Abstract
Glycosphingolipids (GSLs) are amphipathic lipids ubiquitously expressed in all vertebrate cells and body fluids, but they are especially abundant in the nervous system. The synthesis of GSLs generally is initiated in the endoplasmic reticulum and completed in the Golgi apparatus, followed by transportation to the plasma membrane surface as an integral component. The amount and expression patterns of GSLs change drastically in brains during the embryonic to postnatal stages. Recent studies have revealed that GSLs are highly localized in cell surface microdomains and function as important components that mediate signal transduction and cell adhesion. Also in developing brains, GSLs are suggested to play important roles in nervous system formation. Disturbance of GSL expression and metabolism affects brain function, resulting in a variety of diseases, particularly lysosomal storage diseases. In this review, we describe some aspects of the roles of GSLs, especially of gangliosides, in brain development.
Keywords: ganglioside, glycogene, brain function
Glycosphingolipids (GSLs) are amphipathic molecules composed of a hydrophilic carbohydrate chain and a hydrophobic ceramide moiety that contains a sphingosine and a FA residue (1). GSLs containing one or more sialic acid residues in the carbohydrate chain are referred to as gangliosides. Based on the sequences of the core carbohydrate residues, GSLs are classified into a number of series, including gala-, ganglio-, isoganglio-, lacto-, neolacto-, lactoganglio-, globo-, isoglobo-, and muco-series. Structural diversity in their carbohydrate chains is a hallmark of GSLs. At present, 172 neutral GSLs, 24 sulfated GSLs, and 188 gangliosides with variations in the carbohydrate chain have been reported in a variety of vertebrate tissues and organs (2). This complexity is increased manifold when heterogeneity in the lipophilic components is taken into consideration.
GSLs, especially gangliosides, are ubiquitously expressed in vertebrate cells and body fluids but are more abundant in the nervous system. In cells, GSLs are localized primarily, although not exclusively, on the plasma membrane, and they are crucial in determining the properties and biological functions of the cell. Drastic, consistent changes in ganglioside expression are observed during neurodevelopment, particularly during the early stages. Many studies have shown that the qualitative and quantitative changes in ganglioside expression in the nervous system correlate with certain cellular events during development. Because of their spatio-temporal expression patterns, GSLs are considered to be useful stage-specific marker molecules of certain lineages of cells, such as neural cells emanating from neural stem cells (3). Also, mounting evidence supports the notion that GSLs, including gangliosides, serve regulatory roles in the developing nervous system. In this review, we focus on the metabolism and functional roles of GSLs, especially of gangliosides, in the developing brain.
SYNTHESIS AND LOCALIZATION OF GSLS IN BRAIN DEVELOPMENT
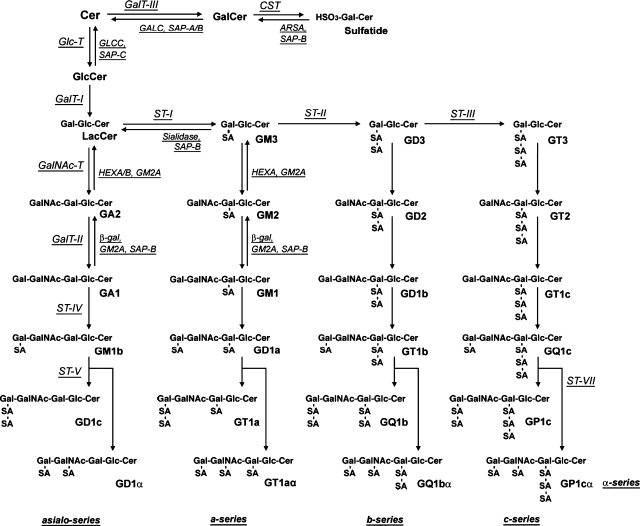
GSLs are primarily synthesized in the endoplasmic reticulum and matured in the Golgi apparatus by sequential addition of a single carbohydrate moiety to an existing acceptor lipid substrate (4). The structures and metabolic pathways of GSLs are shown in Fig. 1.
