Summary
Dendritic cells (DCs) orchestrate a repertoire of immune responses that endows resistance to infection and tolerance to self. Understanding the principles by which DCs control immunity and tolerance has provided a rich basis for studying and improving clinical outcome of human disease treatment. Several features contribute to the complexity of the DC system. Among these, plasticity and existence of subsets are prominent determinants to the quality of the elicited immune responses. Indeed, different DC subsets are distributed in peripheral tissues and the blood and display different microbial receptors, surface molecules and cytokine expression, all of which influence the immunological outcome. The biological raison d’être for separate DC subsets has been the focus of many studies including our own and is being reviewed with an emphasis on human skin DCs.
Keywords: Dendritic cells, Subsets, Human, Vaccines
The challenges of protective immunity
Though our skin and mucosa are covered by considerable amounts of microbes, we stay healthy. However, when the microbes break the skin or mucosal barriers, the immune system faces a number of options. First, it needs to decide whether to respond or not. Second, if a response is made, it must be tailored to fight that particular microbe. Generating the right type of immune response can be a matter of life and death itself. For instance, in leprosy, the tuberculoid form of the disease is characterized by a Type 1 response which keeps the disease in check, while the lepromatous form induces an often fatal Type 2 response [1]. Microbe-specific immunity must therefore limit the spreading of the infection as well as remove the infected cells. This requires the participation of different cells of the innate immune system as well as cells of the adaptive immune system, more specifically T and B lymphocytes which are educated and activated by DCs. Microbiologists, spearheaded by Louis Pasteur have devised multiple ways to inactivate pathogens to generate vaccines that protect us against about twenty different infectious agents. Most if not all of these vaccines are designed to initiate protective humoral immune responses. Unfortunately, many pathogens, for which no efficient vaccines are available, are still affecting mankind with terrible diseases such as HIV-induced AIDS, Plasmodium-induced malaria, Virus-induced Hepatitis C, Mycobacterium-induced tuberculosis. Most of these appear to be chronic diseases for which it is thought that strong cellular immunity, in particular Cytotoxic T cells is necessary to eliminate the cells that are infected with the causative agent. Thus, a more detailed understanding of the mechanisms leading to strong cellular immunity is necessary to design relevant vaccines. There is now evidence that DCs are important in the response to vaccination and that adjuvants are actually activators of DCs. We surmise that a better knowledge of human DCs will permit us to reach this goal. This paper is dedicated to the human DCs subsets which we have been studying for 15 years with the goal of designing vaccines that elicit powerful immunity.
Background on Dendritic Cells
Dendritic cells are a fundamental force for initiating immune responses, by educating B and T lymphocytes, the components of adaptive immunity [2, 3]. First visualized by Paul Langerhans as LCs in the skin some 140 years ago, DC characterization began less than forty years ago [4]. During the first twenty years, DCs were carefully isolated from the tissues and studied by few investigators. However, identification of methods to differentiate large quantities of DCs in vitro [5–8] allowed the discovery of many of their biological and molecular properties. Yet, the discovery of different subsets led investigators to go back to their painful isolation from tissues or to target them specifically with fusion proteins directly in vivo [9, 10].
The initiation of T-cell immunity faces several challenges. The low frequency of microbe-specific T cells, the very few specific peptide-MHC complexes presented by the infected cells (one hundred or less per cell) and the lack of co-stimulatory molecules expression on the infected cells. All these are required and limiting the ability to drive T cell clonal expansion and the generation of cytotoxic/helper T cells. However, these challenges are overcome by DCs, which capture microbes, present their antigens and provide signals necessary for T cell expansion and differentiation. Upon recognition of microbial components or in response to inflammatory cytokines secreted by cells in tissue microenvironment, DCs upregulate co-stimulatory molecules and migrate to secondary lymphoid organs, i.e., spleen and lymph nodes (LNs), where they activate antigen-specific T cells. Thus, DCs have traditionally been viewed as mobile sentinels that bring antigens to T cells and activate them. More recent studies indicate, however, that soluble antigens can directly diffuse into the draining LNs through lymphatics and conduits, thereby reaching the LN-resident DCs [11]. Murine in vivo studies suggest that these two waves of antigen delivery to LNs yield different immune responses [11]. DCs are also important in launching humoral immunity partly through their capacity to directly activate B cells [12, 13]. They also activate innate immune cells, such as natural killer (NK) cells [14, 15] and natural killer T (NKT) cells [16].
In addition to the ability to recognize and eliminate what is foreign or aberrant, the immune system has built-in tolerance mechanisms to ignore components of “self” [17]. DCs appear to be essential in maintenance of immunological tolerance both in the thymus and in the periphery [17]. Thus, their alteration might contribute to the break of tolerance and thereby to the pathogenesis of autoimmune diseases.
