Abstract
Apoprotein E (apoE) is synthesized by a number of tissues including the liver, brain, adipose tissue, and artery wall. The majority of apoE is found in the plasma associated with specific lipoprotein subclasses and is derived primarily from the liver. However the fact that apoE expression is sustained in nonhepatic tissues suggests that the local production must have some unique functional attribute. ApoE is involved in many steps in lipid and lipoprotein homeostasis, for the triglyceride-rich lipoproteins and for HDL. ApoE is also important for lipid homeostasis in the brain, artery wall, and adipose tissue through its synthesis by glial cells, adipocytes, and macrophages. In addition, nonlipid related functions have also been attributed to apoE, including effects on immune response and inflammation, oxidation, and smooth muscle proliferation and migration. Some of these effects have been shown to be dependent upon different domains of the protein, different concentrations, and lipidation state. Thus, this multifunctional protein impacts normal and pathophysiology at multiple levels.
Keywords: adipose tissue, antiinflammatory, antioxidant, proliferation, reverse cholesterol transport
Apolipoprotein E (apoE) has received a great deal of attention as a risk factor for both atherosclerosis and Alzheimer's disease. Human apoE, in contrast to other species, exists in one of three major isoforms, designated E2, E3, and E4. Each of these isoforms exhibit isoform-specific effects on atherosclerosis and Alzheimer's disease (1, 2).
ApoE is a 34 kDa glycoprotein, initially noted as a component of plasma VLDL and HDL. It specifically associates with subsets of lipoproteins and this differs by isoform (3). Although evolutionarily derived from a common soluble apoprotein progenitor gene, apoE has properties that distinguish it from related apoproteins. Its closest structural apoprotein is apoA-I, which is found on bulk HDL and on large triglyceride-rich lipoproteins such as chylomicrons. In the lipid-free state, both proteins form a four-helix bundle with a more random-ordered hydrophobic C terminus. The N-terminal domain of apoE (residues 1–191) contains the LDL receptor binding domain and a major heparan sulfate proteoglycan (HSPG) binding domain (both in the vicinity of residues 140–150). The C-terminal domain is believed to be responsible for the initial binding of the protein to lipid.
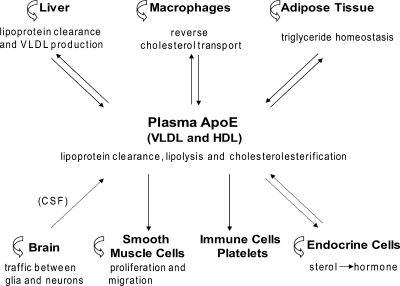
ApoE is characterized by its wide tissue distribution (4) and function (Fig. 1). Some of the protein is degraded prior to secretion, and a portion remains associated with HSPG on the cell surface (5, 6). While the liver is the major source of plasma apoE, apoE produced by other cell types also contribute to plasma levels. Though plasma apoE may enter these tissues, the fact that expression is sustained in the tissues suggests that the local production must have some unique functional attribute. In this brief review, we outline the role of apoE in lipid transport and discuss other functions attributed to apoE that impact on atherosclerosis and energy homeostasis.
Fig. 1.
Roles of Apolipoprotein E (apoE). ApoE synthesized by a number of tissues contributes to plasma levels, with the brain and endocrine cells contributing little if any to plasma apoE levels. Plasma-derived and endogenous apoE (curved arrow) may participate in the various functions of apoE in most of these tissues. CSF, cerebral spinal fluid.
APOE AND PLASMA LIPOPROTEIN HOMEOSTASIS
ApoE is involved in many steps of lipoprotein homeostasis. In the plasma, apoE is associated with VLDL, chylomicron remnants, and a subset of HDL particles. It is a high affinity ligand for the LDL receptor and its family members such as the LDL receptor related protein (LRP1), VLDL receptor, and apoE2 receptor (LPR8). ApoE interacts with these receptors and HSPG promoting the endocytic clearance of plasma lipoproteins, especially VLDL and remnant lipoproteins (5, 7). The liver is the major site for the clearance of apoE-containing lipoproteins. In addition to its ligand function, apoE can also influence other aspects of lipoprotein metabolism in the plasma. ApoE at high levels restricts VLDL lipolysis, in part by displacing the lipoprotein lipase activator apoprotein CII from the particle (8). ApoA-I, the premier activator of LCAT, is much less efficient on large HDL particles and apoB-containing lipoproteins where apoE functions as the LCAT activator (9, 10). ApoE may also influence the activity of hepatic lipase and cholesteryl ester transfer protein (CETP) (11).
