Abstract
The veins that irrigate leaves during photosynthesis are demonstrated to be strikingly more abundant in flowering plants than in any other vascular plant lineage. Angiosperm vein densities average 8 mm of vein per mm2 of leaf area and can reach 25 mm mm−2, whereas such high densities are absent from all other plants, living or extinct. Leaves of non-angiosperms have consistently averaged close to 2 mm mm−2 throughout 380 million years of evolution despite a complex history that has involved four or more independent origins of laminate leaves with many veins and dramatic changes in climate and atmospheric composition. We further demonstrate that the high leaf vein densities unique to the angiosperms enable unparalleled transpiration rates, extending previous work indicating a strong correlation between vein density and assimilation rates. Because vein density is directly measurable in fossils, these correlations provide new access to the physiology of extinct plants and how they may have impacted their environments. First, the high assimilation rates currently confined to the angiosperms among living plants are likely to have been unique throughout evolutionary history. Second, the transpiration-driven recycling of water that is important for bolstering precipitation in modern tropical rainforests might have been significantly less in a world before the angiosperms.
Keywords: venation, transpiration, assimilation, tropical rainforest, tracheophyte
1. Introduction
Broad leaves with a ramifying network of veins maximize resource economy of leaf production and are the predominant photosynthetic organ where competition for light is not superseded by other limitations such as freezing or drought (Smith et al. 1997). Leaves trade large quantities of water for carbon, an exchange that makes rapid transpiration a prerequisite for high productivity (Cowan & Farquhar 1977). Because leaf mesophyll is specialized for photosynthesis rather than hydraulic transport, however, this tissue imposes high resistance upon the transpiration stream after exiting the veins en route to evaporative loss through the stomatal pores (Sack & Holbrook 2006; Brodribb et al. 2007; Mott 2007). Thus, avoiding photosynthesis-inhibiting cell desiccation while maintaining a high transpiration rate requires a vascular plumbing system that minimizes transport in the photosynthetic tissue by delivering water directly from the vein close to the stomata where gas exchange takes place (Brodribb et al. 2007). That requirement is effectively achieved by increasing the abundance of vein ramifications within the mesophyll (vein density), but trade-offs associated with vein production include the displacement of photosynthetic tissue by veins, the investment in thick lignified cell walls and the metabolism of living support cells for the veins. The capacity of the leaf to transpire water must also integrate the evaporative demands of the environment and the ability of the rest of the plant to absorb water from its substrate and transport it to the leaf.
What balances may have been achieved through time between the photosynthetic benefits associated with high leaf vein densities and the limitations on their production are investigated here. First, the phylogenetic distribution of leaf vein densities is established over the almost 400 million year history of leaf evolution including at least four independent originations of laminate leaves (Boyce 2008). Second, the relationship between vein density and transpiration capacity is established through hydraulic analyses that build upon previous work linking the path length of water transport through the mesophyll to transpiration and assimilation rates (Sack & Frole 2006; Brodribb et al. 2007; Noblin et al. 2008).
2. Material and methods
(a) Leaf vein density measurements
Published leaf vein densities were compiled from the literature. In addition, measurements of vein density were made directly from figured specimens and from freshly collected leaves (see the electronic supplementary material, table 1) using digital image analysis (Image J; National Institute of Health, Bethesda, MD, USA).
(b) Vein density and stomatal conductance
For a subsample of the tropical and temperate ferns, conifers and angiosperms (see the electronic supplementary material, table 2), both mean vein density and maximum stomatal conductance were measured in the same plant. Healthy leaves were selected from plants exposed to optimal light growing in natural forest with high soil water availability. Three leaves per species were cleared in 40 per cent aqueous NaOH and three 4 mm2 sections of leaf lamina were excised from the central and marginal regions of each leaf, stained and mounted in glycerol for determination of vein density. Leaf samples were photographed at 20× magnification with two images per sample (18 images per species).
