Abstract
Introduction
The mechanism(s) by which sex steroids regulate bone turnover in humans are unclear, and recent studies have suggested that follicle-stimulating hormone (FSH) may play an important role in regulating bone resorption.
Materials and Methods
Fifty-nine men (median age, 69 yr) underwent suppression of sex steroids using a gonadotropin-releasing hormone (GnRH) agonist and aromatase blocker and were replaced with testosterone (T; 5 mg/d) and estradiol (E; 37.5 μg/d). After assessment of bone resorption markers (serum C-terminal telopeptide of type I collagen [CTX] and TRACP5b), they were randomized to sex steroid deficiency (−T, −E), E alone (−T, +E), T alone (+T, −E), or both (+T, +E) and restudied 3 wk later. Bone marrow aspirates were obtained to isolate osteoblastic, T, and monocytic cells using magnetic-activated cell sorting.
Results
Serum CTX and TRACP5b increased significantly (by 71% and 15%, p < 0.01 and < 0.001, respectively) in the −T, −E group, and these increases occurred despite a 60% suppression of serum FSH levels (p < 0.001) caused by the GnRH agonist. There were significant E (but not T) effects on preventing increases in serum CTx and TRACP levels. There was a nonsignificant trend (p = 0.122) for E to suppress RANKL mRNA levels in bone marrow osteoblastic cells. Changes in mRNA levels for other cytokines (TNF-α, interleukin (IL)-1α, IL-1β, IL-1ra, IFN-γ) in bone marrow cells were not significant.
Conclusions
E has greater suppressive effects on bone resorption than T, and increased bone resorption after sex steroid deficiency can occur independently of changes in FSH secretion. E effects on bone resorption may be mediated by regulation of RANKL production by osteoblastic cells, although further studies using more highly purified cells may reduce the variability of the mRNA measurements and allow for clearer definition of the mediators of sex steroid action in vivo.
Key words: male osteoporosis, cytokines, RANKL, bone turnover
INTRODUCTION
It is apparent that age-related bone loss in men is caused, at least in part, by declining non–sex hormone–binding globulin (SHBG)–bound (or bioavailable) sex steroid levels, with declining bioavailable estradiol (E2) levels playing a particularly important role.(1) In a previous study using suppression of testosterone (T) and estrogen (E) production and selective replacement with T, E, both, or neither, we showed that E played a critical, and perhaps dominant, role in regulating bone resorption in men, with both E and T contributing to the maintenance of bone formation.(2) Using a somewhat different study design, Leder et al.(3) also subsequently showed an important role for E in regulating bone turnover in men, although in their study, T seemed to have effects comparable to E.
Recent work has also indicated that the pituitary hormone, follicle-stimulating hormone (FSH), may be a key regulator of bone resorption,(4) although findings with FSH receptor knockout mice have been conflicting.(5) Nonetheless, osteoclast precursors do possess FSH receptors, and FSH clearly increases osteoclastogenesis in vitro, leading to the suggestion that the increase in bone resorption in the setting of sex steroid deficiency is not caused by the direct effects of sex steroid withdrawal on bone but rather are mediated indirectly through the increased FSH secretion that accompanies primary gonadal failure.(4,6,7)
Despite extensive studies in rodents,(8) the precise mediators of the increase in bone resorption in the setting of sex steroid deficiency in humans remain unclear. Our group previously showed that E deficiency in women was associated with increased expression of RANKL protein (assessed using flow cytometry) on bone marrow alkaline phosphatase (ALP)+ (osteoblastic), CD3+ (T cells), and CD20+ (B cells) cells.(9) However, whether this increase in RANKL expression was a consequence of direct effects of E deficiency on RANKL mRNA and/or protein levels or was caused by increased RANKL production secondary to increased levels of proresorptive cytokines (interleukin [IL]-1α, IL-1β, TNFα),(10,11) reduced levels of the IL-1 receptor antagonist (IL-1ra),(12) or increased sensitivity to the action of these cytokines remain open questions. Alternatively, sex steroids may modulate the production of the decoy receptor for RANKL, osteoprotegerin (OPG),(13) or regulate the production of TRAIL, which binds OPG and may potentially decrease the amount of OPG available to neutralize RANKL.(14) Finally, recent studies in mice have suggested that E deficiency is associated with increased IFNγ production and/or action, which leads to an expansion of T cells, thereby increasing the total production of TNFα in the bone microenvironment, without necessarily altering TNFα production per cell.(11,15)
This study used the factorial design identical to our previous study(2) of replacing elderly men who were made hypogonadal using a gonadotropin-releasing hormone (GnRH) agonist with T alone, E alone, both, or neither to address several of the key unresolved issues regarding the role of sex steroid action on bone. Specifically, because GnRH administration would be expected to suppress FSH secretion, we sought to answer definitively whether bone resorption would increase in the setting of sex steroid deficiency concomitant with a suppression of FSH secretion. We also assessed, using flow cytometry, RANKL protein levels on bone marrow osteoblastic, T, and B cells, as well as the concentration of these cells under the conditions of deficiency of T, E, both, or neither. Finally, using bone marrow cells harvested from these subjects, we evaluated mRNA levels for RANKL, OPG, TRAIL, an IFNγ responsive gene (IFNγ-inducible protein 30 [IFI30]),(16) and a TNFα responsive gene (TNF-α inducible protein 6 [TNFαIP6])(17) in osteoblastic cells, TNF-α and IFNγ in T cells, and TNFα, IL-1α, IL-1β, IL-1ra, IFNγ, and TNFαP6 in monocytic cells.