APOE AND TRIGLYCERIDE-RICH LIPOPROTEIN PRODUCTION
ApoE is also implicated on the anabolic side of VLDL homeostasis. High expression levels of hepatic apoE result in a notable increase in VLDL triglyceride secretion (11, 12). The C-terminal domain is required for the promotion of VLDL triglyceride secretion, particularly the hydrophobic amino acids between residues 260 and 270 (13). The multiple roles of apoE in VLDL homeostasis are concentration dependent with low levels of apoE sufficient to promote receptor-mediated lipoprotein clearance and higher concentrations required to induce hypertriglyceridemia.
Although hepatocytes and Kupffer cells produce apoE, little attention has been paid to the role of the Kupffer cells in lipoprotein homeostasis. In contrast, intestinal enterocytes do not synthesize apoE. The apoE found on circulating chylomicron remnants is acquired either from other circulating lipoproteins or from other tissues.
APOE AND HDL HOMEOSTASIS
ApoE, like apoA-I, interacts with ABCA1 to generate nascent HDL particles (14). The C-terminal hydrophobic domain of apoE (residues 222–299) is necessary for its stimulation of ABCA1-dependent cholesterol efflux, so that apoE isoforms interact equivalently. Nascent apoE-HDL particles may also arise following the endocytosis of VLDL by hepatocytes with apoE being retained in early endosomes and recycled to the cell surface (15). ApoE may move between HDL and postprandial triglyceride-rich particles, probably as a lipid complex. As a consequence, apoE on HDL declines at the height of lipid absorption. While nascent apoE-HDL is produced primarily by hepatocytes or macrophages, the steady-state distribution of apoE among lipoprotein classes is equivalent regardless of the tissue source (7).
ApoE is found on minor HDL subclasses, including particles in which it is the sole apoprotein and particles that also contain apoA-I. In general, HDL particles containing apoE are larger than apoA-I-containing HDL particles. This may be related to differences in the nature of the amphipathic α-helices impacting on their association with phospholipid so that apoE is better able to accommodate LCAT-mediated core expansion of the HDL (16, 17). With low levels of hepatic lipase or high peripheral cholesterol load, apoE-rich HDL accumulates in the plasma. The apoE gene is a target of LXRα in nonhepatic tissues. When LXR is activated, enlarged HDL is generated but only in the presence of apoE and this is abrogated by CETP (18). Hence the HDL accumulating with CETP inhibition or inactive mutants is generally enriched in apoE.
APOE IN THE BRAIN
ApoE plays a significant role in the central nervous system. It is produced in the glial cells, where it functions as a cholesterol transport protein between astrocytes and neurons, especially during growth and repair. As there is limited permeability of the blood brain barrier to intact lipoproteins, endogenous synthesis of cholesterol and this transport protein is important (1).
APOE INFLUENCES ADIPOSE TISSUE HOMEOSTASIS
Adipose tissue is a repository of stored triglyceride and has important metabolic effects, including influencing glucose homeostasis and atherosclerosis. ApoE is expressed in adipose tissue, primarily in adipocytes, but also in macrophages (19). Adipose tissue, because of its mass, is a potential source of significant amounts of this apoprotein. ApoE−/− mice (19, 20) and genetically obese mice deficient in apoE (20, 21) have lower body-fat deposits and smaller adipocytes than control mice and exhibit higher glucose tolerance and improved insulin sensitivity (21, 22). VLDL contributes fatty acid to adipose tissues either by lipoprotein lipolysis or by endocytosis. Lipid uptake by adipose tissue is impaired in apoE−/− mice (22). VLDL induces adipocyte differentiation in vitro even in the presence of a lipoprotein lipase inhibitor. The uptake of VLDL from apoE−/− mice by adipocytes is promoted by preincubation of the VLDL with apoE (20), pointing to apoE-mediated VLDL endocytosis. But this is not the only locus of apoE action in adipocytes. Incubating apoE-containing VLDL with apoE-deficient adipocytes does not normalize VLDL uptake, indicating an important role of endogenous apoE production by the adipocytes for normal homeostasis (19). There appears to be a reciprocal regulation of apoE and adipose tissue homeostasis. Adipose tissue apoE levels decline with obesity and increase with fasting (23). In addition, PPARγ agonists promote and TNFα suppresses apoE synthesis in adipose tissue (24). The influence of apoE in adipose tissue on body energy homeostasis and atherosclerosis is being actively studied (22, 25).