Maximum instantaneous stomatal conductance was measured on 10 healthy, mature leaves of each species using a portable gas analyser (Li-COR 6400; Lincoln, NE, USA) to quantify CO2 uptake under conditions of saturating light and water availability. During all measurements, a high flow rate (500 ml min−1) through the cuvette was maintained such that conditions in the cuvette approximated ambient temperature (kept between 25 and 33°C), vapour pressure deficit (kept below 2 kPa) and CO2 (ranged from 364 to 375 μmol mol−1). All measurements were carried out between 08.00 and 11.00 when CO2 uptake was maximal. Light intensities of either 1800 or 1000 μmol quanta m−1 s−1 were used depending on the saturating photosynthetic photon flux density of the species (as determined by a light response curve made on one individual per species); the lower light intensity was used to avoid over-saturation in species growing in forest understorey.
3. Results
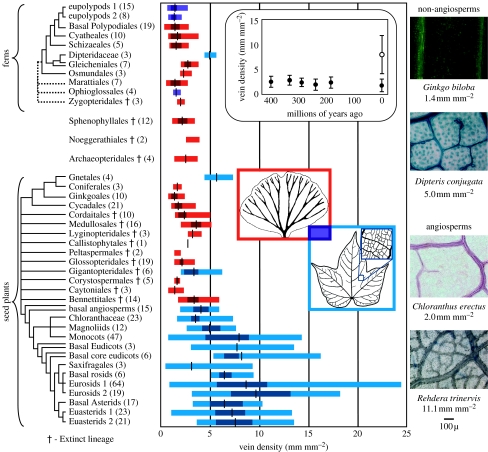
Our measurements of fossil and extant leaf vein densities and compilation of published sources demonstrate that the uniquely high vein densities achieved in angiosperm leaf evolution represent a landmark event. Each of at least four Palaeozoic origins of laminate leaves—archaeopterid progymnosperms, sphenophylls, ferns and seed plants—possessed similarly low vein densities of approximately 2 mm of vein per mm2 of leaf surface area (figure 1). Despite large changes in climate and atmospheric composition (Berner 1999; Ziegler et al. 2003), the vein densities of non-angiosperms have remained stable and generally below 5 mm mm−2 throughout the last 380 million years (figure 1 inset). By contrast, angiosperm vein densities are generally greater than 5 mm mm−2 and can reach 25 mm mm−2 (figure 1).
Figure 1.
Phylogenetic and stratigraphic distribution of leaf vein densities. Full minimum–maximum ranges across four or more evolutions of laminate leaves, including greater phylogenetic resolution in living ferns (Smith et al. 2006) and angiosperms (Angiosperm Phylogeny Group 2003). Whether all ferns represent a single origin is equivocal as are affinities of the fossil Noeggerathiales and relationships among extinct seed plants. Group mean (black lines) and median 50% of values (darker shading) provided where sampling is sufficient. Colours indicate venation architectures: at most two orders of marginally ending open or reticulate veins (red); multiple hierarchical orders of reticulate veins with internally directed veins (blue); or the presence of both types among members of the Ophioglossales and eupolypods (purple). Number of species involved in each measurement indicated parenthetically. Inset graph: vein density mean±s.d. through time for all non-angiosperm taxa (filled circles) compared with extant eudicot angiosperms (open circle). Along right: vein density examples presented at the same scale (higher magnification would be needed for angiosperms with densities much greater than the euasterid Rehdera).
