Abstract
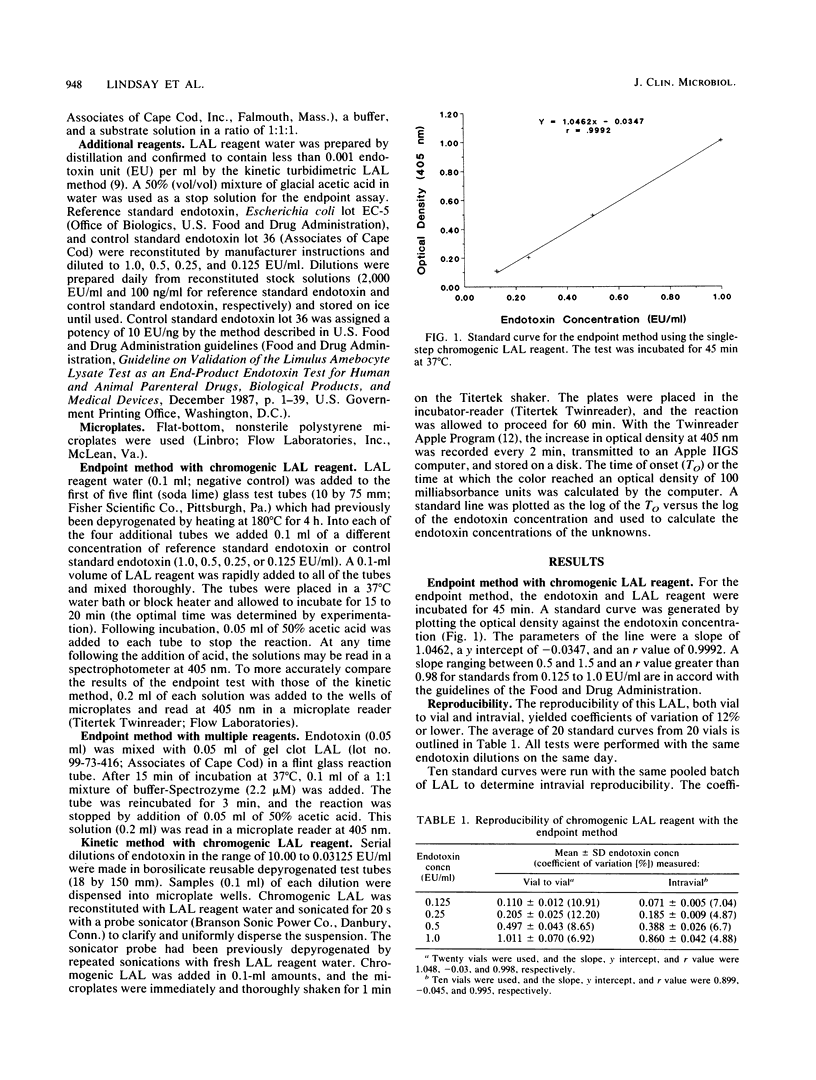
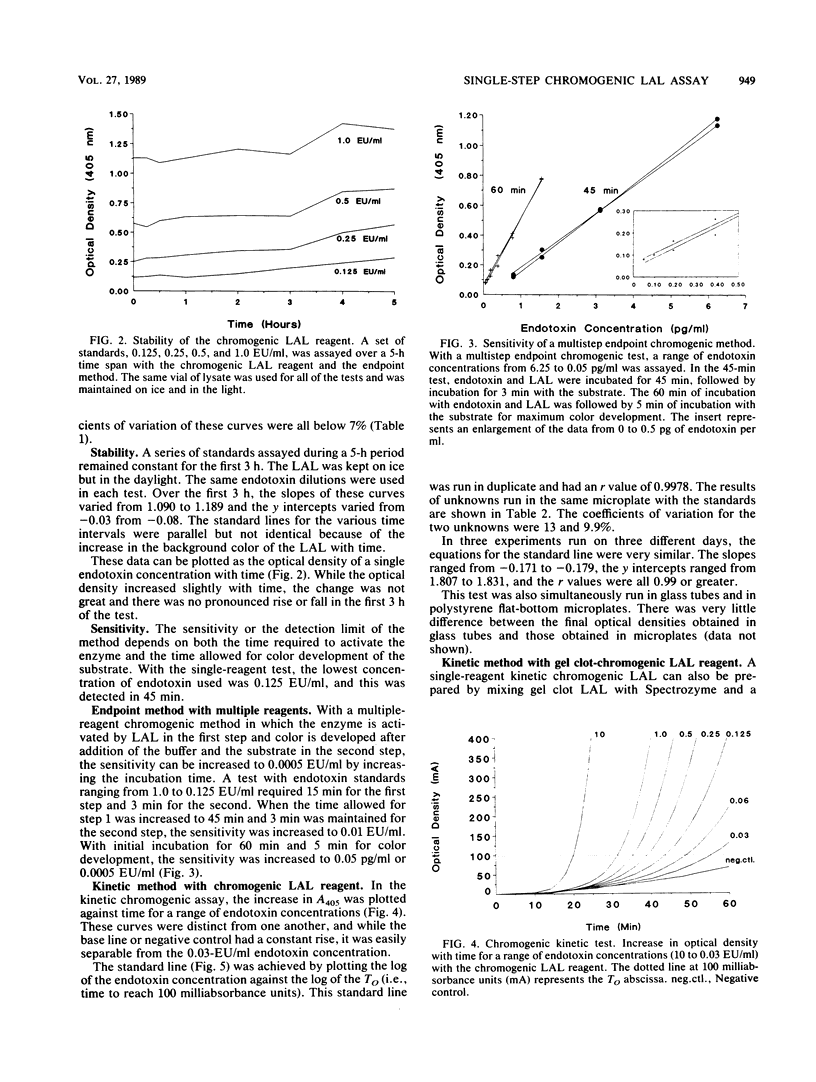
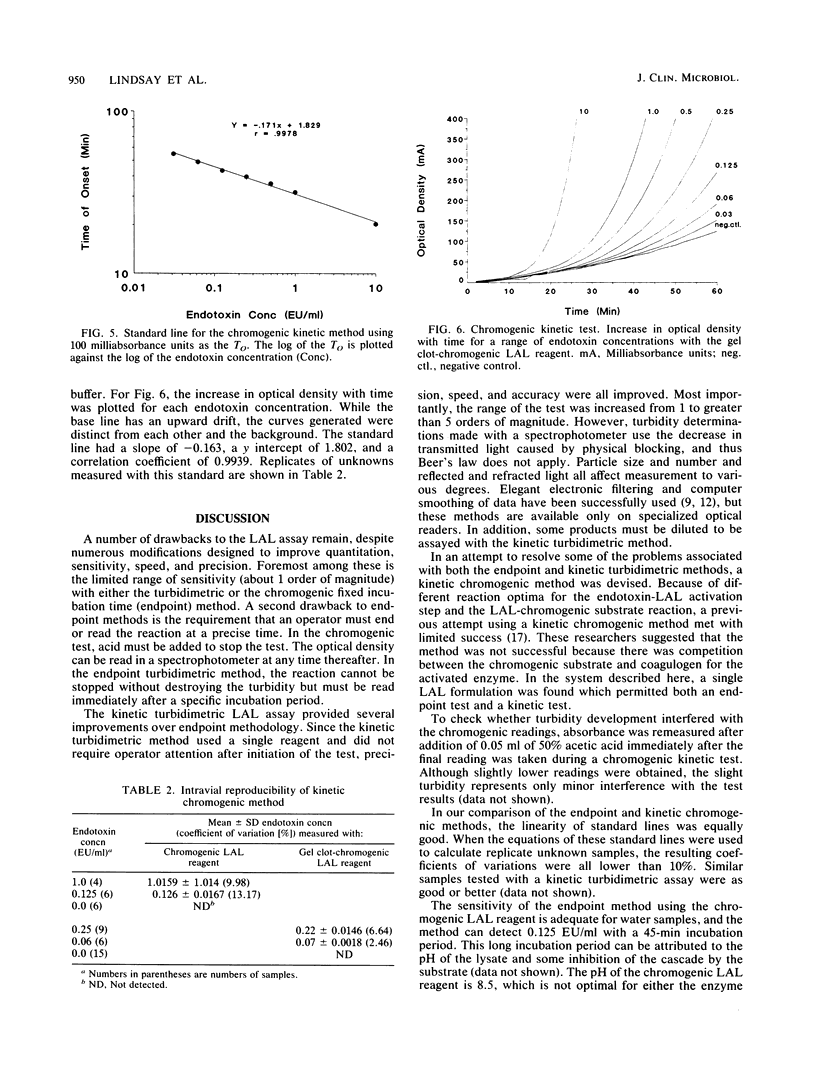
A new reagent for the chromogenic Limulus amebocyte lysate (LAL) assay is described. LAL was formulated for optimal performance in either an endpoint procedure or a kinetic procedure with the chromogenic substrate, buffer, and LAL components colyophilized as a single reagent. The kinetic chromogenic method required an incubating microplate reader coupled to a computer for collection and analysis of data. The kinetic method had a longer incubation time than the endpoint method and spanned a range of over 3 orders of magnitude compared with the 1-order-of-magnitude range of the endpoint assay. The kinetic method was less subject to operator error, since readings were continuous and automatic. The endpoint test was more operator intensive, requiring both addition of acetic acid to stop the reaction and transfer of the sample to the reading device. A single-step chromogenic reagent was also prepared without lyophilization by mixing reconstituted gel clot LAL with a buffer and a chromogenic substrate. The reagent prepared in this manner performed as well as the colyophilized agent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bussey D. M., Tsuji K. Optimization of a chromogenic Limulus amebocyte lysate (LAL) assay for automated endotoxin detection. J Parenter Sci Technol. 1984 Nov-Dec;38(6):228–233. [PubMed] [Google Scholar]
- Cohen J., McConnell J. S. Observations on the measurement and evaluation of endotoxemia by a quantitative limulus lysate microassay. J Infect Dis. 1984 Dec;150(6):916–924. doi: 10.1093/infdis/150.6.916. [DOI] [PubMed] [Google Scholar]