LOCAL INFLUENCE OF APOE ON CELLS OF THE ATHEROSCLEROTIC LESION
Reverse cholesterol transport and macrophage expression
One of the mechanisms by which apoE is thought to be atheroprotective is by promoting cholesterol efflux from lipid loaded macrophages in the artery wall. ApoE is one of the major proteins secreted by macrophages (6) and bone-marrow transplantation studies indicate that macrophage-derived apoE is antiatherogenic (as reviewed in Ref. 26). ApoE is an LXR target gene in macrophages and thus its expression is up-regulated upon cholesterol loading, likely due to the generation of a cholesterol metabolite that functions as an LXR agonist (27).
Cell-culture experiments suggest that apoE promotes cholesterol efflux by three distinct processes: ABCA1-dependent and ABC-independent processes and an ABCG1-dependent process. These two ABC transporters are LXR target genes. ApoE added to the media interacts with ABCA1 to facilitate cholesterol and phospholipid efflux, generating lipidated apoE (28, 29). Macrophage synthesis of apoE stimulates cholesterol efflux from cells not loaded with cholesterol but most of this is ABCA1-independent (29). In cholesterol-loaded cells, stimulation of lipid efflux by macrophage apoE is primarily ABCA1-dependent (28). This is likely the predominant pathway in the artery wall. ApoA-I facilitates the formation of buoyant apoE-containing lipoproteins and this involves ABCA1 (28). In contrast to ABCA1, which promotes efflux to lipid poor apoproteins, ABCG1 promotes efflux to mature HDL particles. ApoE-HDL particles efficiently promote efflux via ABCG1 (9). ApoE, rather than apoA-I, may activate LCAT on these large HDL particles to promote esterification of the effluxed free cholesterol.
The apoE-containing particles generated by lipid efflux can be cleared from the plasma via the LDL receptor family or the cholesteryl esters in the HDL particles selectively taken into cells via the scavenger receptor class B type I (SR-BI). The selective uptake pathway is impaired in the liver and adrenal glands in apoE−/− mice (30). It has been postulated that apoE either on HDL or the surface of cells may facilitate HDL presentation to SR-BI.
Plasma apoE levels <5% of wild-type levels derived either from macrophages, adrenal gland, or liver is sufficient to reduce atherosclerosis even in the absence of any change in plasma lipid levels (31–34). This suggests that the production of apoE by macrophages in the atherosclerotic lesion is not obligatory for atheroprotection. The low levels of plasma-derived apoE are likely functioning as a promoter of macrophage cholesterol efflux or by influencing other functions in the vessel wall (Table 1). It is difficult to reconcile these observations with the supposed importance of macrophage apoE production when studied in culture. Using an inducible system, apoE has been shown to provide partial regression of established lesions but only when expressed at physiological levels (35). Clearly apoE has different functional properties at different levels of expression and more study is required to understand these differences.
Table 1.