Dendritic cell subsets
There are two main DC lineages; the myeloid DCs (mDCs, sometimes called conventional DCs or cDCs) and the plasmacytoid DCs (pDCs).
pDCs circulate in the blood and enter lymphoid organs like lymphocytes through High Endothelial Venules (HEV). They are identified as linnegHLA-DR+ cells expressing IL-3Rα chain (CD123) at high levels and two specific markers: BDCA-2 [18–22] and ILT-7 [23]. They express a unique set of TLRs; TLR1, 6, 7, 9, and 10 and upon exposure to viruses, secrete large amounts of Type I IFN [22] as well as IP-10, TNF, and IL-6 [24]. Viral activation appears to depend on TLR7 and TLR9-triggering.
mDCs exist in various compartments: 1) peripheral tissue resident DCs, 2) secondary lymphoid organ-resident DCs and 3) circulating blood mDCs. Studies in mice have shown that LN-resident DCs capture microbial antigens that are rapidly delivered through lymphatics and conduits, and upon stimulation, these DCs induce the activation and proliferation of antigen-specific T cells [11]. In the steady state, LN-resident DCs that have captured self antigens without being activated, from dying cells for example, prevent the activation of autoreactive T cells through various mechanisms including apoptosis and anergy. While human LNs display a lot of DCs as well, it remains unclear whether the same mechanisms are operating. Mouse studies from the late 90’s using administration of purified DC subsets indicated that CD8α+-spleen DCs and CD8α−-spleen DCs were respectively inducing Type 1 and Type 2 immune responses [25, 26]. This concept of differential regulation of T cell immunity by distinct DC subsets was further demonstrated in elegant studies using fusion proteins targeting these different DCs subsets. There, antigens were selectively loaded in vivo onto distinct DC subsets, CD8α+ mDCs expressing DEC205, or CD8α− mDCs expressing DCIR2, by using specific antibodies conjugated with OVA [9]. CD8α+ mDCs preferentially induce CD8+ T cell immunity, while CD8α− mDCs preferentially induce CD4+ T cell immunity [9]. Accordingly, CD8α− mDCs and CD8α+ mDCs preferentially express distinct sets of genes involved in MHC Class II and Class I presentation, respectively [9].
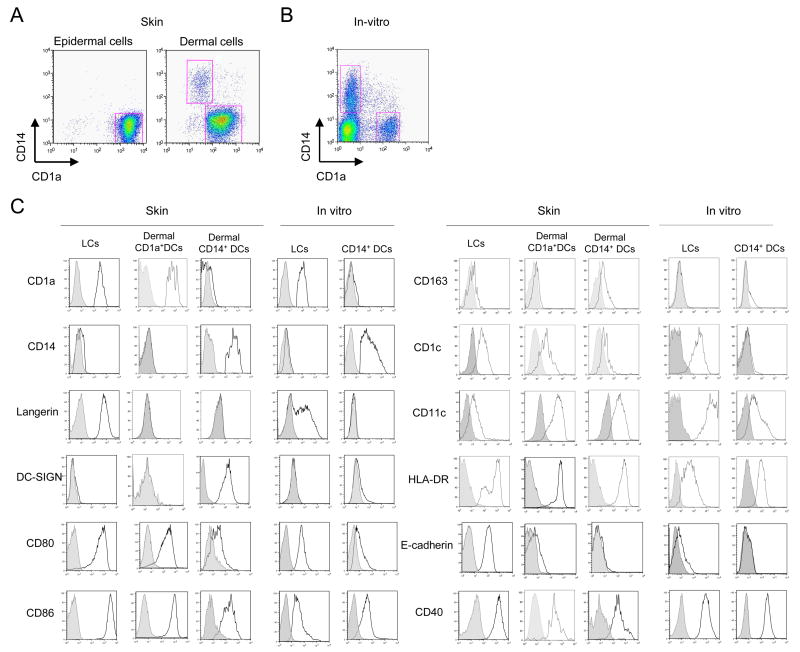
The human skin harbors at least 3 different DC subsets as well as macrophages. Langerhans cells, (LCs) reside in the epidermis, while the dermis hosts at least two main subsets of DCs; CD1a+ DCs and CD14+ DCs [27] alongside with macrophages [28]. (Figure 1A,B,C). Epidermal LCs express CD1a, Langerin and E-cadherin, while dermal CD14+ DCs express DC-SIGN, CD11b and Factor XIIIa [29]. Dermal CD1a+ DCs express an intermediate phenotype. The skin mDC subsets can also be generated in vitro by culturing CD34+-hematopoietic progenitor cells (HPCs), with GM-CSF and TNF-α [5, 30]. Our current view is that dermal CD163+FXIIIa+ cells include both non-migrating CD11c− macrophages and CD11c+CD14+ migratory DCs. It is possible that these macrophages and CD14+ DCs cells are able to transform into each other as was shown earlier with monocyte-derived DCs and macrophages in response to a balance of IL-6 and TNF [31]. Other surface molecules distinguish LCs from dermal DCs. In particular CD14+ DCs express a large number of surface lectins including DC-SIGN, DEC-205, LOX-1, CLEC-6, Dectin-1 and DCIR, while LCs only express Langerin and DCIR. Dermal CD14+ DCs also express regulatory molecules such as PD-L1, ILT-2 and HLA-G while LCs do not (Klechevsky; unpublished data).
Figure 1. Purification and characterization of epidermal and dermal DCs obtained from human skin and of in vitro CD34+-HPCs-derived mDC subsets.