MATERIALS AND METHODS
Study subjects
After approval of the protocol by the Mayo Institutional Board and obtaining written, informed consent, 59 elderly men (median age, 69 yr; age range, 50–80 yr) were recruited for the studies. All subjects were interviewed for medical history and underwent a physical examination. Subjects were excluded if they were taking any medications known to affect calcium metabolism (i.e., glucocorticoids, anticonvulsants, calcium supplements >1000 mg/d, vitamin D >1000 IU/d, T replacement therapy, calcitonin, or bisphosphonates). Other exclusions were subjects who were within 6 mo of a major surgical procedure or traumatic fracture, those with significant medical diseases such as renal failure, malabsorption, active malignancy (including prior history of prostate cancer), or those with congestive heart failure. Subjects were also excluded if they had a history of a nontraumatic fracture of the vertebrae, hip, or distal forearm. All subjects underwent a prestudy laboratory assessment that included a complete blood count, chemistry group, serum 25-hydroxyvitamin D [25(OH)D], and prostate-specific antigen level. Subjects with a significant abnormality in any of the prestudy laboratory screening tests were excluded. If the 25(OH)D level was <25 ng/ml, the men were treated with 1000 units/d of vitamin D3 for 6 wk before study. If the baseline 25(OH)D was <15 ng/ml, the subjects were rechecked after vitamin D treatment to ensure that the prestudy 25(OH)D level was ≥25 ng/ml.
Study protocol
The overall design of the study was similar to that previously reported by our group to evaluate effects of withdrawal and selective replacement with E or T on bone turnover markers.(2) Briefly, before entry into the study, the subjects had a fasting blood sample drawn for measurement of FSH levels. At the time of entry into the study, the subjects were administered a long-acting GnRH agonist (leuprolide acetate, Lupron-Depot; kindly provided by TAP Pharmaceuticals, Lake Forest, IL, USA), 7.5 mg intramuscularly, to suppress endogenous T and E production. They were also started on the aromatase inhibitor, letrozole (Femara; Novartis, East Hanover, NJ, USA), 2.5 mg/d. Physiological T and E2 levels were maintained by starting the subjects on a T gel (AndroGel; Solvay Pharmaceuticals, Marietta GA), 5 g/d (delivering 5 mg/d of testosterone), as well as an E2 patch (VivelleDot; Novartis), 0.0375 mg/d. The doses of the T gel and E2 patch were chosen based on the serum levels previously shown to be achieved by these doses, which were similar to physiologic circulating T and E2 levels in normal elderly men.(2) Three weeks after GnRH agonist administration and while maintaining letrozole, T, and E2 treatment, the subjects were admitted to the Mayo Clinical Research Unit (CRU) for their baseline visit. After an overnight fast, serum samples were drawn at 8:00 a.m. for calcium, phosphorus, amino-terminal propeptide of type I collagen (PINP), C-terminal telopeptide of type I collagen (CTX), and TRACP5b levels. Two consecutive 24-h urine collections were obtained for measurement of urinary N-telopeptide of type I collagen (NTX), and the mean of these urine values was used in the analysis.
After the baseline studies, the subjects were randomized into one of four groups: group A (−T, −E; n = 15) discontinued both T and E replacement; group B (−T, +E; n = 15) discontinued the T gel but continued the E patch; group C (+T, −E; n = 15) discontinued the E patch but continued the T gel; and group D (+T, +E, n = 14) continued both the T gel and E patch. All subjects received a second dose of the GnRH agonist, and all subjects continued letrozole treatment throughout the study period.
Three weeks after randomization, the subjects were readmitted to the CRU for their final visit. The baseline studies were repeated at this time point, and in addition, the subjects underwent a bone marrow aspiration from the iliac crest (30 ml) under local anesthesia for isolation of marrow cell populations (see below). On completion of the study, all study medications were discontinued, with the exception that the subjects were given an intramuscular injection of T (Testosterone Enanthate; Savient Pharmaceuticals, East Brunswick, NJ, USA), 200 mg, to allow time for the effects of the GnRH agonist to wane. Results of changes in skin blood flow after the above hormonal manipulations in these subjects have been recently reported in a separate publication.(18)
Hormonal and biochemical assays
Serum calcium and phosphorus were measured by an automated photometric assay (interassay CV < 10%; Roche Diagnostic, Indianapolis, IN, USA). Serum CTX was measured by a one-step ELISA (interassay CV < 8%; Osteometer BioTech, Herlev, Denmark). Bone TRACP5b was also measured by ELISA (interassay CV < 14%; Immunodiagnostic Systems [IDS], Fountain Hills, AZ, USA). Measurement of the concentration of PINP was done by radioimmunoassay (RIA; interassay CV < 10%; IDS). Urine NTX (expressed as nmol/d in complete 24-h urine collections) was measured by an automated immunoassay (interassay CV < 12%; Ortho-Clinical Diagnostics, Rochester, NY, USA). Total testosterone was measured by RIA (interassay CV < 12%; lower limit of detection 4 ng/dl; Diagnostic Products, Los Angles, CA, USA). High-sensitivity estradiol was measured by a double antibody RIA (interassay CV < 15%; lower limit of detection 5 pg/ml; Diagnostic Products). FSH was measured using a sequential two-step immunoenzymatic “sandwich” assay (interassay CV < 10%; Beckman Coulter, Fullerton, CA, USA).