Functions of apoE
| Lipidation state of apoE | Interaction with LDL receptor family | Influenced by apoE isoform | Signaling mediators | Reference | |
|---|---|---|---|---|---|
| Lipid and lipoprotein metabolism functions | |||||
| Ligand for LDL receptor family | Lipidated | + | yes | 5 | |
| Promotion of triglyceride secretion | — | no | 12 | ||
| Ligand for proteoglycans | Lipidated and lipid-poor | — | yes | 5 | |
| Inhibition of intravascular lipolysis of VLDL | Lipidated | — | no | 8 | |
| Activation of LCAT | Lipidated | — | no | 9, 10 | |
| Promotion of macrophage cholesterol efflux | Lipidated and lipid-poor | — | yes | 9, 28, 29 | |
| Transfer of cholesterol from astrocytes | Lipidated | — | yes (?) | 1 | |
| Selective uptake of cholesteryl esters | Lipidated | — | no | 30 | |
| Nonlipid related functions | |||||
| Promotes biosynthesis of proteoglycans | Lipidated | — | unknown | NO (?) | 38 |
| Adipose tissue homeostasis | Lipidated and lipid-poor | unknown | unknown | 19–22 | |
| Antioxidant | unknown | yes | 46–49 | ||
| Stimulation of Cox-2 expression in SMC | Lipidated and lipid-poor | — | no | unknown | 39, 40 |
| Stimulates SMC proliferation | Lipidated | — | yes | NO, PGI2 | 36, 37, 39 |
| Stimulates SMC migration | Lipidated | + | no | cAMP/PKA | 36 |
| Inhibits T-cell proliferation | Lipidated and lipid-poor | unknown | unknown | unknown | 41 |
| Anti-inflammatory | unknown | yes/no | PKA, NF-kB | 41–45 | |
| Antigen presentation to NKT cells | Lipidated | + | unknown | 43 | |
| Inhibits activation of platelets | Lipidated | + | unknown | NO (?) | |
| Stimulates NO production | unknown | yes | |||
| Apoptosis | Lipidated and lipid-poor | unknown | unknown | ||
apoE, apolipoprotein E; NO, nitric oxide; SMC, smooth muscle cell.
SMCs
Smooth muscle cell (SMC) proliferation contributes to postangioplasty restenosis and to atherogenesis. ApoE can inhibit SMC migration and proliferation. There is profound neointima formation in apoE−/− mice in the injured carotid artery (36) and femoral artery (37). In vitro, platelet derived growth factor (PDGF) promotes SMC migration and proliferation. ApoE inhibits both processes but by different mechanisms (36). SMC proliferation is inhibited by apoE binding to cell surface HSPG, resulting in induction of iNOS gene expression and nitric oxide (NO) formation. The increased level of NO attenuates the increased expression of cyclin D1 stimulated by PDGF, thus inhibiting cell-cycle progression. On the other hand, apoE signals through LRP1 to inhibit PDGF promoted SMC migration. The interaction of apoE with LRP1 activates the cAMP/protein kinase A pathway. The effect of apoE on SMC migration is mediated by its receptor-binding domain, though it does not exhibit apoE isoform preferences. Low concentrations of apoE inhibit migration, while higher concentrations are necessary to inhibit proliferation in vitro. Because subphysiologic apoE plasma levels are able to inhibit neointimal formation following femoral artery injury (37), this suggests that migration is probably the upstream effector in neointimal formation.
The influence of apoE on SMC proliferation is enhanced by the stimulation of the biosynthesis of perlecan HSPG, and its sulfation by apoE and apoE-containing HDL (38). This may involve apoE stimulation of NO production. The stimulation of HSPG synthesis by apoE may also influence apoE-mediated cholesterol efflux from lipid-laden macrophages (6).
In addition to the above signaling pathways, apoE, particularly its N-terminal domain, also exerts antimitogenic effects on SMC by stimulating Cox-2 gene expression, leading to increased prostacyclin production, activation of the prostaglandin receptor IP, and decreased expression of the cyclin A gene (39). Lipid-free apoE is more effective than the lipid associated protein. Whatever receptor mediates this effect, it does not appear to be the LDL receptor, LRP1, or HSPG (40).
APO E INFLUENCES SEVERAL ASPECTS OF THE INFLAMMATORY RESPONSE
Lymphocytes
ApoE has anti-inflammatory effects on T cells. Harmony and collaborators showed that apoE and apoE-containing lipoproteins inhibit the proliferation of stimulated CD4+ and CD8+ T cells via a receptor that is distinct from the conventional lipoprotein receptors (as reviewed in Ref. 41). ApoE down-regulates the bioactivity of the characteristic T-cell growth factor IL-2. Although not precisely known, the decreased activity of IL-2 likely involves modification of intracellular signaling perhaps involving calcium and phosphatidylinositol.
Antigen-presenting cell
Macrophages derived from apoE−/− mice stimulated by exogenous antigen are more effective in the up-regulation of MHC class II and costimulatory molecules CD40 and CD80, with increased IFN-γ secretion in responding T cells (42). In the special case of the activation of natural killer T cells by the presentation of lipid antigen by CD1d, apoE plays an important role in facilitating delivery of the lipid antigen to the antigen-presenting cell (43).