Although the vein density spectra of angiosperms and non-angiosperms overlap, the high vein densities achieved by the majority of angiosperm species are unprecedented. Densities as low as 0.5 mm mm−2 can be found among the angiosperms, but are present in succulent leaves where increasing vein density would have little effect on the mesophyll path length of water transport, which is dominated by leaf thickness (Noblin et al. 2008). The three non-angiosperm lineages with the highest vein densities are the Permian gigantopterid seed plants (2.0–6.2, mean of 3.4 mm mm−2), extant Gnetum (4.4–7.4, mean of 5.7 mm mm−2) and the extant dipteridaceous ferns (4.4–5.6 mm mm−2). Each of these lineages also represents a convergence upon angiosperm-like venation consisting of a reticulate hierarchical network with internal vein endings (Boyce 2005). However, other examples of convergence upon angiosperm venation patterns possess much lower densities—Ophioglossum (1.4–2.0, mean of 1.7 mm mm−2) and various polypod fern lineages (cumulative range of 0.6–2.7, mean of 1.5 mm mm−2)—and the maximum density of any non-angiosperm does not reach the 8 mm mm−2 mean seen across eudicot angiosperms (figure 1). Notably, extant early diverging angiosperm lineages also are characterized by relatively low vein densities (1.7–5.7, mean of 4.1 mm mm−2) that are intermediate between those common among angiosperms and among non-angiosperms. High vein density is uniquely angiospermous, but has probably arisen multiple times within the angiosperm lineage rather than once for angiosperms as a whole. A more detailed dissection of ecological and phylogenetic patterns within the angiosperms would be needed to determine the frequency with which extremely high vein densities and reversions to low vein density have appeared across the radiation of flowering plants.
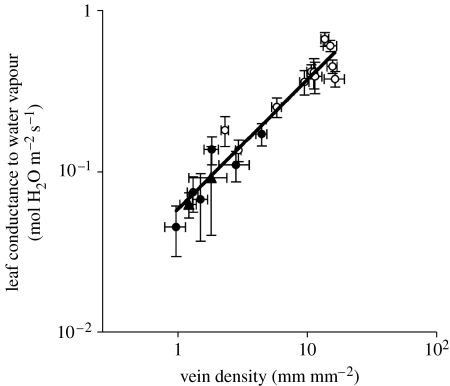
Mirroring the strong relationship already established between vein density and assimilation (Brodribb et al. 2007), a similarly strong relationship is observed here between vein density and transpiration for a diverse sampling of ferns, conifers and angiosperms (figure 2). Consequently, the uniquely high vein densities of angiosperms allow photosynthetic and transpiration capacities that are unmatched by other plants, living or extinct.
Figure 2.
Vein density as a limiter of transpiration. Relationship between vein density and maximum conductance of leaves to water vapour (equivalent to transpiration under standard atmospheric conditions) shown for a mixed sample of tropical and temperate angiosperm (open circles), conifer (filled triangles) and fern (filled circles) leaves measured under natural conditions. Values are means±s.d. A single, highly significant regression (y=0.068 x0.74; r2=0.87) is fitted to the pooled data since group regressions were not significantly different.
4. Discussion
The patterns of vein density and transpiration described here provide striking evidence of a major change in leaf morphology that accompanied the evolutionary diversification of angiosperm leaves. While the basic architecture of the angiosperm leaf vein network has been converged upon by a number of other vascular plant lineages (Boyce 2005), the high vein densities of angiosperm leaves are not found in any other clade of vascular plants, living or extinct. Furthermore, this innovation in angiosperm leaf venation also represents an innovation in leaf physiology, enabling transpiration and photosynthesis rates higher than their competitors. Although the mean vein density for extant non-angiosperms is 1.8 mm mm−2, that of all eudicot angiosperms in our dataset is 8.0 mm mm−2 despite including a number of succulents. Furthermore, vein densities of 12 mm mm−2 or greater are common among subtropical/tropical angiosperms (see the electronic supplementary material, figure 1). Per unit leaf area, three and four times higher transpiration rates can be expected to accompany those respective increases in vein density over the non-angiosperm background (figure 2). Similarly, the rate of maximum carbon assimilation would have increased by a factor of approximately five, given the relationships established previously (Brodribb et al. 2007).