Magnetic-activated cell sorting
Briefly, 20 ml of bone marrow (BM) aspirate was diluted in PBS, incubated with RosetteSep (Human Mesenchymal Cell Enrichment Cocktail; StemCell Technologies, Vancouver, Canada) for 60 min at room temperature and layered over Ficoll/sodium diatrizoate (1.0770–1.0800 g/ml; Lymphocyte Separation Medium; Amersham Biosciences, Piscataway, NJ, USA). After centrifugation and depletion of hematopoietic lineage cells, the mesenchymal cell–enriched fraction was stained with an anti-human alkaline phosphatase (ALP) antibody (clone B4–78; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA) and with a donkey anti-mouse IgG antibody conjugated to phycoerythrin (PE) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Anti-PE Microbeads (Miltenyi Biotec, Auburn, CA, USA) were used to isolate ALP+ cells on a separator (AutoMACS; Miltenyi Biotec). Aliquots of ALP+ and ALP− were analyzed by flow cytometry to determine the relative purity of isolated cells. To isolate CD3+ and CD14+ cells, the sample was not subjected to the RosetteSep step for hematopoietic cell depletion; anti-CD3 and CD14 antibodies were used to isolate these cells using magnetic cell sorting (MASC) as for the ALP+ cells.
Quantitative PCR analyses
To determine the regulation of expression of endogenous genes in different cell types (ALP+, CD3+, and CD14+ cells) isolated from each patient belonging to either of the four different treatment groups, total RNA was isolated from these cell types separately using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA). Five hundred nanograms of total RNA from each cell type was used for the cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). These cDNAs were used as template for the relative quantification of the selected mRNAs by real-time PCR using the iCycler (Bio-Rad). Table 1 provides information on the PCR primers used in the study. After amplification, the mean threshold cycle (Ct) value of a target mRNA from each patient's cDNA (amplified in triplicate) was normalized to the mean Ct of the TBP (the reference mRNA) of the corresponding patient to determine the dCT and 2−dCT values. The relative level of expression of a target mRNA was determined by normalizing the 2−dCT value of each patient with the mean 2−dCT value of group D.
Table 1.
Forward and Reverse PCR Primers, Expected Size of Amplified Product, and GenBank Accession Number for Primers Used in the Study
| Gene | Forward primer | Reverse primer | Expected size of product | Genbank accession number |
| RANKL | 5′-CAAGGAGCTGTGCAAAAGGAATTA-3′ | 5′-GATCATGGTACCAAGAGGACAG-3′ | 202 bp | NM_003701 |
| OPG | 5′-CAAGGAGCTGTGCAAAAGGAATTA-3′ | 5′-GATCATGGTACCAAGAGGACAG-3′ | 202 bp | NM_003701 |
| TRAIL | 5′-ACGAGCTGAAGCAGATGCAGGA-3′ | 5′-TGACGGAGTTGCCACTTGACTT-3′ | 139 bp | U57059 |
| TNFα | 5′-ACGAGCTGAAGCAGATGCAGGA-3′ | 5′-TGACGGAGTTGCCACTTGACTT-3′ | 139 bp | U57059 |
| IFNγ | 5′-GAAGAATTGGAAAGAGGAGAGTG-3′ | 5′-GTTCATGTATTGCTTTGCGTTGG-3′ | 239 bp | NM_000619 |
| IL-1α | 5′-CCAATGACTCAGAGGAAGAAATC-3′ | 5′-CAGCAGCCGTGAGGTACTGAT-3′ | 165 bp | NM_000575 |
| IL-1β | 5′-TTCGACACATGGGATAACGAGG-3′ | 5′-AGGACATGGAGAACACCACTTG-3′ | 172 bp | NM_000576 |
| IL-1ra | 5′-ATACTTGCAAGGACCAAATGTCAA-3′ | 5′-GTCAGTGATGTTAACTGCCTCC-3′ | 163 bp | NM_173841 |
| IFI-30 | 5′-AAAATGTCAGTGGCAGGTGGGA-3′ | 5′-AGCTGCAGGCATAGTGGCAGA-3′ | 175 bp | NM_006332 |
| TNFαIP6 | 5′-GCTAAGGCGGTGTGTGAATTTG-3′ | 5′-GCCACCACACTCCTTTGCGTG-3′ | 252 bp | NM_007115 |
| TBP | 5′-CCCCATGACTCCCATGACCC-3′ | 5′-CGTGGTTCGTGGCTCTCTTATC-3′ | 208 bp | NM_003194 |
Flow cytometry analyses
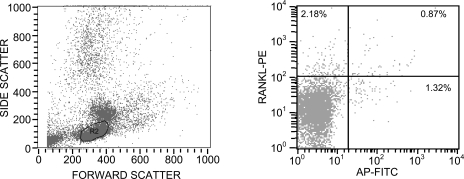
Flow cytometry was performed on bone marrow mononuclear cells as previously described.(9) The primary antibodies consisted of a monoclonal anti-ALP (B4–78; Hybridoma Bank, University of Iowa) and a polyclonal goat anti-human RANKL (N-19; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Corresponding control isotype antibodies at the same concentrations as the primary antibodies were used to measure the background staining. Secondary antibodies included PE donkey anti-goat (Jackson ImmunoResearch) and fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse (Jackson ImmunoResearch). Cells were also stained with the following fluorescent conjugated antibodies: CD3-FITC (Becton Dickinson, Franklin Lakes, NJ, USA) and CD-19 FITC (Becton Dickinson). Cells were analyzed using a Becton Dickinson FACScan cytometer, and data were analyzed using CellQuest Software (Becton Dickinson). A total of 50,000 events were counted, and final data were obtained within the lymphocyte gate. The frequency of positive cells was measured as the percentile of gated cells in fluorescent channels with activities >99% of the corresponding isotype controls, thus including backgrounds <1.0%. Figure 1A shows an example of the gating used in the analysis, which included primarily the lymphocyte gate. We examined other gates, but the vast majority of the cells of interest (ALP+, CD3+, and CD19+ cells) fell within the gate shown in Figure 1. Figure 1B shows an example of the dual flow using the RANKL and ALP antibodies. PE calibration beads (Becton Dickinson) were used to normalize PE fluorescence intensity for RANKL between samples, and the mean RANKL fluorescence intensity (MFI) per cell was used as an index of RANKL protein per cell, as described previously.(9) To validate that the RANKL antibody was selecting a population of RANKL-expressing cells, we used FACS to isolate RANKL+ versus RANKL− cells from waste human peripheral mononuclear cells from the blood bank and, using quantitative PCR, showed that there was a 2.5-fold increase in RANKL mRNA levels in RANKL+ versus RANKL− cells.
