Abstract
The isoprostanes (IsoPs) are a unique series of prostaglandin-like compounds formed in vivo via a nonenzymatic mechanism involving the free radical-initiated peroxidation of arachidonic acid. This article summarizes our current knowledge of these compounds. Herein, a historical account of their discovery and the mechanism of their formation are described. A specific class of IsoPs, the F2-IsoPs, are stable, robust molecules that can be measured as indices of endogenous oxidant stress. The utility of these molecules as biomarkers and methods by which these compounds can be quantified are discussed. In addition to the F2-IsoPs, isoprostanes with other prostane ring structures as well as oxidation products with furan and dioxolane rings can be generated from arachidonic acid. And, in more recent years, isoprostane-like compounds have been shown to be formed from polyunsaturated fatty acids including eicosapentaenoic acid [C20:5, ω-3], docosahexaenoic acid [C22:6, ω-3], and adrenic acid [C22:4, ω-6]. These findings will be summarized as well.
Keywords: oxidative stress, lipid peroxidation, prostaglandins
Free radicals derived primarily from molecular oxygen have been implicated in a variety of human conditions and disorders, including atherosclerosis and associated risk factors, which include smoking and obesity, ischemia/reperfusion injury, and neurodegenerative diseases such as Alzheimer's disease and Huntington's disease (1). Lipids are readily attacked by free radicals resulting in the formation of a number of peroxidation products. One class of oxidation products formed in abundance in vitro and in vivo is the isoprostanes (IsoPs), which were discovered by our laboratory in 1990. IsoPs are a series of prostaglandin (PG)-like compounds produced by the free radical-catalyzed peroxidation of arachidonic acid independent of the cyclooxygenase (2). Over the past 20 years, we and others (3) have carried out a large number of studies defining the basic chemistry and biochemistry involved in the formation and metabolism of the IsoPs. In addition, we have shown that levels of IsoPs are increased in a number of human diseases, and it is currently recognized that measurement of these molecules is the most accurate analytical method to assess oxidative injury in vivo. Further, a number of IsoPs have been found to possess potent biological activity and thus are likely also mediators of oxidant injury (4). In recent years, additional related compounds, derived from various polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) (5), docosahexaenoic acid (DHA) (6), and, more recently, adrenic acid (7), have been discovered to be formed as products of the IsoP pathway. It is the purpose herein to summarize our current knowledge regarding the IsoPs including the chemistry and biochemistry of their formation, the utility of measuring these compounds as markers of in vivo oxidant stress, and their pharmacological properties.
DISCOVERY OF F2-ISOPROSTANES
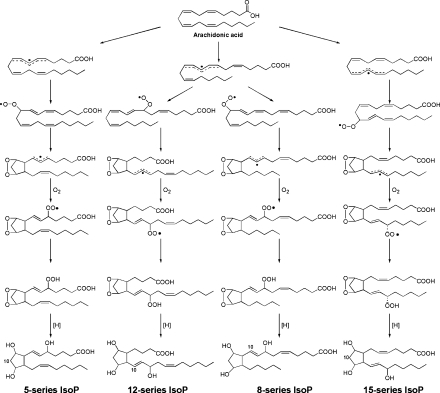
In 1990, Morrow et al. (2) discovered the first class of IsoPs, the F2-IsoPs, so named because they contain F-type prostane rings analogous to PGF2α. A mechanism to explain the formation of the F2-IsoPs from arachidonic acid is outlined in Fig. 1. Based on this mechanism of formation, four F2-IsoP regioisomers are generated; compounds are denoted as 5-, 12-, 8-, or 15-series regioisomers, depending on the carbon atom to which the side chain hydroxyl is attached (8). An alternative nomenclature system for the IsoPs has been proposed by Rokach et al. (9) in which the abbreviation iP is used for isoprostane, and the regioisomers are denoted as III–VI based upon the number of carbons between the omega carbon and the first double bond.