The impact of these leaf-level effects is likely to permeate through the entire landscape. Although scaling up from the leaf to the whole plant or canopy can only be approximate (Jarvis & McNaughton 1986), the angiosperm transformation of most ecosystems has been extreme. The rise of angiosperm dominance has resulted in an abrupt disjunction in vein density (with values as low as the 2–3 mm mm−2 average of all other taxa being restricted among angiosperms only to basal clades and derived drought-adapted succulents) through a near complete replacement of their low vein density predecessors in most forest canopies (Morley 2000), except at higher latitudes and altitudes and more marginal habitats. ‘Big leaf’ models and their modern derivatives have been successfully used to model canopy CO2 and water fluxes (Jarvis & McNaughton 1986), and our investigations show that the characteristics of the individual leaves that constitute the big leaf of the canopy have changed dramatically with angiosperm evolution. Thus, changes in assimilation and transpiration capacities with the advent of high vein density angiosperm leaves are likely to have impacted productivity and transpiration at the canopy scale.
(a) Leaf assimilation rates and productivity through time
One naturally hesitates to assess past ecophysiological diversity from the living exemplars of non-angiospermous groups represented in the fossil record because nearly all terrestrial ecosystems have been restructured by the advent of angiosperms (Wing & Sues 1992). For example, living cycads have low growth rates, but conceivably could have had a higher range of assimilation rates in the deep past when they occupied ecologies from which they have now been displaced by angiosperms. However, cycad leaf vein densities have been stably low throughout their evolutionary history, making such a hypothesis unlikely. Indeed, the physiological model that links gas exchange to the distance between vein and stomata (Brodribb et al. 2007; Noblin et al. 2008) has been verified across a wide phylogenetic sample that extends from mosses to angiosperms, and therefore we have considerable confidence in using relationships between vein architecture and gas exchange (figure 2) as an a priori index of leaf function. Thus, the assimilation rates of living cycads and ferns are likely to be representative of fossil plants that have shared similar leaf vein densities.
Angiosperm leaves in their more derived forms represent a substantial departure from the photosynthetic limits that seem to apply to other plant groups. This pattern suggests that the substantially lower assimilation capacities seen in non-angiosperms relative to angiosperms in modern environments were the norm for pre-angiosperm environments, since vein densities among non-angiosperms have been relatively stable for 380 million years. Fluctuations in global climate and atmospheric CO2 levels complicate interpretation, but the universality of low vein densities among non-angiosperms through deep time suggests the possibility of substantially lower biomass production and input of terrestrial vegetation to a pre-angiosperm global carbon cycle (at least for the many palaeoenvironments not dominated by the subset of conifers with a high leaf area index (LAI), as discussed in the following section).
(b) Transpiration capacity and tropical precipitation
We suggest that the increases in transpiration capacity accompanying the increased vein densities of angiosperms have significantly increased vapour flux at the canopy level. Based purely on the temporal changes in mean vein density (figure 1) and relationship between density and leaf transpiration shown here (figure 2), a three- to fourfold increase in transpiration can be expected to have accompanied angiosperm evolution.
While high vein densities are also found in middle- to high-latitude angiosperms, the evolution of this enhanced angiosperm transpirational engine could have had the largest effects on tropical forests where the recycling of transpiration-derived water today can account for up to 30–50 per cent of precipitation (Worden et al. 2007). This effect is illustrated by the aftermath of lowland rainforest clear-cutting where ecosystem recovery is hampered by the loss of transpiration-driven rainfall emanating from angiosperm tree canopies (Shukla et al. 1990; Pielke et al. 2007). Regardless of vegetation, the intertropical convergence zone (ITCZ) ensures a tropical rainy belt that can be further bolstered by local orography and maritime effects. Indeed, the drought intolerance of early diverging clades encompassing the first five or six nodes of the angiosperm phylogeny (Sperry et al. 2007) suggests that the original evolution of the angiosperms required such pre-existing tropical everwet environments. Many of those basal angiosperms are today largely confined to low insolation upland tropical cloud forests with reliably high precipitation (Feild & Arens 2007) fed by the ITCZ and orographic effects. However, transpiration-fed hydrological cycles extending beyond areas expected for the ITCZ alone, as in the Amazon, may not have been possible before the evolution of high vein density angiosperm leaves.