Fig. 1.
Structures and metabolic pathway of gangliosides (9). The nomenclature for gangliosides and related GSLs is based on that of Svennerholm (49). ARSA, arylsulfatase A; β-gal, lysosomal acid β-galatosidase; CST, cerebroside sulfotransferase; GALC, galactosylceramidase; GalCer, galactosylceramide; GalNAcT, GA2/GM2/GD2/GT2 synthase; GalT-I, LacCer synthase; GalT-II, GA1/GM1/GD1b/GT1c synthase; GalT-III, GalCer synthase; GlcT, GlcCer synthase; GM2A, GM2 activator protein; HEX, β-N-acetylhexosaminidase; SAP, saposin; ST-I, GM3 synthase; ST-II, GD3 synthase; ST-III, GT3 synthase; ST-IV, GM1b/GD1a/GT1b/GQ1c synthase; ST-V, GD1c/GT1a/GQ1b/GT3 synthase; ST-VII, GD1a/GT1aα/GQ1bα/GP1cα synthase.
As the first step of GSL synthesis, ceramide is synthesized in the membranes of the endoplasmic reticulum and transferred to the Golgi apparatus, accompanied by a ceramide transport protein, CERT (5). Ceramide is then converted to glucosylceramide (GlcCer) by the action of glucosylceramide synthase. Most of the complex GSLs in vertebrates are derived from GlcCer. Some GSLs, however, are derived from galactosylceramide (GalCer). Complex GSLs are synthesized by the stepwise addition of carbohydrate molecules (e.g. galactose, N-acetylgalactosamine, and sialic acid) to these simple precursors in the Golgi apparatus. Matured GSLs are transferred via vesicular transport to the cell surface where they become components of the cell membrane. In the plasma membrane, GSLs are additionally affected by catabolic enzymes (6). The constitutive degradation of GSLs occurs primarily in the endosomes and lysosomes. The regulatory mechanisms for GSL metabolism have recently been reviewed (4, 7).
As shown in Fig. 1, complex gangliosides are synthesized sequentially from simple to complex gangliosides in different pathways. The first ganglioside, GM3, is produced from lactosylceramide (4, 7, 8) by CMP-sialic acid:LacCer α2-3 sialyltransferase (ST-I), which adds an α2, 3-linked sialic acid to lactosylceramide. Further addition of sialic acids by cytidine-5′-monophosphate (CMP)-sialic acid: GM3 α2-8 sialyltransferase (ST-II) and CMP-sialic acid: GD3 α2-8 sialyltransferase generates GD3 and GT3, respectively. These simple gangliosides constitute the complex gangliosides belonging to the a-, b-, and c-series, respectively. Elaboration of the ganglioside structures is achieved by other specific glycosyltransferases, such as UDP-GalNAc: LacCer/GM3/GD3/GT3 β1-4 N-acetylgalactosaminyltransferase (GalNAcT), UDP-Gal: GA2/GM2/GD2/GT2 β1-3 galactosyltransferase, CMP-sialic acid: GA1/GM1/GD1b/GT1c α2-3 sialyltransferase, and CMP-sialic acid: GM1b/GD1a/GT1b/GD3 α2-8 sialyltransferase (4, 7).
There is a significant difference in expression levels and patterns of gangliosides in developing brains. For instance, the amount of total gangliosides increases almost 8-fold in adult mouse brains as compared with embryonic mouse brains (9) (Fig. 2A). Simultaneously, the expression pattern of gangliosides shifts from simple gangliosides, such as GM3 and GD3, to complex gangliosides, such as GM1, GD1a, GD1b, and GT1b (Fig. 2B). Similarly, in the human brain, the amount of ganglioside increases approximately 3-fold from the gestational weeks to the infant period. In particular, GM1 and GD1a are increased 12- to 15-fold during the same period (10). In addition to elaboration of the carbohydrate chains, the ceramide components undergo considerably different expression patterns during development (11).