Fig. 1.
Pathway of formation of F2-isoprostanes. IsoP, isoprostane.
An important structural distinction between IsoPs and cyclooxygenase (COX)-derived PGs is that the former contain side chains that are predominantly oriented cis to the prostane ring while the latter possess exclusively trans side chains (2). In this regard, however, we have reported that PGs can be formed via the IsoP pathway as smaller amounts of endoperoxides containing trans side chains are generated in vitro and in vivo by this mechanism (10). In this case, PGs derived via the IsoP pathway can be distinguished from those formed by COX because the former are generated as a racemic mixture while the latter are enantiomerically pure. A second important difference between IsoPs and PGs is that IsoPs are formed primarily in situ esterified to phospholipids and are subsequently released by a phospholipase(s) (11), while PGs are generated only from free arachidonic acid. Molecular modeling of IsoP-containing phospholipids reveals them to be remarkably distorted molecules (12). Thus, the formation of these abnormal phospholipids would be expected to exert profound effects on membrane fluidity and integrity, well-known sequelae of oxidant injury.
In this regard, the biological activity of the F2-IsoPs has been studied. While these molecules are formed in situ on phospholipids as discussed, studies exploring the bioactivity of IsoPs have been performed using unesterified compounds as free acids. One F2-IsoP that is produced abundantly in vivo and has been extensively tested for biological activity is 15-F2t-IsoP (8-iso-PGF2α) (13). This IsoP has been found to be a potent vasoconstrictor in a variety of vascular beds, including the kidney, lung, heart, brain, and others (14, 15). In addition, 15-F2t-IsoP induces endothelin release and proliferation of vascular smooth muscle cells. There is also additional evidence that this molecule can increase resistance to aspirin inhibition of platelet aggregation in platelets as well as inhibit platelet aggregation in human whole blood (16). These vasoactive and platelet effects of 15-F2t-IsoP have been shown to result from interaction with the thromboxane receptor, a G-protein coupled transmembrane eicosanoid receptor, based on the finding that these effects could be abrogated by thromboxane receptor antagonists (17, 18).
QUANTIFICATION OF F2-ISOPS AS AN INDEX OF ENDOGENOUS OXIDANT STRESS
A true utility of the F2-IsoPs is in the quantification of lipid peroxidation and thus oxidant stress status in vivo (19, 20). Because they are stable molecules, measurement of the F2-IsoPs has revolutionized the ability to quantify oxidative injury in vivo. In a recent multi-investigator study, termed the Biomarkers of Oxidative Stress Study, sponsored by the National Institute of Health, it was found that the most accurate method to assess in vivo oxidant stress status is the quantification of plasma or urinary IsoPs, and thus, currently, quantification of these compounds provides the “gold standard” to assess oxidative injury in vivo (3).
A number of methods have been developed to quantify the IsoPs. Our laboratory uses a gas chromatographic/negative ion chemical ionization mass spectrometric (GC/NICI-MS) approach employing stable isotope dilution (21). For quantification purposes, we measure the F2-IsoP, 15-F2t-IsoP, and other F2-IsoPs that coelute with this compound. Several internal standards are available from commercial sources to quantify the IsoPs. The advantages of MS over other approaches include its high sensitivity and specificity, which yield quantitative results in the low picogram range. Its drawbacks are that it is labor intensive and requires considerable expenditures on equipment.
Several alternative GC/MS assays have been developed by different investigators including Pratico et al. (22) that quantify other IsoP regioisomers and Mas et al. (23) who utilize an F-ring IsoP deriving from DHA, termed 4-F4t-NP, as an internal standard. In addition, a number of liquid chromatographic (LC)/MS methods for F2-IsoPs have been developed (24–26). One advantage of LC/MS methods is that the sample preparation for analysis is simpler than that for GC/MS because no derivatization of the molecule is required.