The inflammatory response
ApoE−/− mice are more susceptible to endotoxemia and bacterial infection (11, 41). Type I inflammatory responses are suppressed by apoE. This is seen as increased production of the proinflammatory cytokines TNF-α, IL-6, IL-12, and IFN-γ in apoE−/− mice in response to LPS, a Toll-like receptor (TRL) 4 agonist, that was not due to the effects of the hyperlipidemia per se (44). The LPS-induced increase in IL-12 and TNF-α was suppressed by liver-specific expression of apoE. ApoE also suppresses TLR3-mediated up-regulation of IL-12 synthesis, but not up-regulation mediated by other TLR. ApoE has also been shown to interrupt IL-1β signaling in vascular SMCs by preventing IRAK-1 phosphorylation and its complex formation with TRAF-6 (45). LRP1 stimulation of protein kinase A activity appears to mediate this inhibitory effect of apoE.
Antioxidant capacity
In vitro, apoE has been shown to protect LDL against oxidation (46) and this appears to involve its receptor-binding domain (47). The high levels of oxidized phospholipids in the plasma of chow fed apoE−/− mice (48), and the significantly reduced levels of isoprostanes in LDL receptor deficient mice with hepatic overexpression of apoE (49) are consistent with apoE exerting an antioxidant role in vivo.
CONCLUSIONS
It is clear from this brief review that apoE is a multifunctional protein that has an impact on pathophysiology at many locations in the organism. We have not even discussed the influence of apoE on apoptosis, platelet aggregation, endothelial cells, and the homeostasis in endocrine organs and have barely touched the differential effect of apoE isoforms on these functions. Some of the actions of apoE affect lipoprotein and lipid homeostasis. Some actions are properties of lipid-poor or free apoE. ApoE has several well-defined domains, and some of its properties are distinguished from one another by the requirement for one or other domain. It is also clear from several in vivo studies that some of the functions of apoE require low or modest levels of the protein. The precise mechanisms of action of these highly sensitive effects of apoE have yet to be clarified. Thus in interpreting the effects of apoE detailed account of its lipidation state, its concentration and its location of action must be carefully considered. The apoE-deficient murine model of atherosclerosis is widely employed. The multitude of actions of apoE in local tissues and as a lipid-free apoprotein should alert one to the importance of studying other models of atherosclerosis before resorting to generalizations about widely applicable mechanisms of atherogenesis.
This work is funded by grants from the National Heart Lung Blood Institute of the National Institutes of Health and the Leducq Foundation.
Published, JLR Papers in Press, November 18, 2008.
References
- 1.Mahley, R. W., K. H. Weisgraber, and Y. Huang. 2009. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J. Lipid Res. S183–S188. [DOI] [PMC free article] [PubMed]
- 2.Pendse, A. A., J. M. Arbones-Mainar, L. A. Johnson, M. K. Altenburg, and N. Maeda. 2009. Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J. Lipid Res. S178–S182. [DOI] [PMC free article] [PubMed]
- 3.Dong L. M., and K. H. Weisgraber. 1996. Human apolipoprotein E4 domain interaction. Arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J. Biol. Chem. 271 19053–19057. [DOI] [PubMed] [Google Scholar]
- 4.Driscoll D. M., and G. S. Getz. 1984. Extrahepatic synthesis of apolipoprotein E. J. Lipid Res. 25 1368–1379. [PubMed] [Google Scholar]
- 5.Mahley R. W., and Z. S. Ji. 1999. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J. Lipid Res. 40 1–16. [PubMed] [Google Scholar]
- 6.Kockx M., W. Jessup, and L. Kritharides. 2008. Regulation of endogenous apolipoprotein E secretion by macrophages. Arterioscler. Thromb. Vasc. Biol. 28 1060–1067. [DOI] [PubMed] [Google Scholar]