- Friberger P., Knös M., Mellstam L. A quantitative endotoxin assay utilizing LAL and a chromogenic substrate. Prog Clin Biol Res. 1982;93:195–206. [PubMed] [Google Scholar]
- Fujita Y., Nakahara C. Preparation and application of a new endotoxin determination kit, PYRODICK, using a chromogenic substrate. Prog Clin Biol Res. 1982;93:173–182. [PubMed] [Google Scholar]
- Iwanaga S., Morita T., Harada T., Nakamura S., Niwa M., Takada K., Kimura T., Sakakibara S. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7(2-3):183–188. doi: 10.1159/000214260. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Alexander G. A. Automation of the Limulus amoebocyte lysate test by using the Abbott MS-2 microbiology system. Appl Environ Microbiol. 1981 Jun;41(6):1316–1320. doi: 10.1128/aem.41.6.1316-1320.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- Nakamura S., Morita T., Iwanaga S., Niwa M., Takahashi K. A sensitive substrate for the clotting enzyme in horseshoe crab hemocytes. J Biochem. 1977 May;81(5):1567–1569. [PubMed] [Google Scholar]
- Novitsky T. J., Remillard J. F., Loy N. Design criteria and evaluation of the LAL-4000 for kinetic turbidimetric LAL assay. Prog Clin Biol Res. 1987;231:189–196. [PubMed] [Google Scholar]
- Obayashi T., Tamura H., Tanaka S., Ohki M., Takahashi S., Arai M., Masuda M., Kawai T. A new chromogenic endotoxin-specific assay using recombined limulus coagulation enzymes and its clinical applications. Clin Chim Acta. 1985 Jun 30;149(1):55–65. doi: 10.1016/0009-8981(85)90273-6. [DOI] [PubMed] [Google Scholar]