Alternative methods have also been developed to quantify IsoPs using immunological approaches (27). Antibodies have been generated against 15-F2t-IsoP and several immunoassay kits are commercially available. A potential drawback of these methods is that limited information is currently available regarding their precision and accuracy. In addition, little data exist comparing IsoP levels determined by immunoassay to MS. Despite potential limitations, immunoassays have expanded IsoP research due to their low cost and relative ease of use (20).
Normal levels of F2-IsoPs in healthy humans have been defined (4, 28). Defining these levels is particularly important in that it allows for an assessment of the effects of diseases on endogenous oxidant tone and allows for the determination of the extent to which various therapeutic interventions affect levels of oxidant stress. Elevations of IsoPs in human body fluids and tissues have been found in a diverse array of human disorders, including atherosclerosis (29), diabetes (30), obesity (31), cigarette smoking (32), neurodegenerative diseases (33), and many others. Further, treatments for some of these conditions, including antioxidant supplementation, antidiabetic treaments, cessation of smoking, and even weight loss, have been shown to decrease production of F2-IsoPs (34, 35).
FORMATION OF ISOPS WITH ALTERNATIVE RING STRUCTURES
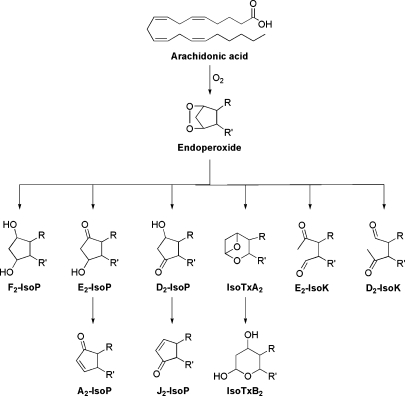
Since the initial discovery of the F2-IsoPs, the laboratories of Roberts and Morrow have shown that the IsoP pathway provides a mechanism for the generation of other classes of IsoPs from arachidonic acid, which differ in regards to the functional groups on the prostane ring. The classes of all known IsoPs are shown in Fig. 2. In addition to undergoing reduction to yield F2-IsoPs, the arachidonoyl endoperoxide intermediate can undergo isomerization to yield E- and D-ring IsoPs, which are isomeric to PGE2 and PGD2, respectively (36). E2/D2-IsoPs are formed competitively with F2-IsoPs, and studies have demonstrated that the depletion of cellular reducing agents, such as glutathione (GSH) or α-tocopherol, favors the formation of E2/D2-IsoPs over that of reduced F2-IsoPs (37).
Fig. 2.
Oxidation of arachidonic acid to yield isoprostanes of differing ring structures. IsoK, isoketal.
E2/D2-IsoPs are not terminal products of the IsoP pathway. These compounds readily dehydrate in vivo to yield A2/J2-IsoPs, which are also known as cyclopentenone IsoPs because they contain an α,β-unsaturated cyclopentenone ring structure (38). A2/J2-IsoPs are highly reactive electrophiles, which readily form Michael adducts with cellular thiols, including those found on cysteine residues in proteins and GSH (39). These cyclopentenone IsoPs are rapidly metabolized in vivo by glutathione transferase enzymes to water-soluble modified glutathione conjugates (40). A major urinary cyclopentenone IsoP metabolite in rats, a 15-A2t-IsoP mercapturic acid sulfoxide conjugate, has been identified (40).
Thromboxane-like molecules have also been reported to be generated via the IsoP pathway, although compounds with structures similar to prostacyclin have not been reported. Further, a series of compounds termed isoketals (IsoKs or isolevuglandins) can be generated via the IsoP pathway and result from opening of the cyclopentane ring (41). These compounds are highly reactive and readily adduct lysine residues on proteins resulting in covalent modification and protein dysfunction and cross-linking.