Fig. 2.
Expression of gangliosides and glycosyltransferases in developing mouse brains (9). Dose (A), molecular species (B) of gangliosides, and expression levels of glycosyltransferases (C) change drastically during development.
The shift of ganglioside patterns from simple to complex gangliosides is regulated primarily by the differential expression of glycogenes, namely glycosyltransferases. Glycosidases, on the other hand, have very little influence at the early stages of brain development. During development, the activity of ST-II decreases, while that of GalNAcT increases (12). The expression level of ST-II, however, does not exhibit a concomitant decrease during development, but the expression of GalNAcT is significantly increased (9, 13, 14) (Fig. 2C). Interestingly, the increase of GalNAcT expression also occurs in primary neural precursor cells during differentiation (9). These results suggest that a shift of ganglioside expression during development is regulated by changes of the expression level and activity of these key glycosyltransferases. Not only gangliosides but also other GSLs, such as GalCer and sulfatide, present drastic pattern shifts during development. The expression of GalCer and sulfatide increases drastically during oligodendrocyte differentiation and myelin formation. Concomitantly, GalCer synthase (GalT-III) and sulfatide synthase genes are upregulated during oligodendrocyte differentiation and myelin formation (9) (Fig. 2C).
FUNCTIONAL ROLES OF GSLS IN DEVELOPING BRAINS
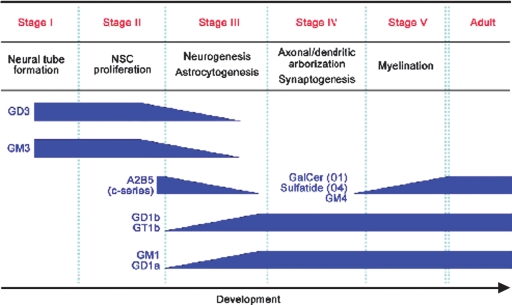
Numerous reports have been published on the relationship between GSL expression and cellular events in the development of the vertebrate nervous system, including that of humans (10, 12, 15–17) (Fig. 3). Available evidence indicates that GSLs have specific functional roles essential for survival, proliferation, and differentiation during brain development. Mouse embryos deficient in GlcCer synthase and lacking GlcCer and all GlcCer-based GSLs are able to differentiate into endoderm, mesoderm, and ectoderm but are unable to form more differentiated tissues because of embryonic lethality, probably caused by massive apoptosis in the ectodermal layer (18). Conditional knockout mice lacking GlcCer synthase in neural cells show structural and functional dysfunction in the cerebellum and peripheral nerves and die 3 weeks after birth, which indicates that GSLs are essential for brain maturation (19).
Fig. 3.
Neurodevelopmental milestones and concurrent changes in GSL expression.
Recently, the developmental role of each ganglioside has been analyzed using glycosyltransferase knockout mice (20–24). ST-II-knockout mice lacking expression of all b-series gangliosides reveal apparently normal nerve phenotypes except the delay of hypoglossal nerve regeneration after experimental lesioning (20, 21). GD3 is a predominant ganglioside in embryonic brains and in primary neural precursor cells (9, 25), but the neural precursor cells prepared from ST-II-knockout mice exhibit little or no phenotype (26). These data strongly suggest that the functions of b-series gangliosides may be compensated for by the remaining GSLs, such as a-series gangliosides. On the other hand, GalNAcT-knockout mice expressing only simple gangliosides, GM3 and GD3, display decreased myelination and axonal degeneration in the central and peripheral nervous systems, demyelination in the peripheral nervous system, and a reduction in neural conduction velocity from the tibial nerve to the somatosensory cortex (23, 27). In addition, it has been reported that maintenance of calcium homeostasis, which is related to apoptosis, is one of the functions of complex gangliosides during neuronal development (28). Moreover, ST-II-/GalNAcT-double knockout mice expressing only GM3 die suddenly in response to lethal sound-induced seizures (20). In ST-I-/GalNAcT-double knockout mice lacking all gangliosides, vacuolar pathology in the white matter regions in the central nervous system with axonal degeneration and perturbed axon-glia interaction have been observed. Embryonic development and morphogenesis in the brain, on the other hand, are not affected (24). In humans, a nonsense mutation of ST-I and depletion of all complex gangliosides other than α-series gangliosides cause autosomal recessive infantile-onset symptomatic epilepsy syndrome (29). These studies suggest that GSLs may have a role in modulating membrane properties, such as signaling, electrical conduction, maintenance, and stability (30).