The impact of increased leaf-level transpiration on canopy transpiration could be overestimated if the trees of pre-angiosperm forests had much more leaf area, but this seems unlikely. Although the LAI of temperate dicot trees (mean 3.3) and tropical forest canopies (means ranging from 6.0 to 7.2) is considerably less than the maxima seen among some conifers (i.e. 16 in Pinus radiata), the LAI of non-angiosperms other than the conifers can be considerably lower: Ginkgo averages 2.5 and plants with a distal rosette of leaves—representing Medullosans, Bennettitales and a diversity of other extinct seed plant groups, cycads and tree ferns—can be even less (i.e. 1.7 in tree ferns; Harrington et al. 2001; Teske & Thistle 2004). In fact, the only group that exhibits a generally higher-than-angiosperm LAI is specifically the Pinaceae (mean 6.5, range 0.8–16); other conifers possess low to moderate LAI (i.e. 2.2 in Taxodium, 2.9 in Dacrydium, 3.0 in Podocarpus and 4.9 in Thuja; Oren et al. 2001; DeLucia et al. 2003; Fetene & Beck 2004; Teske & Thistle 2004). The Pinaceae appear to have always been poor competitors in the tropics due to low shade tolerance and a subsequent reliance on large-scale disturbance (Brodribb & Feild 2008), and the clade was absent from the Mesozoic tropics (Brenner 1976; Rees et al. 2000). Thus, whether past LAI would have been somewhat higher or lower frequently may be unclear, but is unlikely to have been drastically higher in most palaeoenvironments versus the modern equivalent inhabited by angiosperms—particularly in the tropics. By contrast, vein density is directly measurable and has increased by a factor of four.
The evolution of angiosperm lineages with high transpiration rates enabled by high leaf vein densities would have enhanced local convective rainfall and eventually may have established a positive feedback by increasing the area in which the same angiosperms continued to dominate and diversify. The various mechanisms previously proposed for the high diversity of angiosperms (Hickey & Doyle 1977) generally do not explain how the existence of angiosperm rainforests fostered diversification of other lineages aside from generalized evocations of increased ecological complexity in angiosperm-dominated forests (Schneider et al. 2004). However, since precipitation is the single greatest determinant of plant diversity in these environments (Kreft & Jetz 2007), whatever incremental increase in rainfall that can be attributed to angiosperm transpiration should have had a positive impact on tropical diversity. As one example, epiphytes are important in the maintenance of biodiversity and nutrient cycling in tropical rainforests (Nadkarni 1984) and their diversity is strongly correlated with precipitation levels (Cardelús et al. 2006). Vines and lianas are important by the Carboniferous (Kerp & Krings 1998), but, with few possible exceptions (e.g. Rothwell 1991), epiphytes are absent. The radiation of extant epiphytic lineages—among ferns, bryophytes, lycopods and, of course, diverse angiosperm clades (Wikstrom & Kenrick 1997; Givnish et al. 2007; Heinrichs et al. 2007; Schuettpelz 2007)—is essentially a Cenozoic phenomenon. Since the diversity of other tropical lineages correlates with plant diversity (e.g. Novotny et al. 2006), the impact of this restructuring would have propagated throughout rainforest ecosystems.
The various ecosystems of the deep past that are often considered as analogous to modern tropical rainforests (Ziegler et al. 2003; DiMichele et al. 2007), including some mid-latitude environments during warmer palaeoclimates, may well have presented the warmest consistently wet environments of their world. However, they may have been poor analogues of modern tropical rainforests, having had a different forest structure and lower sample-standardized diversity. We argue that this divergence can ultimately be linked to the fundamentally different physiological capacities of the plants that grew in these palaeoecosystems relative to those found in modern tropical rainforests, as borne witness to by their leaves.