FORMATION OF OTHER PEROXIDATION PRODUCTS FROM ARACHIDONIC ACID
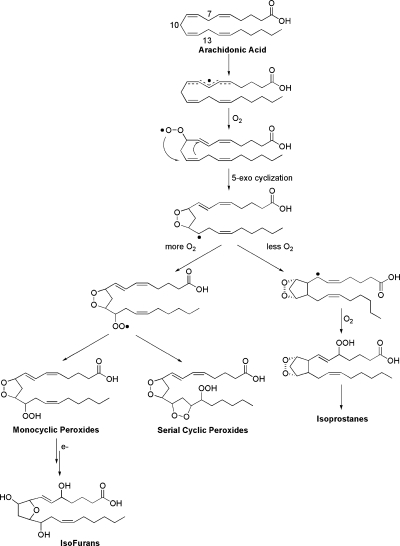
In addition to being able to form PG-like molecules, arachidonic acid can be oxidized to yield molecules with cyclic peroxide and furan ring structures. These molecules are formed in competition with isoprostanes from the key intermediate 1 as can be seen in Fig. 3. This radical intermediate can either undergo a 5-exo cyclization reaction, which does not require oxygen, to yield IsoPs or react with molecular oxygen to yield monocyclic peroxides and serial cyclic peroxides (42), which contain cyclic peroxide ring structures, or compounds termed isofurans (IsoFs), which contain a substituted tetrahydrofuran ring (43). Based upon this mechanism of formation, one would hypothesize that as oxygen tension increases, the formation of cyclic peroxides and IsoFs would be favored compared with IsoPs. Indeed our laboratory has shown that when arachidonic acid is oxidized at differing oxygen tensions (1% O2, 21% O2, and 100% O2) levels of IsoFs increase with oxygen tension, while no significant increase in IsoPs is observed between 21% O2 and 100% O2 (44).
Fig. 3.
Oxidation of arachidonic acid to yield isoprostanes, mono and serial cyclic peroxides, and isofurans.
Considering these findings, IsoFs may provide a better index of oxidant stress than IsoPs in conditions associated with higher oxygen levels. Indeed, in an experimental setting of hyperoxia-induced lung injury in mice, we found that IsoFs esterified in the lung tissues of these animals are significantly increased compared with controls while F2-IsoPs remained unchanged (44). Further, in a study in which rats were administered kainic acid, it was found that seizures induced increases F2-IsoPs and IsoFs in a manner dependent upon cellular levels of O2 in the hippocampus (45). F2-IsoPs were increased in the peak hypoxia phase of the seizure while IsoFs were increased in the “reoxygenation” phase of the seizure.
FORMATION OF ISOPS FROM OTHER POLYUNSATURATED FATTY ACIDS
Arachidonic acid is not the only polyunsaturated fatty acid that can be oxidized to generate IsoPs. F-ring IsoPs have been shown to be generated from the peroxidation of the omega-3 polyunsaturated fatty acids EPA [C20:5, ω-3, F3-IsoPs] and DHA [C22:6, ω-3, F4-neuroprostanes (NPs)] (5, 6). Our interest in examining the formation of IsoP-like compounds from EPA and DHA stems from emerging evidence that has implicated increased dietary intake of fish oil, which contains large amounts of EPA and DHA, as being beneficial in the prevention and treatment of a number of diseases, including atherosclerotic cardiovascular disease and sudden death, neurodegeneration, and various inflammatory disorders, among others. Further, recent data have suggested that the anti-inflammatory effects and other biologically relevant properties of ω-3 fatty acids are due, in part, to the generation of various bioactive oxidation products (46). We thus hypothesized that EPA- and DHA-derived IsoPs could contribute to the beneficial biological effects of fish oil supplementation. Indeed, one report states that the EPA-derived IsoP, 15-F3t-IsoP, possesses activity that is different from 15-F2t-IsoP in that it does not affect human platelet shape change or aggregation (47).