Genetically engineered mice lacking other GSLs also have been established. GalCer synthase-knockout mice lack the expression of GalCer and GalCer-based GSLs such as sulfatide, which are well-known marker GSLs of oligodendrocytes (31, 32). Phenotypically, the mice exhibit enhanced differentiation of oligodendrocytes for the formation of physiologically abnormal “pseudo-myelin.” In mice deficient in sulfatide synthase (cerebroside sulfotransferase), axons are well myelinated, but myelin vacuolation is observed (33). This finding suggests that GalCer and sulfatide may act as negative regulators of oligodendrocyte differentiation and as positive regulators of the long-term maintenance of myelin. Mice deficient in fucosyltransferase IX, which catalyzes the synthesis of stage-specific embryonic antigen-1 (SSEA-1; also known as Lewis x antigen), a marker carbohydrate of neural stem cells on GSLs and glycoproteins, have also been reported (34). These mice have no discernible structural differences in their brains but exhibit increased anxiety-like responses, suggesting a role for the SSEA-1 antigen in the emotional behavior of mice. In mice deficient in glucuronyltransferase that catalyzes human natural killer-1 (HNK-1) antigen synthesis, brain development is generally normal; reduced long-term potentiation and defective spatial memory formation, however, have been observed (35).
The functional roles of GSLs, including gangliosides, in the developing nervous system have been analyzed at the cellular level. Gangliosides are expressed in much greater amounts in isolated neurons and in the neuropil teased from areas immediately around the neuron cell body and the dendrites than they are in isolated glial cells (36). This finding suggests that gangliosides are enriched in terminal axons and synaptic endings and that they may be involved in the maintenance, formation, and development of the nervous system. Several studies have demonstrated that exogenously applied gangliosides enhance the effect of neurotrophic factors in cultured neuronal cell lines (37, 38). It also has been demonstrated that there is a significant increase in the synthesis of complex gangliosides during axonogenesis in cultured hippocampal neurons (39). Depletion of sphingolipids and GSLs with Fumonisin B1 (a ceramide synthase inhibitor) and d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) (a GlcCer synthase inhibitor), respectively, affects the axonal outgrowth in cultured hippocampal neurons (40). GSL depletion with PDMP repress the proliferation of mouse embryonic neural precursor cells via suppression of the Ras-mitogen-activated protein kinase (Ras-MARP) pathway (41). In neural precursor cells, Ras-MAPK pathway activation is dependent on GSL-enriched microdomains, and molecules mediating the signaling in this pathway are localized in the microdomains (41). Therefore, GSLs may have functional roles in microdomains. In addition, gangliosides are proposed to maintain cellular survival and differentiation by modulating ceramide-induced apoptosis in the developing brain (42). All of these studies reinforce the notion that GSLs, including gangliosides, are functionally important in cell-cell interaction and/or in signal transduction during formation of the nervous system at various developmental stages.