FIG. 1.
Example of the flow cytometry analysis used in the study. Shown is a representative analysis using the ALP and RANKL antibodies. (A) Forward/side scatter diagram, indicating the gate used for the analyses. (B) Staining with the ALP and RANKL antibodies, with the thresholds set based on the appropriate isotype controls. Shown also are the percentage of gated cells that were present in each quadrant.
Statistical analysis
The primary method of analysis to dissect out effects of E versus T on the various parameters took advantage of the factorial design. Thus, as in our previous study,(2) we used a two-factor ANOVA model to compare the changes in the variables in the +E (groups B and D) versus the –E (groups A and C) and the +T (groups C and D) versus the –T (groups A and C) groups. A one-sample t-test was used to assess percent change from baseline for variables where we had baseline and final measurements. Baseline clinical characteristics and values related for the bone markers and parameters related to calcium metabolism were compared between groups using a one-factor ANOVA model. If the ANOVA was significant, the Tukey method was used for pairwise comparisons. The relationships between MFI for RANKL in ALP+/RANKL+ cells with serum CTX, urine NTX, and serum TRACP5b were studied using the Pearson correlation. For variables that were not normally distributed, a log2 transformation was done before the statistical analyses. Results were considered significant at the p < 0.05 level. The data are reported as median and interquartile range for consistency, given that many of the measurements needed the log2 transformation to meet statistical assumptions.
RESULTS
Baseline clinical and biochemical data
Table 2 shows the baseline clinical and biochemical data in the four groups. As is evident, the study subjects were well matched for age and anthropometric variables, as well as for baseline serum calcium and phosphorus levels and the bone turnover markers.
Table 2.
Baseline Clinical and Biochemical Data in the Study Subjects
| Group A (−T, −E) | Group B (−T, +E) | Group C (+T, −E) | Group D (+T, +E) | p | |
| n | 15 | 15 | 15 | 14 | |
| Age (yr) | 71 (55, 75) | 67 (58, 72) | 69 (67, 73) | 68 (54, 76) | 0.603 |
| Height (m) | 1.75 (1.74, 1.79) | 1.78 (1.69, 1.83) | 1.78 (1.76, 1.84) | 1.79 (1.74, 1.82) | 0.701 |
| Weight (kg) | 84.6 (78.5, 98.7) | 86.4 (71.2, 99.6) | 88.2 (81.9, 93.5) | 87.3 (82.6, 95.4) | 0.879 |
| Calcium (mg/dl) | 9.4 (9.1, 9.6) | 9.6 (9.2, 9.8) | 9.4 (9.2, 9.6) | 9.3 (9.2, 9.7) | 0.467 |
| Phosphorus (mg/dl) | 3.4 (3.3, 3.5) | 3.5 (3.3, 3.7) | 3.2 (3.0, 3.5) | 3.2 (2.8, 3.4) | 0.128 |
| Bone resorption markers | |||||
| Serum CTX (ng/ml) | 0.53 (0.36, 0.67) | 0.45 (0.38, 0.73) | 0.51 (0.30, 0.60) | 0.50 (0.33, 0.65) | 0.940 |
| Serum TRACP5b (U/liter) | 3.0 (2.6, 3.8) | 2.6 (2.1, 3.3) | 2.8 (2.5, 3.8) | 2.9 (2.6, 3.0) | 0.502 |
| Urine NTX (nmol/d)* | 268 (171, 333) | 290 (242, 329) | 274 (224, 357) | 272 (250, 410) | 0.644 |
| Bone formation marker | |||||
| PINP (μg/liter) | 36 (30, 50) | 40 (32, 51) | 39 (31, 47) | 36 (27, 53) | 0.880 |
p value is for comparison across groups by ANOVA. Data are median (IQR).
* Log2 was used.
Serum sex steroid and FSH levels at the completion of the interventions
To document that the subjects in the respective groups had the expected changes in serum sex steroid levels, we measured T and E2 levels at the end of the 3-wk interventions in the four groups. We also measured serum FSH levels at this visit, because we expected that the GnRH agonist would result in suppression of these levels. As shown in Table 3, serum sex steroid levels were as would be predicted by the specific intervention. In addition, as anticipated, serum FSH levels were suppressed in all four groups into the low-normal range for FSH levels in men (normal range for men, 1–18 mIU/liter). These FSH levels were all significantly lower (p < 0.001) compared with the respective FSH levels in each of the groups before receiving the first injection of GnRH [median (IQR)]: group A (−T, −E), 9.6 mIU/liter (3.5, 12.6 mIU/liter); group B (−T, +E), 9.4 mIU/liter (7.5, 26.3 mIU/liter); group C (+T, −E), 9.6 mIU/liter (5.7, 13.6 mIU/liter); group D (+T, +E), 6.6 mIU/liter (3.8, 10.5 mIU/liter).
Table 3.
Sex Steroid and FSH Levels in the Study Subjects at the Time of the Final Visit
| Group A (−T, −E) | Group B (−T, +E) | Group C (+T, −E) | Group D (+T, +E) | |
| T (ng/dl) | 15.3 (11.7, 28.4) | 19.4 (11.0, 24.8) | 379.6 (268.2, 478.5) | 369.5 (217.8, 450.5) |
| E2 (pg/ml) | <5 | 34.5 (19.0, 48.5) | <5 | 23.5 (15.0, 30.0) |
| FSH (mIU/liter) | 3.4 (1.9, 4.4) | 1.2 (0.8, 2.5) | 4.0 (3.2, 6.0) | 1.2 (1.1, 2.7) |
Data are median (IQR).