(A) Epidermal- and dermal-resident DCs were allowed to migrate from their respective tissues and were harvested after 2 days. The cells were enriched with a Ficoll-diatrizoate gradient, stained with CD1a and CD14 mAbs and analyzed by flow cytometry. Epidermal sheets yielded CD1ahiCD14−cells. Dermis yielded two distinct populations: CD1a−CD14+ cells (dermal CD14+ DCs) and CD1adimCD14− cells (dermal CD1a+ DCs).
(B) CD34+-HPC cultured with GM-CSF and TNF-α for 9 days were stained with CD1a and CD14 mAbs to identify two subpopulations of mDCs: LCs and CD14+ DCs.
(C) Flow cytometry analysis of isolated epidermal and dermal cells, or in vitro-generated CD34-HPCs-derived DC subsets. Cells were gated on CD1a-positive or CD14-positive populations and analyzed for the expression of Langerin, DC-SIGN, CD80, CD86, CD163, CD1c, CD11c, HLA-DR, E-cadherin and CD40.
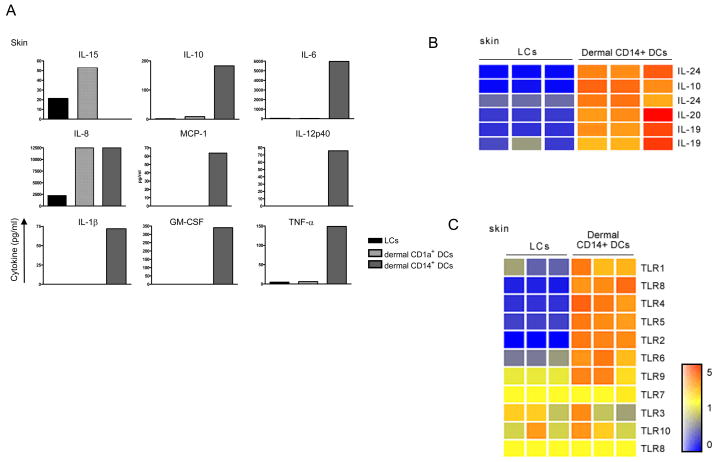
CD14+ DCs, but not LCs, either generated in vitro [30] or isolated from human skin [10] produce, upon activation through CD40 a large set of cytokines including IL-1β, IL-6, IL-8, IL-10, IL-12, GM-CSF, MCP and TGF-β (Figure 2A). Microarray analysis also reveals that CD14+ DCs uniquely express other members of the IL-10-family IL-19, IL-20 and IL-24 (Figure 2B) [32, 33] whose role remains to be established. In contrast, LCs produce only a few cytokines, a notable exception being the CD8+ T cell-enhancing cytokine; IL-15 (Figure 2A and B).
Figure 2. Characterization of epidermal and dermal DCs obtained from human skin .
(A) IL-15, IL-10, IL-6, IL-12p40, MCP-1, GM-CSF, IL-1β, and TNF-α were measured by a multiplex bead assay (Luminex) in the culture supernatant of sorted LCs, dermal CD1a+ DCs and dermal CD14+ DCs after activation with CD40L for 24 h. Data are representative of 3 independent experiments.
(B) Gene expression analysis of IL-10 family related cytokines: IL-10, IL-19, IL-20 and IL-24 by skin DCs isolated from 3 different specimens. RNA was prepared from FACS-sorted migrated skin mDC subsets: epidermal LCs and dermal CD14+ DCs. Expression values are normalized per gene to the median of the 6 samples. Transformed expression levels are indicated by color scale, with red representing relative high expression and blue indicating relative low expression.
(C) Gene expression analysis of Toll-Like Receptor genes by skin DCs isolated from 3 different specimens. RNA was prepared from FACS-sorted migrated skin mDC subsets: epidermal LCs and dermal CD14+ DCs. Expression values are normalized per gene to the median of the 6 samples. Transformed expression levels are indicated by color scale, with red representing relative high expression and blue indicating relative low expression.
Dermal CD14+ DCs Preferentially Initiate Humoral Immunity
Initial studies carried more than a decade ago using CD34-HPCs-derived DCs gave the first insight regarding the preferential control of humoral immunity by dermal CD14+ DCs [33]. There, it was shown that CD14+ DCs themselves but not LCs, induce naïve B cells-activated through their CD40 receptor to produce large amounts of IgM. The process was found to be dependent on the secretion of IL-6, IL-12 and sgp80 by CD14+ DCs [33][34]. Furthermore, CD4+ T cells that were primed by dermal CD14+ DCs were able to induced naïve B cells to produce large amounts of IgM while CD4+ T cells cultured with LCs were less efficient. However, most remarkably, only CD4+ T cells that were primed by dermal CD14+ DCs were able to induce naïve B cells to switch isotypes towards the secretion of both IgG and IgA. In contrast, CD4+ T cells cultured with LCs did not allow isotype switching by naïve B cells.