DISEASES RELATED TO GSLS
So far, it has been revealed that GSLs are important molecules not only in physiological but also in pathological conditions. For instance, some GSLs are potent antigens that may underlie certain autoimmune neurodegenerative diseases, such as Guillain-Barré syndrome and related neuropathies (43). Alzheimer's disease may also arise as a result of the aggregation of amyloid-β-proteins initiated by GM1 (44). Human autosomal recessive infantile-onset symptomatic epilepsy syndrome is caused by a nonsense mutation of ST-I (29). Research has shown that Huntington's disease may involve disruption of GSL/ganglioside metabolic pathways, and metabolic imbalances of GSLs may be involved in the development of this disease (45).
A group of inherited disorders referred to as lysosomal storage diseases also are caused by defects of GSL catabolism (46). GSLs are degraded by glycosidases in cooperation with certain cofactors in lysosomes. Defects in the lysosomal glycosidases or in the cofactors result in accumulation of undegraded GSL substrates in the lysosomes, and those defects cause the disorders. Among the known typical lysosomal storage diseases associated with GSL accumulation and neuropathological symptoms are gangliosidosis (including Tay-Sachs disease and Sandhoff disease), Fabry disease, metachromatic leukodystrophy, Gaucher disease, and Krabbe disease. These disorders clearly demonstrate the importance of the precise amount and appropriate timing of the expression of GSLs in the development and maintenance of the nervous system. The molecular mechanisms underlying the onset of those disorders by GSL accumulation are complicated and not yet fully understood. Interestingly, neuronal cell death found in GM1 gangliosidosis has been proposed to be caused by an unfolded protein response activated by accumulated gangliosides (47).
CLOSING REMARK
Since the discovery in the 18th century of cerebroside by J. L. W. Thudichum, a pioneer of neurochemistry, a large body of knowledge in the biology and chemistry of GSLs has been accumulated (48). The progress made in the last 50 years is tremendous. With the advent of modern biochemistry, many GSLs, including gangliosides, have been characterized and their chemical structures identified. The biosynthetic and catabolic pathways of GSLs have been almost fully clarified. Recent molecular and cellular biological approaches have had remarkable success in cloning glycogenes (glycosyltransferases, glycosidases, and their cofactors) and in understanding the functional roles of GSLs. Our studies on the metabolism and the functional roles of GSLs, however, are still fragmentary. We have no clear idea how and why these diverse and complex molecules are expressed in a spatio-temporally accurate manner. Future studies over the next 50 years should be most rewarding for seeking a better understanding of the control mechanisms of glycogenes that regulate the specific expression of GSLs during development. Additionally, efforts should focus on the molecular basis and signaling pathways modulating their biological functions in both normal and pathologic brains.
Acknowledgments
We thank Diana Westbrook for her expert editorial assistance.
Abbreviations
GalCer, galactosylceramide
GalNAcT, LacCer/GM3/GD3/GT3 β1-4 N-acetylgalactosaminyltransferase
GalT-III, GalCer synthase
GlcCer, glucosylceramide
GSL, glycosphingolipid
SSEA-1, stage-specific embryonic antigen-1
ST-I, LacCer α2-3 sialyltransferase
ST-II, GM3 α2-8 sialyltransferase
This work was supported in part by grants from the National Institutes of Health (NS11853, AG027199, and NS26994) and a grant from the Children's Medical Research Foundation, Chicago, IL.
Published, JLR Papers in Press, October 14, 2008.
References
- 1.Hakomori S. 1990. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J. Biol. Chem. 265 18713–18716. [PubMed] [Google Scholar]
- 2.Yu, R. K., M. Yanagisawa, and T. Ariga. 2007. Glycosphingolipid structures. In Comprehensive Glycoscience. J. P. Kamerling, editor. Elsevier, Oxford. 73–122.
- 3.Yanagisawa M., and R. K. Yu. 2007. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 17 57R–74R. [DOI] [PubMed] [Google Scholar]
- 4.Yu, R. K., T. Ariga, M. Yanagisawa, and G. Zeng. 2008. Biosynthesis and degradation of gangliosides in the nervous system. In Glycoscience. B. O. Fraser-Reid, K. Tatsuka, and J. Thiem, editors. Springer Berlin-Heidelberg, Germany. 1671–1695.