Interestingly, our laboratory has found that the levels of IsoPs generated from the oxidation of EPA significantly exceeds those of F2-IsoPs generated from arachidonic acid, perhaps because EPA contains more double bonds and is therefore more easily oxidizable (5). Additionally, in vivo in mice, levels of F3-IsoPs in tissues such as heart were virtually undetectable at baseline but supplementation of animals with EPA markedly increased quantities up to 27.4 ± 5.6 ng/g heart. But, of particular note, we found that EPA supplementation also markedly reduced levels of arachidonate-derived F2-IsoPs mouse heart tissues by over 60% (P < 0.05). These observations are significant because F2-IsoPs are generally considered to be pro-inflammatory molecules associated with the pathophysiological sequelae of oxidant stress. It is thus intriguing to propose that part of the mechanism by which EPA prevents certain diseases is its ability to decrease F2-IsoP generation. In addition, it suggests that supplementation with fish oil may be of benefit to populations associated with increased levels of F2-IsoPs.
More recently, our laboratory has examined the formation of F-ring IsoPs generated from adrenic acid (C22:4, ω-6) (7). These compounds are termed F2-dihomo-IsoPs. Adrenic acid, like DHA, is highly enriched in the brain but is primarily found in white matter and is associated with myelin. White matter is commonly damaged by ischemic stroke and is uniformly damaged in multiple sclerosis. We reported that F2-dihomo-IsoPs are formed in significant amounts from adrenic acid and levels are markedly increased in settings of oxidant stress occurring in the white matter portion of the brain in humans. Proportionally, levels of dihomo-IsoPs in white matter undergoing oxidative injury increase to a greater extent than IsoPs and NPs derived from arachidonic acid and DHA, respectively. These studies suggest that quantification of dihomo-IsoPs may be a selective marker of white matter injury in vivo.
SUMMARY AND THOUGHTS FOR THE FUTURE
The discovery of the IsoPs as products of nonenzymatic lipid peroxidation has been a major contribution to lipid oxidation and free radical chemistry. Our understanding of the IsoP pathway continues to expand, providing new insights into the nature of lipid peroxidation in vivo and revealing new molecules that exert potent biological actions and might serve as unique indices of disease. Basic research into the biochemistry and pharmacology of the IsoPs, coupled with clinical studies employing these molecules as biomarkers, should continue to provide important insights into the role of oxidant stress in human physiology and pathophysiology.
Abbreviations
COX, cyclooxygenase
DHA, docosahexaenoic acid
EPA, eicosapentaenoic acid
GC, gas chromatography
GSH, glutathione
IsoF, isofuran
IsoK, isoketal
IsoP, isoprostane
LC, liquid chromatography
MS, mass spectrometry
NICI, negative ion chemical ionization
NP, neuroprostane
PG, prostaglandin
Supported by National Institutes of Health Grants GM15431, GM42056, AG023597, DK48831, ES13125 and ES00267.
Published, JLR Papers in Press, October 28, 2008.