Acknowledgments
We thank D. Jablonksi and P. Crane for their helpful discussions and P. Hudson, M. Stevenson, A. Hartley and D. Chatelet for assisting with vein measurements. Collection of basal angiosperms permitted by INRENA (Peru), MAF (French Polynesia) and Province Nord (New Caledonia). Research supported by NSF grants to T. S. F. and M. A. Z. and an Australian Research Fellowship grant to T. J. B.
Supplementary Material
Distribution of leaf vein densities among subtropical/tropical dicots
Vein densities, phylogenetic affinities, growth habits, and stratigraphic distribution of fossil and extant leaves
Maximum stomatal conductance and vein density
References
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003;141:399–436. doi:10.1046/j.1095-8339.2003.t01-1-00158.x [Google Scholar]
- Berner R.A. The carbon cycle and CO2 over Phanerozoic time: the role of land plants. Phil. Trans. R. Soc. B. 1999;353:75–82. doi:10.1098/rstb.1998.0192 [Google Scholar]
- Boyce C.K. Patterns of segregation and convergence in the evolution of fern and seed plant leaf morphologies. Paleobiology. 2005;31:117–140. doi:10.1666/0094-8373(2005)031<0117:POSACI>2.0.CO;2 [Google Scholar]
- Boyce C.K. The fossil record of plant physiology and development—what leaves can tell us. Paleontol. Soc. Papers. 2008;14:133–146. [Google Scholar]
- Brenner G.J. Middle Cretaceous floral provinces and early migrations of angiosperms. In: Beck C.B., editor. Origin and early evolution of angiosperms. Columbia University Press; New York, NY: 1976. pp. 23–47. [Google Scholar]
- Brodribb T.J., Feild T.S. Evolutionary significance of a flat-leaved Pinus in Vietnamese rainforest. New Phytol. 2008;178:201–209. doi: 10.1111/j.1469-8137.2007.02338.x. doi:10.1111/j.1469-8137.2007.02338.x [DOI] [PubMed] [Google Scholar]
- Brodribb T.J., Feild T.S., Jordan G.J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007;144:1890–1898. doi: 10.1104/pp.107.101352. doi:10.1104/pp.107.101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelús C.L., Colwell R.K., Watkins J.E. Vascular epiphyte distribution patterns: explaining the mid-elevation richness peak. Ecology. 2006;94:144–156. doi:10.1111/j.1365-2745.2005.01052.x [Google Scholar]
- Cowan I.R., Farquhar G.D. Stomatal function in relation to leaf metabolism and environment. Symp. Soc. Exp. Biol. 1977;31:471–505. [PubMed] [Google Scholar]
- DeLucia E.H., Turnbull M.H., Walcroft A.S., Griffin K.L., Tissue D.T., Glenny D., McSeveny T.M., Whitehead D. The contribution of bryophytes to the carbon exchange for a temperate rainforest. Glob. Chang. Biol. 2003;9:1158–1170. doi:10.1046/j.1365-2486.2003.00650.x [Google Scholar]
- DiMichele W.A., Falcon-Lang H.J., Nelson W.J., Elrick S.D., Ames P.R. Ecological gradients within a Pennsylvanian mire forest. Geology. 2007;35:415–418. doi:10.1130/G23472A.1 [Google Scholar]
- Feild T.S., Arens N.C. The ecophysiology of early angiosperms. Plant Cell Environ. 2007;30:291–309. doi: 10.1111/j.1365-3040.2006.01625.x. doi:10.1111/j.1365-3040.2006.01625.x [DOI] [PubMed] [Google Scholar]
- Fetene M., Beck E.H. Water relations of indigenous versus exotic tree species, growing at the same site in a tropical montane forest in southern Ethiopia. Trees. 2004;18:428–435. doi:10.1007/s00468-004-0321-3 [Google Scholar]