- 5.Hanada K., K. Kumagai, S. Yasuda, Y. Miura, M. Kawano, M. Fukasawa, and M. Nishijima. 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 426 803–809. [DOI] [PubMed] [Google Scholar]
- 6.Miyagi T., T. Wada, K. Yamaguchi, K. Hata, and K. Shiozaki. 2008. Plasma membrane-associated sialidase as a crucial regulator of transmembrane signalling. J. Biochem. 144 279–285. [DOI] [PubMed] [Google Scholar]
- 7.Yu R. K., E. Bieberich, T. Xia, and G. Zeng. 2004. Regulation of ganglioside biosynthesis in the nervous system. J. Lipid Res. 45 783–793. [DOI] [PubMed] [Google Scholar]
- 8.Svennerholm L. 1994. Designation and schematic structure of gangliosides and allied glycosphingolipids. Prog. Brain Res. 101 XI–XIV. [DOI] [PubMed] [Google Scholar]
- 9.Ngamukote S., M. Yanagisawa, T. Ariga, S. Ando, and R. K. Yu. 2007. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem. 103 2327–2341. [DOI] [PubMed] [Google Scholar]
- 10.Svennerholm L., K. Bostrom, P. Fredman, J. E. Mansson, B. Rosengren, and B. M. Rynmark. 1989. Human brain gangliosides: developmental changes from early fetal stage to advanced age. Biochim. Biophys. Acta. 1005 109–117. [DOI] [PubMed] [Google Scholar]
- 11.Valsecchi M., P. Palestini, V. Chigorno, S. Sonnino, and G. Tettamanti. 1993. Changes in the ganglioside long-chain base composition of rat cerebellar granule cells during differentiation and aging in culture. J. Neurochem. 60 193–196. [DOI] [PubMed] [Google Scholar]
- 12.Yu R. K., L. J. Macala, T. Taki, H. M. Weinfield, and F. S. Yu. 1988. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J. Neurochem. 50 1825–1829. [DOI] [PubMed] [Google Scholar]
- 13.Ishii A., T. Ikeda, S. Hitoshi, I. Fujimoto, T. Torii, K. Sakuma, S. Nakakita, S. Hase, and K. Ikenaka. 2007. Developmental changes in the expression of glycogenes and the content of N-glycans in the mouse cerebral cortex. Glycobiology. 17 261–276. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto A., M. Haraguchi, S. Yamashiro, S. Fukumoto, K. Furukawa, K. Takamiya, M. Atsuta, H. Shiku, and K. Furukawa. 1996. Heterogeneity in the expression pattern of two ganglioside synthase genes during mouse brain development. J. Neurochem. 66 26–34. [DOI] [PubMed] [Google Scholar]
- 15.Irwin L. N., and C. C. Irwin. 1982. Development changes and regional variation in the ganglioside composition of the rat hippocampus. Brain Res. 256 481–485. [DOI] [PubMed] [Google Scholar]
- 16.Kotani M., T. Terashima, and T. Tai. 1995. Developmental changes of ganglioside expressions in postnatal rat cerebellar cortex. Brain Res. 700 40–58. [DOI] [PubMed] [Google Scholar]
- 17.Kracun I., H. Rosner, V. Drnovsek, Z. Vukelic, C. Cosovic, M. Trbojevic-Cepe, and M. Kubat. 1992. Gangliosides in the human brain development and aging. Neurochem. Int. 20 421–431. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita T., R. Wada, T. Sasaki, C. Deng, U. Bierfreund, K. Sandhoff, and R. L. Proia. 1999. A vital role for glycosphingolipid synthesis during development and differentiation. Proc. Natl. Acad. Sci. USA. 96 9142–9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennemann R., R. Sandhoff, S. Wang, E. Kiss, N. Gretz, C. Zuliani, A. Martin-Villalba, R. Jager, H. Schorle, M. Kenzelmann, et al. 2005. Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth. Proc. Natl. Acad. Sci. USA. 102 12459–12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai H., M. L. Allende, R. Wada, M. Kono, K. Sango, C. Deng, T. Miyakawa, J. N. Crawley, N. Werth, U. Bierfreund, et al. 2001. Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J. Biol. Chem. 276 6885–6888. [DOI] [PubMed] [Google Scholar]