References
- 1.Halliwell B., and J. M. Gutteridge. 1990. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 186 1–85. [DOI] [PubMed] [Google Scholar]
- 2.Morrow J. D., K. E. Hill, R. F. Burk, T. M. Nammour, K. F. Badr, and L. J. Roberts 2nd. 1990. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA. 87 9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadiiska M. B., B. C. Gladen, D. D. Baird, D. Germolec, L. B. Graham, C. E. Parker, A. Nyska, J. T. Wachsman, B. N. Ames, S. Basu, et al. 2005. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 38 698–710. [DOI] [PubMed] [Google Scholar]
- 4.Fam S. S., and J. D. Morrow. 2003. The isoprostanes: unique products of arachidonic acid oxidation–a review. Curr. Med. Chem. 10 1723–1740. [DOI] [PubMed] [Google Scholar]
- 5.Gao L., H. Yin, G. L. Milne, N. A. Porter, and J. D. Morrow. 2006. Formation of F-ring isoprostane-like compounds (F3-isoprostanes) in vivo from eicosapentaenoic acid. J. Biol. Chem. 281 14092–14099. [DOI] [PubMed] [Google Scholar]
- 6.Roberts L. J., T. J. Montine, W. R. Markesbery, A. R. Tapper, P. Hardy, S. Chemtob, W. D. Dettbarn, and J. D. Morrow. 1998. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 273 13605–13612. [DOI] [PubMed] [Google Scholar]
- 7.VanRollins M., R. L. Woltjer, H. Yin, J. D. Morrow, and T. J. Montine. 2008. F2-dihomo-isoprostanes arise from free radical attack on adrenic acid. J. Lipid Res. 49 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taber D. F., J. D. Morrow, and L. J. Roberts 2nd. 1997. A nomenclature system for the isoprostanes. Prostaglandins. 53 63–67. [DOI] [PubMed] [Google Scholar]
- 9.Rokach J., S. P. Khanapure, S. W. Hwang, M. Adiyaman, J. A. Lawson, and G. A. FitzGerald. 1997. Nomenclature of isoprostanes: a proposal. Prostaglandins. 54 853–873. [DOI] [PubMed] [Google Scholar]
- 10.Yin H., L. Gao, H. H. Tai, L. J. Murphey, N. A. Porter, and J. D. Morrow. 2007. Urinary prostaglandin F2alpha is generated from the isoprostane pathway and not the cyclooxygenase in humans. J. Biol. Chem. 282 329–336. [DOI] [PubMed] [Google Scholar]
- 11.Stafforini D. M., J. R. Sheller, T. S. Blackwell, A. Sapirstein, F. E. Yull, T. M. McIntyre, J. V. Bonventre, S. M. Prescott, and L. J. Roberts 2nd. 2006. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 281 4616–4623. [DOI] [PubMed] [Google Scholar]
- 12.Morrow J. D., J. A. Awad, H. J. Boss, I. A. Blair, and L. J. Roberts 2nd. 1992. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA. 89 10721–10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow J. D., T. A. Minton, K. F. Badr, and L. J. Roberts 2nd. 1994. Evidence that the F2-isoprostane, 8-epi-prostaglandin F2 alpha, is formed in vivo. Biochim. Biophys. Acta. 1210 244–248. [DOI] [PubMed] [Google Scholar]
- 14.Morrow J. D. 2006. The isoprostanes - unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr. Pharm. Des. 12 895–902. [DOI] [PubMed] [Google Scholar]
- 15.Hou X., L. J. Roberts 2nd, F. Gobeil, Jr., D. Taber, K. Kanai, D. Abran, S. Brault, D. Checchin, F. Sennlaub, P. Lachapelle, et al. 2004. Isomer-specific contractile effects of a series of synthetic f2-isoprostanes on retinal and cerebral microvasculature. Free Radic. Biol. Med. 36 163–172. [DOI] [PubMed] [Google Scholar]
- 16.Patrignani P. 2003. Aspirin insensitive eicosanoid biosynthesis in cardiovascular disease. Thromb. Res. 110 281–286. [DOI] [PubMed] [Google Scholar]
- 17.Habib A., and K. F. Badr. 2004. Molecular pharmacology of isoprostanes in vascular smooth muscle. Chem. Phys. Lipids. 128 69–73. [DOI] [PubMed] [Google Scholar]