CD14+ DCs induce naïve T cells to differentiate into cells with properties of folicullar helper T cells (Tfh). The CD14+ DC-polarized T cells secrete the cytokine CXCL13, a unique feature of the follicular helper CD4+ T cell population isolated from Germinal Centers. This newly identified property of CD14+ DCs, together with our earlier demonstration of the capacity of CD14+ DCs from cultures of CD34-derived DCs to induce naive B cells to secrete IgM [33], indicate that CD14+ DCs specialize in the control of mature B cell differentiation. This novel function discovered with human DCs might also apply to mouse dermal DCs which migrate into the outer paracortex, just beneath the B cell follicles, whereas LCs migrate into the T cell rich inner paracortex [35]. While able to induce naïve CD4+ T cells to potently help B cells, CD14+ DCs, unlike LCs, are not inducing CD4+ T cells to secrete typical Type 2 cytokines.
When considering CD8+ T cells, dermal CD14+ DCs show a very poor ability to induce their differentiation into potent CTL effectors. This is observed during a Mixed Lymphocyte Reaction using allogeneic naïve CD8+ T cells and during the priming of naïve autologous CD8+ T cells using Mart-1-derived HLA-A201+ binding peptides [10]. Indeed, this is not due to the inability of the CD14+ DCs to present peptide-MHC Class I complexes but rather due to their inability to induce the expression of the cytotoxic effector molecules (Granzymes A/B, perforin) on the differentiating T cells. Indeed, the CD14+ DCs, when pulsed with Class I-restricted peptide, can permit the expansion of memory CD8+T cells, such as those specific for the immunodominant Influenza Matrix Protein peptide suggesting that they can efficiently load peptides on HLA-Class I molecules and present these peptide MHC complexes to memory CD8+ T cells. However, these CD14+ DCs display a reduced ability to cross-present proteins such as Influenza Matrix Protein, which is not due to their lower ability to process proteins as they can efficiently process tetanus toxoid and present its peptides to memory CD4+ T cells. As recently documented with mouse lymphoid organ resident DC subsets [9], a microarray analysis reveals the higher expression of MHC class II processing machinery and reduced expression of the MHC Class I processing machinery in CD14+ DCs when compared to LCs.
Thus, our current data together with our previous observation that CD14+ DCs can induce B cell priming [33], indicate that CD14+ DCs have a special capacity to directly, and indirectly, through T cell polarization, affect B cell differentiation towards antibody secretion. Thus we conclude that CD14+ DCs preferentially control humoral immunity.
LCs Preferentially Initiate Cellular Immunity
The initial observation using CD34-HPCs-derived DCs showed that LCs have an enhanced capacity to expand allogeneic T lymphocytes in a mixed lymphocyte reactions (MLR) compared to CD14+ DCs [33]. Detailed analysis further showed a marked differences in the ability of these two subsets to expand naïve CD4+ or CD8+ T cells. Indeed, LCs, either generated in vitro or isolated from human epidermis, showed a strong ability to induce proliferation of allogeneic naive T cells in mixed lymphocyte reactions (MLR) with less than 100 cells showing a considerable T cell proliferation. CD14+ DCs however, were able to induce a limited proliferation of naïve CD4+ and CD8+ T cells. To further study the quality of the primed CD8+ T cells, melanoma antigen-specific CD8+ T cells were expanded by exposing naïve CD8+ T cells to MART-1-peptide-loaded LCs or CD14+ DCs. Similarly to the allogeneic response, LCs were able to induce a more robust expansion of peptide-specific CD8+ T cells when compared to CD14+ DCs. In addition, when examined for the capacity of each DC subset to present in the context of MHC Class I peptides derived from external unprocessed protein antigens such as Influenza Matrix Protein and MART-1 to CD8+ T cells (cross-presentation/priming), in vitro-cultured LCs were able to more efficiently process and present protein to naïve and memory CD8+ T cells compared to CD14+ DCs.
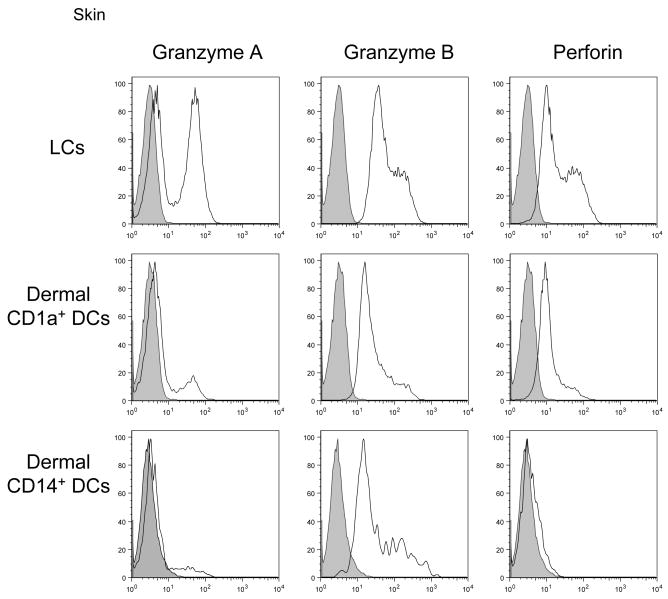
These studies of primary CD8+ T cell responses with Class I-binding peptides as well as cross-priming with proteins and polypeptides which require processing, indicate that LCs are strong inducers of the differentiation of naive CD8+ T cells into potent cytotoxic effectors. Indeed naïve CD8+ T cells, which do not express Granzymes A/B or perforin, acquire high levels of these effector molecules when primed by LCs but not CD14+DCs (Figure 3). This phenotype of effector Cytotoxic T cells is consistent with their ability to efficiently kill tumor cell lines which express on their surface low levels of TCR ligand. Interestingly, though LCs express lower levels of TCR ligands than CD14+ DCs, the antigen-specific T cells that they elicit display a higher avidity than those generated with CD14+DCs. Studies performed with CD34-HPCs-derived DCs loaded with killed tumor cells [36, 37] also indicated that LCs and CD14+ DCs were able to induce some expansion and differentiation of naive CD8+ T cells provided several culture cycles were performed. Under these conditions, LCs were not significantly more potent than CD14+ DCs, in a variance with our studies with MHC-Class I binding peptides or with proteins. This may be best explained by a differential processing of dead tumor cells and proteins. In particular, CD14+ DCs are far more efficient at phagocytosing killed tumor cells than LCs [37]. Further studies to better characterize the quality of the CD8+ T cells primed by the dermal CD14+ DCs are currently being performed.