Changes in bone turnover markers
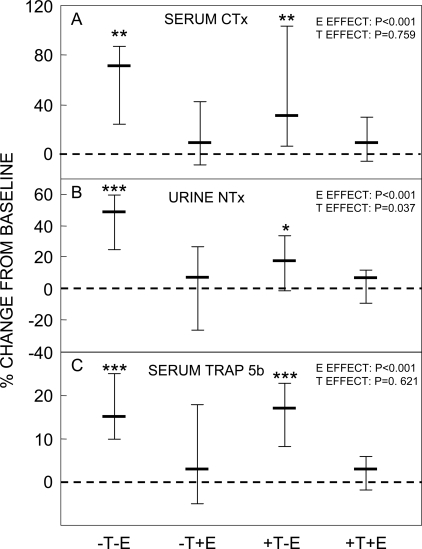
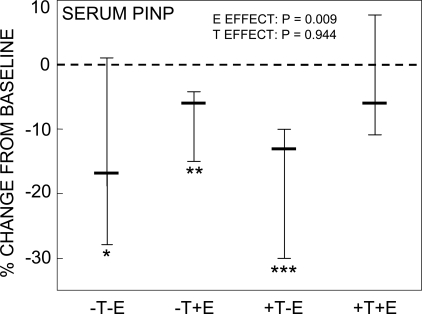
Figure 2 shows the percent change from baseline in the bone resorption markers in the four study groups. Entirely consistent with our previous study,(2) we found highly significant effects of E on preventing increases in all three resorption markers assessed. For the collagen degradation markers, serum CTX and urine NTX, which may reflect a combination of osteoclast numbers and activity,(19) E seemed to be at least twice as effective as T, although the T effect was significant only for urine NTX (Figs. 2A and 2B). In contrast, T seemed to have no effect on changes in a marker principally reflecting osteoclast numbers,(19) serum TRACP5b (Fig. 2C). Of note, changes in bone resorption markers, particularly the highly significant increase in all three resorption markers in the group with complete sex steroid deficiency (group A), occurred despite suppressed FSH levels in the subjects in all four groups (Table 3). Also consistent with our previous study,(2) we found a highly significant E effect on preventing decreases in serum PINP levels, with no demonstrable T effect (Fig. 3).
FIG. 2.
Percent change from baseline in (A) serum CTX, (B) urine NTX, and (C) serum TRACP5b levels in the four study groups between the baseline and final visits. *p < 0.05, **p < 0.01, and ***p < 0.001 for change from baseline. The overall effect of E and T on the bone markers was analyzed using the two-factor ANOVA model described in the Materials and Methods section. Shown are the medians and interquartile ranges.
FIG. 3.
Percent change from baseline in serum PINP levels. **p < 0.01, and ***p < 0.001 for change from baseline. The overall effect of E and T on serum PINP levels was analyzed using the two-factor ANOVA model described in the Materials and Methods section. Shown are the medians and interquartile ranges.
RANKL mRNA and protein levels
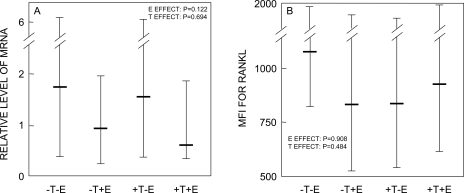
Using MACS, we isolated ALP+ cells from the bone marrow samples. We have previously shown that these cells express bone-related genes (ALP, osteocalcin, collagen I) and can form mineralized nodules in vitro.(9) Because we wanted to be certain we had sufficient numbers of cells for the mRNA analyses, we did not perform a further round of purification by fluorescent activated cell sorting (FACS) after MACS. Hence, a caveat to these analysis is that the purity of the MACS-sorted ALP+ cells (assessed using flow cytometry from aliquots of the ALP+ and ALP− cell populations) in each of the groups was considerably <100% [median (IQR)]: group A (−T, −E), 39.3% (34.0%, 45.3%); group B (−T, +E), 46.2% (40.1%, 53.1%); group C (+T, −E), 42.5% (31.2%, 57.3%); group D (+T, +E), 44.5% (38.7%, 48.3%). Figure 4A shows the normalized (to the control group, group D) RANKL mRNA levels in each of the groups. As is evident, RANKL mRNA levels followed the general pattern seen with the bone resorption markers (Fig. 2), with group A (−T, −E) having the greatest increase in the resorption markers and the highest expression of RANKL mRNA and protein, and group D (+T, +E) having the lowest levels of both the resorption markers and RANKL mRNA levels in ALP+ cells. However, because of the variance of the RANKL mRNA analyses, the two-factor ANOVA model was not significant for either E or T effects on RANKL mRNA levels (p = 0.122 and 0.694, respectively). Similarly, Fig. 4B shows the RANKL MFI (an index of RANKL protein levels per cell(9)) on ALP+ cells, and whereas the subjects in group A (−T, −E) did have the highest MFI for RANKL, the ANOVA model was not significant for E or T effects. In all subjects combined, RANKL mRNA levels did correlate weakly with MFI for RANKL on ALP+ cells (R = 0.26, p = 0.094). Using similar methods, we also assessed RANKL mRNA levels in CD3+ cells (T cells) and RANKL protein concentrations on CD3+/RANKL+ cells and on CD19+/RANKL+ cells (B cells co-expressing RANKL; Table 4), but could not detect any significant E or T effects on these parameters.
FIG. 4.
(A) Normalized (to the control group, group D) RANKL mRNA levels in MACS-sorted ALP+ cells and (B) MFI for RANKL Ab in ALP+/RANKL+ cells analyzed by flow cytometry in the four groups. The overall effect of E and T was analyzed using the two-factor ANOVA model described in the Materials and Methods section. Shown are the medians and interquartile ranges.