- 18.Benndorf R. A., E. Schwedhelm, A. Gnann, R. Taheri, G. Kom, M. Didie, A. Steenpass, S. Ergun, and R. H. Boger. 2008. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A2 receptor. A potential link between oxidative stress and impaired angiogenesis. Circ. Res. 103 1037–1046. [DOI] [PubMed] [Google Scholar]
- 19.Milne G. L., E. S. Musiek, and J. D. Morrow. 2005. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 10 (Suppl 1): S10–S23. [DOI] [PubMed] [Google Scholar]
- 20.Milne G. L., H. Yin, J. D. Brooks, S. Sanchez, L. Jackson Roberts 2nd, and J. D. Morrow. 2007. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 433 113–126. [DOI] [PubMed] [Google Scholar]
- 21.Milne G. L., S. C. Sanchez, E. S. Musiek, and J. D. Morrow. 2007. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2 221–226. [DOI] [PubMed] [Google Scholar]
- 22.Pratico D., O. P. Barry, J. A. Lawson, M. Adiyaman, S. W. Hwang, S. P. Khanapure, L. Iuliano, J. Rokach, and G. A. FitzGerald. 1998. IPF2alpha-I: an index of lipid peroxidation in humans. Proc. Natl. Acad. Sci. USA. 95 3449–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mas E., F. Michel, A. Guy, V. Bultel, Y. Falquet, P. Chardon, J. C. Rossi, J. P. Cristol, and T. Durand. 2008. Quantification of urinary F(2)-isoprostanes with 4(RS)-F(4t)-neuroprostane as an internal standard using gas chromatography-mass spectrometry application to polytraumatized patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 872 133–140. [DOI] [PubMed] [Google Scholar]
- 24.Liang Y., P. Wei, R. W. Duke, P. D. Reaven, S. M. Harman, R. G. Cutler, and C. B. Heward. 2003. Quantification of 8-iso-prostaglandin-F(2alpha) and 2,3-dinor-8-iso-prostaglandin-F(2alpha) in human urine using liquid chromatography-tandem mass spectrometry. Free Radic. Biol. Med. 34 409–418. [DOI] [PubMed] [Google Scholar]
- 25.Bohnstedt K. C., B. Karlberg, L. O. Wahlund, M. E. Jonhagen, H. Basun, and S. Schmidt. 2003. Determination of isoprostanes in urine samples from Alzheimer patients using porous graphitic carbon liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 796 11–19. [DOI] [PubMed] [Google Scholar]
- 26.Taylor A. W., R. S. Bruno, and M. G. Traber. 2008. Women and smokers have elevated urinary F(2)-isoprostane metabolites: a novel extraction and LC-MS methodology. Lipids. 43 925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu S. 1998. Radioimmunoassay of 8-iso-prostaglandin F2alpha: an index for oxidative injury via free radical catalysed lipid peroxidation. Prostaglandins Leukot. Essent. Fatty Acids. 58 319–325. [DOI] [PubMed] [Google Scholar]
- 28.Morrow J. D., Y. Chen, C. J. Brame, J. Yang, S. C. Sanchez, J. Xu, W. E. Zackert, J. A. Awad, and L. J. Roberts. 1999. The isoprostanes: unique prostaglandin like products of free radical-catalyzed lipid peroxidation. Drug Metab. Rev. 31 117–139. [DOI] [PubMed] [Google Scholar]
- 29.Morrow J. D. 2005. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 25 279–286. [DOI] [PubMed] [Google Scholar]
- 30.Davi G., F. Chiarelli, F. Santilli, M. Pomilio, S. Vigneri, A. Falco, S. Basili, G. Ciabattoni, and C. Patrono. 2003. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation. 107 3199–3203. [DOI] [PubMed] [Google Scholar]
- 31.Keaney J. F., Jr., M. G. Larson, R. S. Vasan, P. W. Wilson, I. Lipinska, D. Corey, J. M. Massaro, P. Sutherland, J. A. Vita, and E. J. Benjamin. 2003. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 23 434–439. [DOI] [PubMed] [Google Scholar]
- 32.Morrow J. D., B. Frei, A. W. Longmire, J. M. Gaziano, S. M. Lynch, Y. Shyr, W. E. Strauss, J. A. Oates, and L. J. Roberts 2nd. 1995. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 332 1198–1203. [DOI] [PubMed] [Google Scholar]