- 21.Okada M., M. Itoh Mi, M. Haraguchi, T. Okajima, M. Inoue, H. Oishi, Y. Matsuda, T. Iwamoto, T. Kawano, S. Fukumoto, et al. 2002. b-series Ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem. 277 1633–1636. [DOI] [PubMed] [Google Scholar]
- 22.Proia R. L. 2004. Gangliosides help stabilize the brain. Nat. Genet. 36 1147–1148. [DOI] [PubMed] [Google Scholar]
- 23.Sheikh K. A., J. Sun, Y. Liu, H. Kawai, T. O. Crawford, R. L. Proia, J. W. Griffin, and R. L. Schnaar. 1999. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc. Natl. Acad. Sci. USA. 96 7532–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita T., Y. P. Wu, R. Sandhoff, N. Werth, H. Mizukami, J. M. Ellis, J. L. Dupree, R. Geyer, K. Sandhoff, and R. L. Proia. 2005. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc. Natl. Acad. Sci. USA. 102 2725–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagisawa M., K. Nakamura, and T. Taga. 2004. Roles of lipid rafts in integrin-dependent adhesion and gp130 signalling pathway in mouse embryonic neural precursor cells. Genes Cells. 9 801–809. [DOI] [PubMed] [Google Scholar]
- 26.Yu R. K., and M. Yanagisawa. 2007. Glycosignaling in neural stem cells: involvement of glycoconjugates in signal transduction modulating the neural stem cell fate. J. Neurochem. 103 (Suppl. 1): 39–46. [DOI] [PubMed] [Google Scholar]
- 27.Takamiya K., A. Yamamoto, K. Furukawa, S. Yamashiro, M. Shin, M. Okada, S. Fukumoto, M. Haraguchi, N. Takeda, K. Fujimura, et al. 1996. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. USA. 93 10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G., X. Xie, Z. H. Lu, and R. W. Ledeen. 2001. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc. Natl. Acad. Sci. USA. 98 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson M. A., H. Cross, C. Proukakis, D. A. Priestman, D. C. Neville, G. Reinkensmeier, H. Wang, M. Wiznitzer, K. Gurtz, A. Verganelaki, et al. 2004. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat. Genet. 36 1225–1229. [DOI] [PubMed] [Google Scholar]
- 30.Brigande J. V., and T. N. Seyfried. 1998. Glycosphingolipid biosynthesis may not be necessary for vertebrate brain development. Ann. N. Y. Acad. Sci. 845 215–218. [DOI] [PubMed] [Google Scholar]
- 31.Bosio A., E. Binczek, and W. Stoffel. 1996. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc. Natl. Acad. Sci. USA. 93 13280–13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coetzee T., J. L. Dupree, and B. Popko. 1998. Demyelination and altered expression of myelin-associated glycoprotein isoforms in the central nervous system of galactolipid-deficient mice. J. Neurosci. Res. 54 613–622. [DOI] [PubMed] [Google Scholar]
- 33.Honke K., Y. Hirahara, J. Dupree, K. Suzuki, B. Popko, K. Fukushima, J. Fukushima, T. Nagasawa, N. Yoshida, Y. Wada, et al. 2002. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc. Natl. Acad. Sci. USA. 99 4227–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudo T., T. Fujii, S. Ikegami, K. Inokuchi, Y. Takayama, Y. Ikehara, S. Nishihara, A. Togayachi, S. Takahashi, K. Tachibana, et al. 2007. Mice lacking alpha1,3-fucosyltransferase IX demonstrate disappearance of Lewis x structure in brain and increased anxiety-like behaviors. Glycobiology. 17 1–9. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S., S. Oka, M. Inoue, M. Shimuta, T. Manabe, H. Takahashi, M. Miyamoto, M. Asano, J. Sakagami, K. Sudo, et al. 2002. Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J. Biol. Chem. 277 27227–27231. [DOI] [PubMed] [Google Scholar]