Table 4.
Normalized RANKL mRNA Levels in MACS-Sorted CD3+ Cells (T Cells) and MFI for RANKL in CD3+/RANKL+ and CD19+/RANKL+ Cells
| Group A (−T, −E) | Group B (−T, +E) | Group C (+T, −E) | Group D (+T, +E) | E effect | T effect | |
| RANKL mRNA expression | ||||||
| CD3+ cells* | 0.51 (0.24, 1.25) | 1.18 (0.39, 1.50) | 0.41 (0.28, 1.02) | 0.59 (0.34, 1.90) | 0.134 | 0.393 |
| RANKL MFI | ||||||
| CD3+/RANKL+ cells | 940 (599, 1268) | 1009 (614, 1676) | 712 (303, 1062) | 884 (556, 1532) | 0.233 | 0.343 |
| CD19+/RANKL+ cells* | 291 (204, 729) | 303 (186, 752) | 285 (209, 421) | 493 (209, 635) | 0.558 | 0.740 |
Last two columns indicate the p values for E and T effects using the two-factor ANOVA model described in the Materials and Methods section. Data are median (IQR).
* Log2 was used in models.
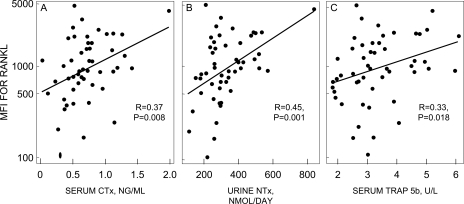
Even though we could not show effects of E or T on MFI for RANKL on ALP+, CD3+, or CD19+ cells, the bone resorption markers at the final visit in all of the subjects combined did correlate with the MFI for RANKL on ALP+ cells (Fig. 5) but not on CD3+ or CD19+ cells (data not shown).
FIG. 5.
Correlation of MFI for RANKL in ALP+/RANKL+ cells with (A) serum CTX, (B) urine NTX, and (C) serum TRACP5b. Note the log2 scale for RANKL MFI. Note that in A and B, the correlations were not driven by the apparent extreme point in each panel, because the correlations were virtually identical using Spearman correlations (i.e., the correlation computed from ranks instead of actual values, data not shown). In addition, the Pearson correlations were still significant after removal of the apparent extreme points (for CTX, R = 0.29, p = 0.047; for NTX, R = 0.38, p = 0.007).
Expression of other relevant genes
Additional gene analyses were performed in MACS-sorted ALP+ (osteoblastic), CD3+ cells (T cells), and CD14+ cells (monocytic cells). Specifically, we assessed expression of OPG, TRAIL, IFI30, and TNFαIP6 in the ALP+ cells, TNFα and IFNγ in CD3+ cells, and TNFα, IL-1α, IL-1β, IL-1ra, IFNγ, and TNFαIP6 in CD14+ cells. As shown in Table 5, we could not show any clear effects of E or T on any of these genes in the various cell populations, except for a borderline (p = 0.059) E effect on increasing TNFαIP6 mRNA levels in CD14+ cells. The only other (nonsignificant) pattern was higher TNFαIP6 mRNA levels in ALP+ cells in the –E groups (medians, 1.21 and 1.86 in the –T, −E and +T, −E groups, respectively) compared with the +E groups (medians, 0.94 and 0.98 in the –T, +E and +T, +E groups, respectively).
Table 5.
Normalized mRNA Levels in MACS-Sorted ALP+ (Osteoblastic) Cells, CD3+ Cells (T Cells), and CD19+ Cells (B Cells)
| Group A (−T, −E) | Group B (−T, +E) | Group C (+T, −E) | Group D (+T, +E) | E effect | T effect | |
| ALP+ cells | ||||||
| OPG* | 1.72 (0.38, 4.10) | 0.26 (0.17, 5.43) | 2.08 (0.15, 3.04) | 0.80 (0.46, 1.45) | 0.371 | 0.780 |
| TRAIL* | 0.73 (0.44, 1.39) | 0.64 (0.22, 1.07) | 0.45 (0.22, 0.94) | 0.93 (0.25, 1.22) | 0.475 | 0.643 |
| IF30* | 0.58 (0.40, 0.96) | 0.44 (0.24, 1.52) | 0.50 (0.26, 0.80) | 0.63 (0.29, 1.47) | 0.484 | 0.351 |
| TNFαP6* | 1.21 (0.92, 1.71) | 0.94 (0.74, 4.31) | 1.86 (0.62, 2.36) | 0.98 (0.63, 1.10) | 0.570 | 0.496 |
| CD3+ cells | ||||||
| TNFα | 0.87 (0.69, 1.37) | 1.12 (0.81, 1.25) | 0.87 (0.69, 1.34) | 1.02 (0.79, 1.14) | 0.721 | 0.932 |
| IFNγ * | 0.84 (0.38, 1.50) | 0.80 (0.39, 1.22) | 1.04 (0.67, 1.40) | 0.97 (0.62, 1.40) | 0.602 | 0.315 |
| CD14+ cells | ||||||
| TNFα * | 0.96 (0.64, 1.33) | 0.99 (0.86, 1.24) | 0.86 (0.80, 1.16) | 0.95 (0.84, 1.21) | 0.263 | 0.784 |
| IL-1α * | 1.13 (0.35, 1.53) | 0.88 (0.42, 1.60) | 0.67 (0.41, 1.17) | 0.84 (0.39, 1.39) | 0.641 | 0.415 |
| IL-1β * | 0.82 (0.50, 1.29) | 0.71 (0.50, 1.15) | 0.78 (0.63, 0.98) | 0.71 (0.40, 1.29) | 0.885 | 0.932 |
| IL-1ra* | 0.98 (0.65, 1.27) | 1.08 (0.73, 1.50) | 1.08 (0.72, 1.25) | 0.87 (0.70, 1.25) | 0.677 | 0.516 |
| IFNγ * | 0.84 (0.48, 2.41) | 0.63 (0.28, 1.12) | 0.80 (0.24, 1.45) | 0.79 (0.36, 1.38) | 0.267 | 0.361 |
| TNFαP6* | 0.65 (0.37, 1.23) | 1.37 (1.07, 3.04) | 0.73 (0.45, 1.29) | 0.82 (0.51, 1.59) | 0.059 | 0.285 |
Last two columns indicate the p values for E and T effects using the two-factor ANOVA model described in the Materials and Methods section. Data are median (IQR).
