivation effects in visual cortex. Eur. J. Neurosci. 2007;25:270–280. doi: 10.1111/j.1460-9568.2006.05273.x. doi:10.1111/j.1460-9568.2006.05273.x [DOI] [PubMed] [Google Scholar]
- Simmers A.J., Gray L.S. Improvement of visual function in an adult amblyope. Optom. Vis. Sci. 1999;76:82–87. doi: 10.1097/00006324-199902000-00014. doi:10.1097/00006324-199902000-00014 [DOI] [PubMed] [Google Scholar]
- Simmers A.J., Gray L.S., McGraw P.V., Winn B. Functional visual loss in amblyopia and the effect of occlusion therapy. Invest. Ophthalmol. Vis. Sci. 1999;40:2859–2871. [PubMed] [Google Scholar]
- Smirnakis S.M., Brewer A.A., Schmid M.C., Tolias A.S., Schüz A., Augath M., Inhoffen W., Wandell B.A., Logothetis N.K. Lack of long-term cortical reorganization after macaque retinal lesions. Nature. 2005;435:300–307. doi: 10.1038/nature03495. doi:10.1038/nature03495 [DOI] [PubMed] [Google Scholar]
- Stankovic B., Milenkovic S. Continuous full-time occlusion of the sound eye vs full-time occlusion of the sound eye periodically alternating with occlusion of the amblyopic eye in treatment of amblyopia: a prospective randomized study. Eur. J. Ophthalmol. 2007;17:11–19. doi: 10.1177/112067210701700103. [DOI] [PubMed] [Google Scholar]
- Stewart C.E., Moseley M.J., Stephens D.A., Fielder A.R. Treatment dose–response in amblyopia therapy: the monitored occlusion treatment of amblyopia study (MOTAS) Invest. Ophthalmol. Vis. Sci. 2004;45:3048–3054. doi: 10.1167/iovs.04-0250. doi:10.1167/iovs.04-0250 [DOI] [PubMed] [Google Scholar]
- Stewart C.E., Fielder A.R., Stephens D.A., Moseley M.J. Treatment of unilateral amblyopia: factors influencing visual outcome. Invest. Ophthalmol. Vis. Sci. 2005;46:3152–3160. doi: 10.1167/iovs.05-0357. doi:10.1167/iovs.05-0357 [DOI] [PubMed] [Google Scholar]
- Stewart C.E., Stephens D.A., Fielder A.R., Moseley M.J. Modeling dose–response in amblyopia: toward a child-specific treatment plan. Invest. Ophthalmol. Vis. Sci. 2007;48:2589–2594. doi: 10.1167/iovs.05-1243. doi:10.1167/iovs.05-1243 [DOI] [PubMed] [Google Scholar]
- Teller D.Y., Movshon J.A. Visual development. Vision Res. 1986;26:1483–1506. doi: 10.1016/0042-6989(86)90169-0. doi:10.1016/0042-6989(86)90169-0 [DOI] [PubMed] [Google Scholar]
- Thompson B., Mansouri B., Koski L., Hess R.F. Brain plasticity in the adult: modulation of function in amblyopia with rTMS. Curr. Biol. 2008;18:1067–1071. doi: 10.1016/j.cub.2008.06.052. doi:10.1016/j.cub.2008.06.052 [DOI] [PubMed] [Google Scholar]
- Tytla M.E., Labow-Daily L. Evaluation of the CAM treatment for amblyopia: a controlled study. Invest. Ophthalmol. Vis. Sci. 1981;20:400–406. [PubMed] [Google Scholar]
- Vereecken E.P., Brabant P. Prognosis for vision in amblyopia after the loss of the good eye. Arch. Ophthalmol. 1984;102:220–224. doi: 10.1001/archopht.1984.01040030170019. [DOI] [PubMed] [Google Scholar]
- von Noorden G.K. New clinical aspects of stimulus deprivation amblyopia. Am. J. Ophthalmol. 1981;92:416–421. doi: 10.1016/0002-9394(81)90534-1. [DOI] [PubMed] [Google Scholar]
- Wang H., Levi D.M., Klein S.A. Spatial uncertainty and sampling efficiency in amblyopic position acuity. Vision Res. 1998;38:1239–1251. doi: 10.1016/s0042-6989(97)00278-2. doi:10.1016/S0042-6989(97)00278-2 [DOI] [PubMed] [Google Scholar]
- Wick B., Wingard M., Cotter S., Scheiman M. Anisometropic amblyopia: is the patient ever too old to treat? Optom. Vis. Sci. 1992;69:866–878. doi: 10.1097/00006324-199211000-00006. doi:10.1097/00006324-199211000-00006 [DOI] [PubMed] [Google Scholar]
- Wiesel T.N. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. doi:10.1038/299583a0 [DOI] [PubMed] [Google Scholar]
- Worth C.A. The Blakiston Company; Philadelphia, PA: 1903. Squint: its causes, pathology and treatment. [Google Scholar]
- Zhou Y., Huang C., Xu P., Tao L., Qiu Z., Li X., Lu Z. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res. 2006;46:739–750. doi: 10.1016/j.visres.2005.07.031. doi:10.1016/j.visres.2005.07.031 [DOI] [PubMed] [Google Scholar]