- 33.Montine K. S., J. F. Quinn, J. Zhang, J. P. Fessel, L. J. Roberts 2nd, J. D. Morrow, and T. J. Montine. 2004. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem. Phys. Lipids. 128 117–124. [DOI] [PubMed] [Google Scholar]
- 34.Davi G., G. Ciabattoni, A. Consoli, A. Mezzetti, A. Falco, S. Santarone, E. Pennese, E. Vitacolonna, T. Bucciarelli, F. Costantini, et al. 1999. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 99 224–229. [DOI] [PubMed] [Google Scholar]
- 35.Roberts L. J., J. A. Oates, M. F. Linton, S. Fazio, B. P. Meador, M. D. Gross, Y. Shyr, and J. D. Morrow. 2007. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic. Biol. Med. 43 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao L., W. E. Zackert, J. J. Hasford, M. E. Danekis, G. L. Milne, C. Remmert, J. Reese, H. Yin, H. H. Tai, S. K. Dey, et al. 2003. Formation of prostaglandins E2 and D2 via the isoprostane pathway: a mechanism for the generation of bioactive prostaglandins independent of cyclooxygenase. J. Biol. Chem. 278 28479–28489. [DOI] [PubMed] [Google Scholar]
- 37.Montine T. J., K. S. Montine, E. E. Reich, E. S. Terry, N. A. Porter, and J. D. Morrow. 2003. Antioxidants significantly affect the formation of different classes of isoprostanes and neuroprostanes in rat cerebral synaptosomes. Biochem. Pharmacol. 65 611–617. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y., J. D. Morrow, and L. J. Roberts 2nd. 1999. Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J. Biol. Chem. 274 10863–10868. [DOI] [PubMed] [Google Scholar]
- 39.Milne G. L., G. Zanoni, A. Porta, S. Sasi, G. Vidari, E. S. Musiek, M. L. Freeman, and J. D. Morrow. 2004. The cyclopentenone product of lipid peroxidation, 15–A2t-isoprostane, is efficiently metabolized by HepG2 cells via conjugation with glutathione. Chem. Res. Toxicol. 17 17–25. [DOI] [PubMed] [Google Scholar]
- 40.Milne G. L., L. Gao, A. Porta, G. Zanoni, G. Vidari, and J. D. Morrow. 2005. Identification of the major urinary metabolite of the highly reactive cyclopentenone isoprostane 15-A(2t)-isoprostane in vivo. J. Biol. Chem. 280 25178–25184. [DOI] [PubMed] [Google Scholar]
- 41.Brame C. J., R. G. Salomon, J. D. Morrow, and L. J. Roberts 2nd. 1999. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J. Biol. Chem. 274 13139–13146. [DOI] [PubMed] [Google Scholar]
- 42.Yin H., and N. A. Porter. 2005. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxid. Redox Signal. 7 170–184. [DOI] [PubMed] [Google Scholar]
- 43.Fessel J. P., N. A. Porter, K. P. Moore, J. R. Sheller, and L. J. Roberts 2nd. 2002. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc. Natl. Acad. Sci. USA. 99 16713–16718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts L. J., and J. P. Fessel. 2004. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem. Phys. Lipids. 128 173–186. [DOI] [PubMed] [Google Scholar]
- 45.Patel M., L. P. Liang, H. Hou, B. B. Williams, M. Kmiec, H. M. Swartz, J. P. Fessel, and L. J. Roberts 2nd. 2008. Seizure-induced formation of isofurans: novel products of lipid peroxidation whose formation is positively modulated by oxygen tension. J. Neurochem. 104 264–270. [DOI] [PubMed] [Google Scholar]
- 46.Serhan C. N., C. B. Clish, J. Brannon, S. P. Colgan, N. Chiang, and K. Gronert. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pratico D., E. M. Smyth, F. Violi, and G. A. FitzGerald. 1996. Local amplification of platelet function by 8-Epi prostaglandin F2alpha is not mediated by thromboxane receptor isoforms. J. Biol. Chem. 271 14916–14924. [DOI] [PubMed] [Google Scholar]