- Givnish T.J., Millam K.C., Berry P.E., Sytsma L.J. Phylogeny, adaptive radiation, and historical biogeography of Bromeliaceae inferred from ndhF sequence data. Aliso. 2007;23:3–26. [Google Scholar]
- Harrington R.A., Fownes J.H., Vitousek P.M. Production and resource use efficiencies in N- and P-limited tropical forests: a comparison of responses to long-term fertilization. Ecosystems. 2001;4:646–657. doi:10.1007/s10021-001-0034-z [Google Scholar]
- Heinrichs J., Hentschel J., Wilson R., Feldberg K., Schneider H. Evolution of leafy liverworts (Jungermanniidae Marchantiophyta): estimating divergence times from chloroplast DNA sequences using penalized likelihood with integrated fossil evidence. Taxon. 2007;56:31–44. [Google Scholar]
- Hickey L.J., Doyle J.A. Early Cretaceous fossil evidence for angiosperm evolution. Bot. Rev. 1977;43:3–104. doi:10.1007/BF02860849 [Google Scholar]
- Jarvis P.G., McNaughton K.G. Stomatal control of transpiration—scaling up from leaf to region. Adv. Ecol. Res. 1986;15:1–49. doi:10.1016/S0065-2504(08)60119-1 [Google Scholar]
- Kerp H., Krings M. Climbing and scrambling growth habits: common life strategies among Late Carboniferous seed ferns. Compte Rendus de l'Académie des Sciences, Série deux, Sciences de la Terre et des Planètes. 1998;326:583–588. [Google Scholar]
- Kreft H., Jetz W. Global patterns and determinants of vascular plant diversity. Proc. Natl Acad. Sci. USA. 2007;104:5925–5930. doi: 10.1073/pnas.0608361104. doi:10.1073/pnas.0608361104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley R.J. John Wiley & Sons; Chichester, UK: 2000. Origin and evolution of tropical rain forests. [Google Scholar]
- Mott K.A. Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves. Plant Cell Environ. 2007;30:1444–1449. doi: 10.1111/j.1365-3040.2007.01720.x. doi:10.1111/j.1365-3040.2007.01720.x [DOI] [PubMed] [Google Scholar]
- Nadkarni N.M. Epiphyte biomass and nutrient capital of a neotropical elfin forest. Biotropica. 1984;16:249–256. doi:10.2307/2387932 [Google Scholar]
- Noblin X., Mahadevan L., Coomaraswamy I.A., Weitz D.A., Holbrook N.M., Zwieniecki M.A. Optimal vein density in artificial and real leaves. Proc. Natl Acad. Sci. USA. 2008;105:9140–9144. doi: 10.1073/pnas.0709194105. doi:10.1073/pnas.0709194105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny V., Drozd P., Miller S.E., Kulfan M., Janda M., Basset Y., Weiblen G.D. Why are there so many species of herbivorous insects in tropical rainforests? Science. 2006;313:1115–1118. doi: 10.1126/science.1129237. doi:10.1126/science.1129237 [DOI] [PubMed] [Google Scholar]
- Oren R., Sperry J.S., Ewers B.E., Pataki D.E., Phillips N., Megonigal J.P. Sensitivity of mean canopy stomatal conductance to vapor pressure deficit in a flooded Taxodium distichum L. forest: hydraulic and non-hydraulic effects. Oecologia. 2001;126:21–29. doi: 10.1007/s004420000497. doi:10.1007/s004420000497 [DOI] [PubMed] [Google Scholar]
- Pielke R.A., Adegoke J., Beltran-Przekurat A., Hiemstra C.A., Lin J., Nair U.S., Niyogi D., Nobis T.E. An overview of regional land-use change and land-cover impacts on rainfall. Tellus B Chem. Phys. Meteorol. 2007;59:587–601. doi:10.1111/j.1600-0889.2007.00251.x [Google Scholar]