Figure 3. LCs are more efficient than CD14+ DCs at priming antigen-specific high-avidity effector CD8+ T cells.
Allogeneic naïve CD8+ T cells primed by each skin mDC subset: LCs (upper row), dermal CD1a+ DCs or CD14+ DCs (middle and lower rows, respectively) for 7 days, were stained and analyzed by flow cytometry for the expression of the effector molecules; Granzyme A, Granzyme B, and perforin. Data are representative of 4 independent experiments.
The limited activation of Tetanus toxoid-specific memory CD4+ T cells indicates that LCs appear less efficient in MHC Class II peptide presentation than CD14+ DCs, however, they are able to polarize naïve CD4+ T cells into cells secreting Type 2 cytokines such as IL-4, IL-5 and IL-13. Indeed, this might explain the preferential skewing towards Th2 responses that was observed in T cells from mice receiving antigen in the epidermis (rich in LCs) using antigen-loaded gold particles delivered with a gene gun [38]. Both LCs and CD14+DCs are capable of inducing IFNγ-secreting CD4+ T cells. However, further studies are necessary to establish whether the IFNγ secreting CD4+ T cells generated in response to CD14+ DCs are qualitatively equivalent to those made in the presence of LCs. Also, it remains to be assessed which type of helper T cell is required for CTL priming. We surmised that the LCs will generate powerful helpers for CD8+ T cell priming. While IL-21 has been found to be such a powerful inducer [39], it is remains to be established whether LCs can induce the generation of IL-21 secreting T cells. Indeed, the localization of LCs to the inner paracortex is consistent with their function ability to prime CD8+ T cells and CD4+ T cells.
For many years, LCs have been viewed as a paradigm population in DC biology, whereby DCs survey and sense pathogens, become activated, and migrate to the draining lymph node where they present antigens to lymphocytes, resulting in pathogen-specific immune responses. Recent studies in mice have challenged this concept, however. Studies with Herpes Simplex viruses (HSV) suggested that LCs played no role in the development of an immune response against the virus [40, 41]. Rather, dermal DCs appeared to transport the viral antigens into the draining lymph nodes and deliver them to the resident DCs [42]. Genetic depletion of LCs in mice resulted in either normal [35], increased [43] or decreased [44] hypersensitivity reactions. Our findings are however in line with studies performed in mice that involved peptide-loaded epidermal DCs injected subcutaneously [45] or lentiviral vectors delivered to LCs by genetic immunization [46]. Furthermore, consistent with our data, another recent mouse study showed that LCs actually cross present antigens to CD8+ T cells in vivo [47]. Thus, while studies in mice have not yet settled with respect to the role of LCs, our data indicate that human LCs preferentially activate cell-mediated immunity.
Dermal CD1a+ DCs might be the equivalent to the mouse Langerin+ Dermal DCs
Our studies have shown that dermal CD1a+ DCs represent a significant population of DCs which are present in the upper layer of the dermis. They are most likely the dermal BDCA1+ cells described by Zaba et al. [28] as they express CD1c, the antigen recognized by the BDCA-1 antibody. This population is a potent inducer of the proliferation of allogeneic CD4+ T cells [10, 28] and CD8+ T cells though less efficient than LCs. Accordingly, their phenotype is closer to that of LCs than that of CD14+ DCs (Figure 1C). A major exception is the lack of Langerin and E-Cadherin, characteristic of LCs. Indeed, Langerin expression could be detected solely on LCs in the epidermis in situ. As LCs, dermal CD1a+ DCs essentially produce IL-15 and little of the proinflammatory cytokines and chemokines expressed by dermal CD14+ DCs with the possible exception of IL-8 (Figure 2A). They are, however, less potent than LCs in inducing the polarization of CD4+ T cells into Th2 cells and that of naïve CD8+T cells into highly potent Granzyme A/B and Perforin -positive CTLs. They might represent the precursors of LCs, and might also correspond to the recently identified mouse Langerin+ dermal DCs that are capable of participating in skin immune response [48–50].