* Log2 was used in models.
Percentages of various cell populations in the study groups
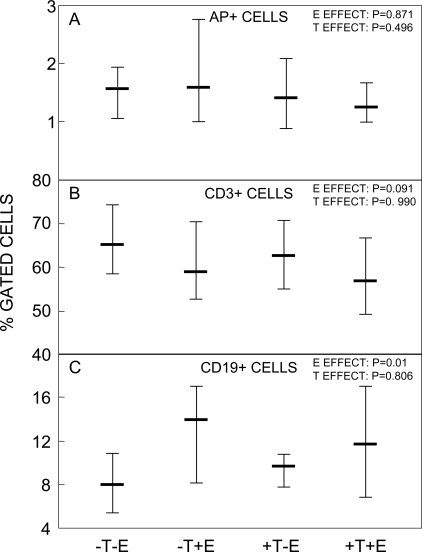
Finally, we also assessed whether any of our interventions altered the percentage of ALP+, CD3+, or CD19+ in bone marrow (Fig. 6). E or T did not affect the percentage of ALP+ cells (Fig. 6A); however, there was a trend for E to reduce the percentage of CD3+ (T) cells (Fig. 6B), with a significant E effect on increasing the percentage of CD19+ (B) cells (Fig. 6C).
FIG. 6.
(A) ALP+, (B) CD3+, and (C) CD19+ cells as a percent of gated cells in the four groups. The overall effect of E and T was analyzed using the two-factor ANOVA model described in the Materials and Methods section. Shown are the medians and interquartile ranges.
DISCUSSION
In this study, we replicated almost entirely the findings of our previous, analogous study in terms of changes in bone turnover markers after selective E or T replacement in men,(2) with both studies showing dominant effects of E on bone resorption and formation markers. The findings of our two studies are somewhat different, particularly with regards to E versus T effects on bone formation markers, from the work of Leder et al.,(3) and likely have to do with the fact that the longer duration of the latter study (12 wk of sex steroid deficiency) led to compensatory changes in bone formation markers in that study because of the coupling of bone formation with bone resorption. We also showed unequivocally that, at least in humans, increases in bone resorption can occur with sex steroid deficiency despite a suppression of FSH levels. Thus, sex steroids must modulate bone resorption independent of FSH action. However, because our study design did not allow us to compare bone resorption markers in the setting of sex steroid deficiency with low versus high FSH levels, we cannot exclude the possibility that sex steroid deficiency with high FSH levels may result in higher levels of bone resorption as compared with sex steroid deficiency with low FSH levels (as was present in our study subjects).
We also found that, whereas T seemed to have some effect in preventing increases in serum CTX and urine NTX (although this was significant only for urine NTX), there seemed to be no effect at all of T for changes in serum TRACP5b activity. It has been suggested that CTX and NTX, which are collagen degradation products, might reflect both the number and activity of osteoclasts, whereas TRACP5b may more exclusively reflect osteoclast numbers.(19) If that is the case, our findings would suggest possible differential effects of E and T on these parameters, with E reducing both osteoclast numbers and activity, whereas T appearing to principally reduce osteoclast activity, without altering osteoclast numbers. However, further studies are needed to address this issue.
We had previously shown that chronically E-deficient women had increased RANKL protein (assessed by flow cytometry) on ALP+, T, and B cells.(9) Because the probe used in that study (fluorescence-labeled OPG-Fc) was no longer available, we had to use a RANKL antibody in this study, along with mRNA analyses in the sorted ALP+ and T cells (for logistic reasons, we could not also isolate B cells from the marrow samples). We found a similar pattern for RANKL mRNA levels on ALP+ cells as was present for the bone resorption markers, with the subjects with complete sex steroid deficiency having ∼3-fold higher RANKL mRNA levels in ALP+ cells compared with the subjects with sex steroid sufficiency; however, because of the variance of these measurements, the changes did not achieve statistical significance (p = 0.122 and 0.694 for E and T effects, respectively). Similarly, whereas we could not show E or T effects on RANKL protein levels (assessed by MFI), in all subjects combined, RANKL MFI per cell did correlate with the bone resorption markers, attesting to the biological validity of the RANKL MFI measurement. It is possible that the different RANKL probe or the longer duration of sex steroid deficiency in the women studied previously(9) allowed us to detect changes with greater precision and/or resulted in the larger changes in RANKL MFI we observed in our previous study.(9) In addition, our mRNA analyses were performed in relatively enriched, but not pure, ALP+ cells. Thus, further studies, perhaps using better methods (FACS) for isolation of pure ALP+ cells are needed to address whether the trends observed in this study for RANKL mRNA levels in ALP+ cells are, in fact, reproducible and ultimately achieve statistical significance. Nonetheless, the lack of any change in RANKL protein levels in the B cells or in RANKL mRNA and protein levels in the T cells suggests that changes in RANKL production by these cells may lag behind alterations in RANKL levels in ALP+ cells (i.e., ALP+ cells may represent the primary site of regulation of RANKL production, at least early in the course of sex steroid deficiency).