- Rees P.M., Ziegler A.M., Valdes P.J. Jurassic phytogeography and climates: new data and models. In: Huber B.T., MacLeod K.G., Wing S.L., editors. Warm climates in Earth history. Cambridge University Press; Cambridge, UK: 2000. pp. 297–318. [Google Scholar]
- Rothwell G.W. Botryopteris forensis (Botryopteridaceae), a trunk epiphyte of the tree fern Psaronius. Am. J. Bot. 1991;78:782–788. doi:10.2307/2445068 [Google Scholar]
- Sack L., Frole K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology. 2006;87:483–491. doi: 10.1890/05-0710. doi:10.1890/05-0710 [DOI] [PubMed] [Google Scholar]
- Sack L., Holbrook N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. doi:10.1146/annurev.arplant.56.032604.144141 [DOI] [PubMed] [Google Scholar]
- Schneider H., Schuettpelz E., Pryer K.M., Cranfill R., Magallón S., Lupia R. Ferns diversified in the shadow of angiosperms. Nature. 2004;428:553–557. doi: 10.1038/nature02361. doi:10.1038/nature02361 [DOI] [PubMed] [Google Scholar]
- Schuettpelz E. Duke University; Durham, NC: 2007. The evolution and diversification of epiphytic ferns. [Google Scholar]
- Shukla J., Nobre C., Sellers P. Amazon deforestation and climate change. Science. 1990;247:1322–1325. doi: 10.1126/science.247.4948.1322. doi:10.1126/science.247.4948.1322 [DOI] [PubMed] [Google Scholar]
- Smith W.K., Vogelmann T.C., DeLucia E.H., Bell D.T., Shepherd K.A. Leaf form and photosynthesis. BioScience. 1997;47:785–793. doi:10.2307/1313100 [Google Scholar]
- Smith A.R., Pryer K.M., Schuettpelz E., Korall P., Schneider H., Wolf P.G. A classification of extant ferns. Taxon. 2006;55:705–731. [Google Scholar]
- Sperry J.S., Hacke U.W., Feild T.S., Yano Y., Sikkema E.H. Hydraulic consequences of vessel evolution in angiosperms. Int. J. Plant Sci. 2007;168:1127–1139. doi:10.1086/520726 [Google Scholar]
- Teske M.E., Thistle H.W. A library of forest canopy structure for use in interception modeling. For. Ecol. Manage. 2004;198:341–350. doi:10.1016/j.foreco.2004.05.031 [Google Scholar]
- Wikstrom N., Kenrick P. Phylogeny of Lycopodiaceae (Lycopsida) and the relationships of Phylloglossum drummondii Kunze based on rbcL sequences. Int. J. Plant Sci. 1997;158:862–871. doi:10.1086/297501 [Google Scholar]
- Wing S.L., Sues H.-D. Mesozoic and early Cenozoic terrestrial ecosystems. In: Behrensmeyer A.K., Damuth J.D., DiMichele W.A., Potts R., Sues H.-D., Wing S.L., editors. Terrestrial ecosystems through time. University of Chicago Press; Chicago, IL: 1992. pp. 327–418. [Google Scholar]
- Worden J., Noone D., Bowman K., The Tropospheric Emission Spectrometer science team and data contributors Importance of rain evaporation and continental convection in the tropical water cycle. Nature. 2007;445:528–532. doi: 10.1038/nature05508. doi:10.1038/nature05508 [DOI] [PubMed] [Google Scholar]
- Ziegler A.M., Eshel G., Rees P.M., Rothfus T.A., Rowley D.B., Sunderlin D. Tracing the tropics across land and sea: permian to present. Lethaia. 2003;36:227–254. doi:10.1080/00241160310004657 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of leaf vein densities among subtropical/tropical dicots
Vein densities, phylogenetic affinities, growth habits, and stratigraphic distribution of fossil and extant leaves
Maximum stomatal conductance and vein density