Molecular control of DC functions
As observed earlier with CD34+-HPCs-derived in vitro generated DCs [32], skin LCs and CD14+ DCs produce a dramatically distinct panel of cytokines. In particular as discussed earlier, CD14+ DCs produce spontaneously and in response to CD40-ligation IL-10 and TGF-β as well as multiple proinflammatory cytokines. In contrast, LCs produce only a limited set of cytokines (IL-6 and IL-8) and most prominently IL-15 (Figure 2A) [10]. Our attempts to identify the molecular mechanisms endowing CD14+ DCs and LCs with their specialized functions have thus far only been partly conclusive. Looking at the cell surface, LCs express more 4-1BB-L [51], but anti-CD137 blocked T cell priming by both subsets. Furthermore, LCs, but not CD14+ DCs, express IL-15, which is known to enhance CD8+ T cell responses [52]. However, none of the mAbs we have tried were convincingly able to inhibit LCs-induced CD8+ T cells priming while they were able to inhibit the proliferation of T cells induced by exogenous IL-15 (Klechevsky; unpublished data). We could, however, enhance CD14+ DCs-mediated priming CD8+ T cells by adding external IL-15 to the culture, which in this case was blocked by anti-IL15 mAb (Klechevsky; unpublished data). CD14+ DCs, on the other hand, produce IL-10 and TGF-β and polarize naïve CD8+ T cells into poor effector cells expressing little Granzyme A/B and Perforin. Addition of IL-10 can induce LCs to polarize CD8+ T cells into cells with low Granzyme A/B and perforin (Klechevsky; unpublished data). Indeed, the earlier demonstration that addition of IL-10 to cocultures of monocyte-derived DCs allows the generation of regulatory CD4+ T cells is a step in this direction [53, 54]. However, blocking IL-10 appears to be insufficient for CD14+ DCs to induce potent effector CD8+ T cells.
In addition to IL-10, microarray analysis permitted us to identify the expression of other IL-10 family-related cytokines by the dermal CD14+ DCs including IL-19, IL-20 and IL-24 (Figure 2B). These cytokines act on epithelial cells and fibroblasts and might just represent an important component of dermis homeostasis. They were shown to mediate diverse activities, including immune suppression and enhance antibacterial and antiviral immunity [55]. The distinct biological effect of each of these cytokines as produced by dermal CD14+ DCs is yet to be fully assessed and which will be dependent on the state of activation, the target cells and the phase of the immune response.
Another level of regulation is provided by the differential sensitivity of different DCs to environmental stimuli [56]. For such, TLRs respond to different ligands and are expressed on distinct DC subsets. The distinct repertoire of TLRs allows DC subsets to respond differentially to microbes. Different TLRs recognize different PAMPs, and deliver distinct molecular signals, thereby yielding distinct types of DC maturation and consequently distinct immune responses [57]. For instance, Escherichia coli LPS stimulates through TLR4, inducing dermal CD14+ DCs to secrete IL-10, which may eventually enhance Type 2 or regulatory T cell development [58]. Conversely, TLR7/TLR8-activation, enhance the production of IL-12 and may lead to generation of distinct Th development.
Epidermal LCs isolated from skin have been reported to express TLR1, TLR2, TLR3, TLR6 and TLR10, but lack TLR4 and TLR5 expression. The expression of TLR7/8 remains unclear [59, 60]. Our own data using microarray of highly purified cells failed to show much TLR expression by LCs (Figure 2C). Thus we feel that additional studies are necessary for concluding on the expression of Pathogen Recognition Receptors (PRRs) by LCs. In contrast to LCs, dermal CD14+ DCs express TLRs recognizing bacterial PAMPs, such as TLR2, 4, and 5 [60] and as we demonstrate TLR 6,8 and 10 (Figure 2C). Therefore dermal CD14+ DC may represent a DC subset specialized for bacterial recognition in the skin.
When considering TLR-activation, particularly in the context of vaccine design, it is important to take into consideration the significant differences that are found between human and murine DC subsets. For instance, both myeloid and plasmacytoid DCs express TLR9 in the mouse [56, 61], while only pDCs express TLR9 in the human [62, 63].
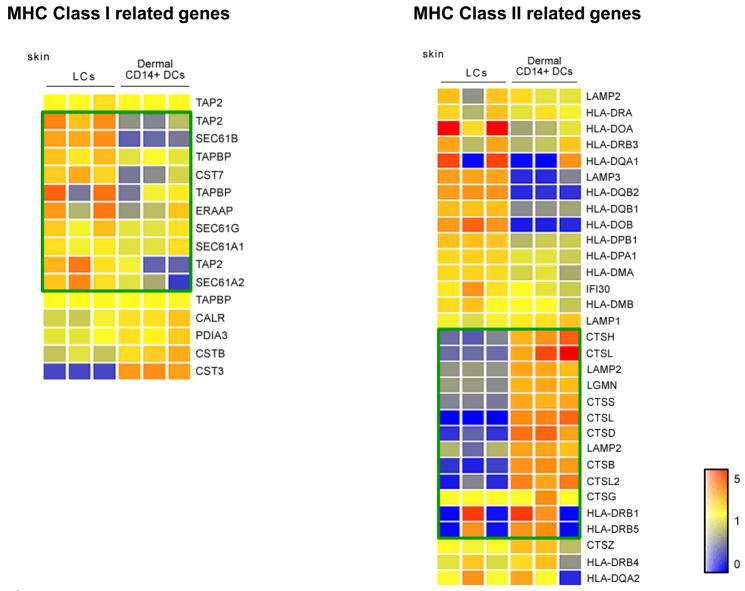
Finally, as observed with mouse spleen DC subsets [9], we found that LCs express more genes related to the MHC Class I pathway and CD14+ DCs more genes related to the MHC Class II pathway (Figure 4) which may explain the superior ability of LCs to crosspresent peptides on Class I molecules to CD8+ T cells.