- Piotrowicz B. I., Edlin S. E., McCartney A. C. A sensitive chromogenic Limulus amoebocyte lysate micro-assay for detection of endotoxin in human plasma and in water. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Aug;260(1):108–112. doi: 10.1016/s0176-6724(85)80105-x. [DOI] [PubMed] [Google Scholar]
- Remillard J. F., Gould M. C., Roslansky P. F., Novitsky T. J. Quantitation of endotoxin in products using the LAL kinetic turbidimetric assay. Prog Clin Biol Res. 1987;231:197–210. [PubMed] [Google Scholar]
- Scully M. F., Newman Y. M., Clark S. E., Kakkar V. V. Evaluation of a chromogenic method for endotoxin measurement. Thromb Res. 1980 Oct 15;20(2):263–270. doi: 10.1016/0049-3848(80)90391-6. [DOI] [PubMed] [Google Scholar]
- Sullivan J. D., Jr, Watson S. W. Factors affecting the sensitivity of Limulus lysate. Appl Microbiol. 1974 Dec;28(6):1023–1026. doi: 10.1128/am.28.6.1023-1026.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller J. D., Kelly K. M. A turbidimetric Limulus amebocyte assay for the quantitative determination of Gram negative bacterial endotoxin. Prog Clin Biol Res. 1979;29:423–433. [PubMed] [Google Scholar]
- Tsuji K., Martin P. A., Bussey D. M. Automation of chromogenic substrate Limulus amebocyte lysate assay method for endotoxin by robotic system. Appl Environ Microbiol. 1984 Sep;48(3):550–555. doi: 10.1128/aem.48.3.550-555.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaschek B., Ditter B., Becker K. P., Urbaschek R. Protective effects and role of endotoxin in experimental septicemia. Circ Shock. 1984;14(4):209–222. [PubMed] [Google Scholar]