We did not find evidence for regulation of TNFα or IFNγ mRNA levels by bone marrow T cells or TNFα, IL-1α, IL-1β, IL-1ra, or IFNγ mRNA levels by bone marrow monocytes. In contrast to the larger variance of the RANKL mRNA measurements, these mRNA measures were relatively robust; indeed, our posthoc sample size estimates indicate that we should have had 90% power to detect E or T effects of ∼30% and 60% on TNFα or IFNγ mRNA levels in T cells, respectively, and ∼20% effects on TNFα, ∼40% on IL-1ra, and ∼100% (i.e., a doubling) on IL-1α or IL-1β mRNA levels in monocytes. Thus, in contrast to studies in rodents,(8) our data do not support an important role for sex steroid regulation of these cytokines in mediating increases in bone resorption, at least over the time course of sex steroid deficiency in this experimental model. However, our findings do not exclude the possibility that direct effects of E deficiency on osteoclast lineage cells(20,21) are primarily responsible for the increase in bone resorption observed after 3 wk of sex steroid deficiency. Indeed, as recently shown by Nakamura et al.,(22) the early effects of estrogen deficiency on bone resorption seem to be caused by a decrease in osteoclast apoptosis as a result of direct effects of estrogen withdrawal on osteoclasts, and future studies to address this issue in humans need to be performed.
We did, however, find a trend for E to reduce the concentration of T cells in bone marrow, consistent with the hypothesis that E deficiency may lead to an expansion of T cells and an overall increase in bone marrow TNFα levels, without an actual increase in TNFα mRNA levels per cell.(11) Moreover, we also found some increases (albeit not statistically significant) in the mRNA for the TNFα responsive gene, TNFαIP6, in ALP+ cells in the subjects that were E deficient. In recent studies, we have shown that the TNFα blocker, etanercept, can prevent increases in bone resorption in the setting of short-term (3 wk) E deficiency in postmenopausal women.(23) Combined with the findings of this study, a plausible hypothesis is that, at least early in the course of E deficiency, there may well be an expansion of T cells in bone marrow in humans, leading to increased TNFα action in osteoblastic cells, with a subsequent induction of RANKL production. In contrast, whereas studies in rodents have shown that estrogen deficiency is associated with an increase in B cells (which also produce RANKL) in bone marrow,(24) our data indicate that the converse is the case in human males, arguing against an important role for increased B lymphopoiesis in mediating effects of estrogen deficiency on bone in humans.
In summary, our findings showed that, in vivo in humans, E has greater suppressive effects on bone resorption than T and that increased bone resorption in the setting of sex steroid deficiency can occur independently of changes in FSH secretion. E effects on bone resorption may be mediated by regulation of RANKL production by osteoblastic cells, although further studies using more highly purified cell populations may reduce the variability of the mRNA measurements and allow for clearer definition of the mediators of sex steroid action in vivo.
ACKNOWLEDGMENTS
We thank Dr Matthew Drake for helpful comments and suggestions. This work was supported in part by NIH Grants P01 AG004875 and 1UL1RR024150.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Khosla S, Melton LJ, Riggs BL. Estrogen and the male skeleton. J Clin Endocrinol Metab. 2002;87:1443–1450. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 2.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–210. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 4.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone AZ, Sairam MR, Kumar TR, Bo W, Braun JJ, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Tiwari-Pandey R, Samadfam R, Yang Y, Miao D, Karaplis AC, Sairam MR, Goltzman D. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone (FSH) Endocrinology. 2007;148:2613–2621. doi: 10.1210/en.2006-1404. [DOI] [PubMed] [Google Scholar]
- 6.Zaidi M, Sun L, Kumar TR, Sairam MR, Blair HC. Both FSH and sex steroids influence bone mass (response) Cell. 2006;127:1080–1081. [Google Scholar]
- 7.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 8.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling: Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 9.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacifici R, Rifas L, McCracken R, Vered I, McMurtry C, Avioli LV, Peck WA. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci USA. 1989;86:2398–2402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimble RB, Vannice JL, Bloedow DC, Thompson RC, Hopfer W, Kung VT, Brownfield C, Pacifici R. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J Clin Invest. 1994;93:1959–1967. doi: 10.1172/JCI117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 14.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luster AD, Weinshank RL, Feinman R, Ravetch JV. Molecular and biochemical characterization of a novel gamma-interferon-inducible protein. J Biol Chem. 1988;263:12036–12043. [PubMed] [Google Scholar]
- 17.He H, Tan CK, Downey KM, So AG. A tumor necrosis factor alpha and interleukin 6-inducible protein that interacts with the small subunit of DNA polymerase delta and proliferating cell nuclear antigen. Proc Natl Acad Sci USA. 2001;98:11979–11984. doi: 10.1073/pnas.221452098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokolnicki L, Khosla S, Charkondian N. Effects of testosterone and estradiol on cutaneous vasodilation during local warming in older men. Am J Physiol Endocrinol Metab. 2007;293:E1426–E1429. doi: 10.1152/ajpendo.00535.2007. [DOI] [PubMed] [Google Scholar]
- 19.Henriksen K, Tanko LB, Qvist P, Delmas PD, Christiansen C, Karsdal MA. Assessment of osteoclast number and function: Application in the development of new and improved treatment modalities for bone diseases. Osteoporos Int. 2007;18:681–685. doi: 10.1007/s00198-006-0286-8. [DOI] [PubMed] [Google Scholar]
- 20.Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava S, Toraldo G, Weitzmann MN, Cenci S, Ross FP, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-kB ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276:8836–8840. doi: 10.1074/jbc.M010764200. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-a and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res. 2007;22:724–729. doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- 24.Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]