Figure 4. Characterization of epidermal and dermal DCs obtained from human skin and of in vitro CD34+-HPCs-derived mDC subsets.
Relative amounts of mRNAs associated with the MHC class I (left panel) and MHC class II (Right panel) processing pathways expressed by FACS-sorted, migrated skin mDC subsets: LCs and Dermal CD14+ DCs. Figure shows three individual gene arrays prepared from distinct mRNA samples. Expression values are normalized per gene to the median of the 6 samples. Transformed expression levels are indicated by color scale, with red representing relative high expression and blue indicating relative low expression.
Summary and future directions
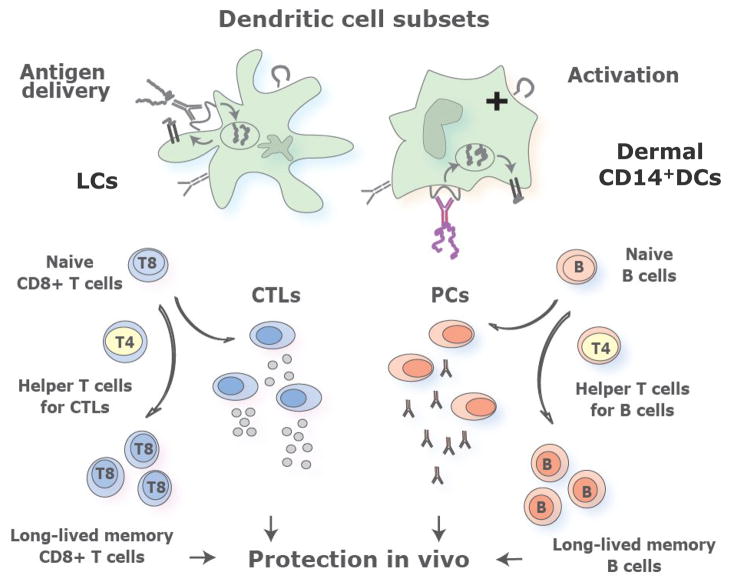
Studies performed in the last decade have highlighted the commonalities and uniqueness of the various DC subsets. This new knowledge represents a fertile ground to work on to design better strategies for intervening in numerous clinical situations. The capacity of LCs and CD14+ DCs to preferentially prime cellular immunity and humoral immunity respectively has significant implications, most particularly in the context of novel human vaccines. The effective vaccines developed against a variety of infectious agents, including polio, measles and Hepatitis B, certainly represent major achievements in medicine. Yet these vaccines are all specific for acute infections and their protective capacity arises largely from their induction of humoral immune responses [64]. Given both the methods by which these vaccines are delivered and the data discussed here, it is likely that they principally deliver antigen to and activate CD14+ DCs and possibly CD1a+ DCs but not LCs. Therefore, targeting LCs will be important for the design of vaccines that aim at eliciting strong cellular immunity. Such vaccines might be particularly useful at preventing, and perhaps even treating, chronic diseases including viral (HIV, Hepatitis C Virus), bacterial (mycobacteria) and parasitic (malaria) diseases, as well as cancer [65]. The most efficient vaccines might actually be those that will target both CD14+ DCs and LCs, thereby allowing the maximal stimulation of both humoral and cellular immune responses. For instance, one would imagine that the ideal HIV vaccine should target Env protein to the dermal DCs to elicit potent humoral responses that will prevent virus entry and target Nef and Gag proteins to LCs to elicit potent T cell responses that will eradicate cells harbouring the virus thereby reducing the spreading of the virus (Figure 5). It is difficult to imagine that a modified Adenoviral vector might optimally deliver each of these antigens to the appropriate DC subset. In this regard it is intriguing to consider that one of the most effective vaccines, smallpox vaccine [64, 66], acts through a combination of strong cellular and humoral immunity and requires scarification of the skin, a procedure that injures both epidermis and dermis and that is likely to mobilize and activate LCs as well as dermal DCs. Likewise, one of the most potent vaccines ever generated against Yellow Fever (YF17D) activate multiple dendritic cell subsets [67] and leads to integrated immune response that include both humoral and cellular immunity [68].
Figure 5. Understanding human myeloid dendritic cell subsets for the rational design of novel DC-targeting vaccines.