- 36.Derry D. M., and L. S. Wolfe. 1967. Gangliosides in isolated neurons and glial cells. Science. 158 1450–1452. [DOI] [PubMed] [Google Scholar]
- 37.Cuello A. C., L. Garofalo, R. L. Kenigsberg, and D. Maysinger. 1989. Gangliosides potentiate in vivo and in vitro effects of nerve growth factor on central cholinergic neurons. Proc. Natl. Acad. Sci. USA. 86 2056–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrari G., A. Batistatou, and L. A. Greene. 1993. Gangliosides rescue neuronal cells from death after trophic factor deprivation. J. Neurosci. 13 1879–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirschberg K., R. Zisling, G. van Echten-Deckert, and A. H. Futerman. 1996. Ganglioside synthesis during the development of neuronal polarity. Major changes occur during axonogenesis and axon elongation, but not during dendrite growth or synaptogenesis. J. Biol. Chem. 271 14876–14882. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz A., E. Rapaport, K. Hirschberg, and A. H. Futerman. 1995. A regulatory role for sphingolipids in neuronal growth. Inhibition of sphingolipid synthesis and degradation have opposite effects on axonal branching. J. Biol. Chem. 270 10990–10998. [DOI] [PubMed] [Google Scholar]
- 41.Yanagisawa M., K. Nakamura, and T. Taga. 2005. Glycosphingolipid synthesis inhibitor represses cytokine-induced activation of the Ras-MAPK pathway in embryonic neural precursor cells. J. Biochem. 138 285–291. [DOI] [PubMed] [Google Scholar]
- 42.Bieberich E., S. MacKinnon, J. Silva, and R. K. Yu. 2001. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J. Biol. Chem. 276 44396–44404. [DOI] [PubMed] [Google Scholar]
- 43.Ariga T., T. Miyatake, and R. K. Yu. 2001. Recent studies on the roles of antiglycosphingolipids in the pathogenesis of neurological disorders. J. Neurosci. Res. 65 363–370. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi H., N. Kimura, H. Yamaguchi, K. Hasegawa, T. Yokoseki, M. Shibata, N. Yamamoto, M. Michikawa, Y. Yoshikawa, K. Terao, et al. 2004. A seed for Alzheimer amyloid in the brain. J. Neurosci. 24 4894–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desplats P. A., C. A. Denny, K. E. Kass, T. Gilmartin, S. R. Head, J. G. Sutcliffe, T. N. Seyfried, and E. A. Thomas. 2007. Glycolipid and ganglioside metabolism imbalances in Huntington's disease. Neurobiol. Dis. 27 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Futerman A. H., and G. van Meer. 2004. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5 554–565. [DOI] [PubMed] [Google Scholar]
- 47.Tessitore A., M. del P Martin, R. Sano, Y. Ma, L. Mann, A. Ingrassia, E. D. Laywell, D. A. Steindler, L. M. Hendershot, and A. d'Azzo. 2004. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol. Cell. 15 753–766. [DOI] [PubMed] [Google Scholar]
- 48.Yamakawa, T. 1987. History of ganglioside research. In Gangliosides and Modulation of Neuronal Functions (NATO ASI Series, Vol. H7). H. Rahmann, editor. Springer-Verlag, Berlin-Heidelberg, Germany. 3–15.
- 49.Svennerholm L. 1963. Chromatographic separation of human brain gangliosides. J. Neurochem. 10 613–623. [DOI] [PubMed] [Google Scholar]