Novel vaccines that will prevent and treat chronic diseases such as HIV relay on rational immunological approaches and aim at activating both the cellular and the humoral arm. We envision that targeting antigens and activation of distinct mDC subsets, with different specializations, will result in the generation of a broad and long lived immune protection. Thus, the most efficient vaccines might be those that will target both LCs and dermal CD14+ DCs thereby allowing the maximal stimulation of cellular and humoral immune responses and the generation of long-term memory protection.
We foresee that the translation of this new knowledge on how DCs regulate the immune system into medicine will result in a wealth of new treatments that target DCs to improve the quality of life of many patients. We foresee that the improved vaccines that target DCs will permit us to treat and prevent many chronic diseases, and likewise, manipulation of DCs will also permit to dampen overly enhanced immune responses as occurs in allergy and autoimmunity possibly by turning on regulatory mechanisms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bleharski JR, Li H, Meinken C, Graeber TG, Ochoa MT, Yamamura M, Burdick A, Sarno EN, Wagner M, Rollinghoff M, Rea TH, Colonna M, Stenger S, Bloom BR, Eisenberg D, Modlin RL. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science. 2003;301(5639):1527. doi: 10.1126/science.1087785. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360(6401):258. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180(1):83. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 10.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19(1):47. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 12.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 13.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312(5780):1672. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 14.Munz C, Dao T, Ferlazzo G, de Cos MA, Goodman K, Young JW. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105(1):266. doi: 10.1182/blood-2004-06-2492. [DOI] [PubMed] [Google Scholar]
- 15.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic Cells Prime Natural Killer Cells by trans-Presenting Interleukin 15. Immunity. 2007;26(4):503. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3(9):867. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 17.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 18.O’Doherty U, Steinman RM, Peng M, Cameron PU, Gezelter S, Kopeloff I, Swiggard WJ, Pope M, Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178(3):1067. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185(6):1101. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olweus J, BitMansour A, Warnke R, Thompson PA, Carballido J, Picker LJ, Lund-Johansen F. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc Natl Acad Sci U S A. 1997;94(23):12551. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strobl H, Scheinecker C, Riedl E, Csmarits B, Bello-Fernandez C, Pickl WF, Majdic O, Knapp W. Identification of CD68+lin- peripheral blood cells with dendritic precursor characteristics. J Immunol. 1998;161(2):740. [PubMed] [Google Scholar]
- 22.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 23.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203(6):1399. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-{alpha} in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102(9):3372. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189(3):587. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A. 1999;96(3):1036. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17(4):273. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117(9):2517. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nestle FO, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest. 1994;94(1):202. doi: 10.1172/JCI117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184(2):695. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK. TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol. 2003;171(5):2262. doi: 10.4049/jimmunol.171.5.2262. [DOI] [PubMed] [Google Scholar]
- 32.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, Banchereau J, Liu YJ, Lebecque S, Caux C. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160(4):1666. [PubMed] [Google Scholar]
- 33.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90(4):1458. [PubMed] [Google Scholar]
- 34.King C, Tangye SG, Mackay CR. T Follicular Helper (T(FH)) Cells in Normal and Dysregulated Immune Responses. Annu Rev Immunol. 2008;26:741. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 35.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22(5):643. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL, Munz C, Heller G, Young JW. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173(4):2780. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 37.Cao T, Ueno H, Glaser C, Fay JW, Palucka AK, Banchereau J. Both Langerhans cells and interstitial DC cross-present melanoma antigens and efficiently activate antigen-specific CTL. Eur J Immunol. 2007;37(9):2657. doi: 10.1002/eji.200636499. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez D, Harder G, Fattouh R, Sun J, Goncharova S, Stampfli MR, Coyle AJ, Bramson JL, Jordana M. Cutaneous antigen priming via gene gun leads to skin-selective Th2 immune-inflammatory responses. J Immunol. 2005;174(3):1664. doi: 10.4049/jimmunol.174.3.1664. [DOI] [PubMed] [Google Scholar]
- 39.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197(2):153. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301(5641):1925. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 42.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25(1):153. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23(6):611. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169(4):569. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celluzzi CM, Falo LD., Jr Epidermal dendritic cells induce potent antigen-specific CTL-mediated immunity. J Invest Dermatol. 1997;108(5):716. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- 46.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24(5):643. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103(20):7783. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204(13):3133. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204(13):3119. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204(13):3147. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 52.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(42):15154. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 54.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen- specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93(5):1634. [PubMed] [Google Scholar]
- 55.Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121(5):1108. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 56.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3(12):984. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 58.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167(9):5067. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells--changing views on their function in vivo. Immunol Lett. 2006;106(2):119. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 60.van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178(4):1986. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 61.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O’Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197(1):101. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31(11):3388. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 64.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124(4):849. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 65.Letvin Correlates of Immune protection and the Development of Human Immunodeficiency Virus Vaccine. Immunity. 2007;27:366. doi: 10.1016/j.immuni.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Frey SE, Newman FK, Cruz J, Shelton WB, Tennant JM, Polach T, Rothman AL, Kennedy JS, Wolff M, Belshe RB, Ennis FA. Dose-related effects of smallpox vaccine. N Engl J Med. 2002;346(17):1275. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- 67.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203(2):413. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, 3, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Kelvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Haddad EK, Sekaly RP. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205(13):3119. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]