- 7.Raffai R. L., A. H. Hasty, Y. Wang, S. E. Mettler, D. A. Sanan, M. F. Linton, S. Fazio, and K. H. Weisgraber. 2003. Hepatocyte-derived apoE is more effective than non-hepatocyte-derived apoE in remnant lipoprotein clearance. J. Biol. Chem. 278 11670–11675. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Z. S. Ji, W. J. Brecht, S. C. Rall, Jr., J. M. Taylor, and R. W. Mahley. 1999. Overexpression of apolipoprotein E3 in transgenic rabbits causes combined hyperlipidemia by stimulating hepatic VLDL production and impairing VLDL lipolysis. Arterioscler. Thromb. Vasc. Biol. 19 2952–2959. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura F., N. Wang, W. Chen, X. C. Jiang, and A. R. Tall. 2006. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J. Clin. Invest. 116 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y., F. E. Thorngate, K. H. Weisgraber, D. L. Williams, and J. S. Parks. 2005. Apolipoprotein E is the major physiological activator of lecithin-cholesterol acyltransferase (LCAT) on apolipoprotein B lipoproteins. Biochemistry. 44 1013–1025. [DOI] [PubMed] [Google Scholar]
- 11.Greenow K., N. J. Pearce, and D. P. Ramji. 2005. The key role of apolipoprotein E in atherosclerosis. J. Mol. Med. 83 329–342. [DOI] [PubMed] [Google Scholar]
- 12.Mensenkamp A. R., M. C. Jong, H. van Goor, M. J. A. van Luyn, V. Bloks, R. Havinga, P. J. Voshol, M. H. Hofker, K. W. van Dijk, L. M. Havekes, et al. 1999. Apolipoprotein E participates in the regulation of very low density lipoprotein-triglyceride secretion by the liver. J. Biol. Chem. 274 35711–35718. [DOI] [PubMed] [Google Scholar]
- 13.Kypreos K. E., K. W. Van Dijk, L. M. Havekes, and V. I. Zannis. 2005. Generation of a recombinant apolipoprotein E variant with improved biological functions. Hydrophobic residues (Leu-261, Trp-264, Phe-265, Leu-268, Val-269) of apoE can account for the apoE-induced hypertriglyceridemia. J. Biol. Chem. 280 6276–6284. [DOI] [PubMed] [Google Scholar]
- 14.Vedhachalam C., V. Marayanaswami, N. Neto, T. M. Forte, M. C. Phillips, S. Lund-Katz, and J. M. Bielicki. 2007. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high density lipoproteins. Biochemistry. 46 2583–2593. [DOI] [PubMed] [Google Scholar]
- 15.Heeren J., U. Beisiegel, and T. Grewal. 2006. Apolipoprotein E recycling: implications for dyslipidemia and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26 442–448. [DOI] [PubMed] [Google Scholar]
- 16.Saito H., S. Lund-Katz, and M. C. Phillips. 2004. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog. Lipid Res. 43 350–380. [DOI] [PubMed] [Google Scholar]
- 17.Mahley R. W., Y. Huang, and K. H. Weisgraber. 2006. Putting cholesterol in its place: apoE and reverse cholesterol transport. J. Clin. Invest. 116 1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X. C., T. P. Beyer, Z. Li, J. Liu, W. Quan, R. J. Schmidt, Y. Zhang, W. R. Bensch, P. I. Eacho, and G. Cao. 2003. Enlargement of high density lipoprotein in mice via Liver X receptor activation requires apolipoprotein E and is abolished by cholesteryl ester transfer protein expression. J. Biol. Chem. 278 49072–49078. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z. H., C. A. Reardon, and T. Mazzone. 2006. Endogenous apoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 55 3394–3402. [DOI] [PubMed] [Google Scholar]
- 20.Chiba T., T. Nakazawa, K. Yui, E. Kaneko, and K. Shimokado. 2003. VLDL induces adipocyte differentiation in apoE-dependent manner. Arterioscler. Thromb. Vasc. Biol. 23 1423–1429. [DOI] [PubMed] [Google Scholar]
- 21.Gao J., H. Katagiri, Y. Ishigaki, T. Yamada, T. Ogihara, J. Imai, K. Uno, Y. Hasegawa, M. Kanzaki, T. T. Yamamoto, et al. 2007. Involvement of apolipoprotein E in excess fat accumulation and insulin resistance. Diabetes. 56 24–33. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann S. M., D. Perez-Tilve, T. M. Greer, B. A. Coburn, E. Grant, J. E. Basford, M. H. Tschöp, and D. Y. Hui. 2008. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes. 57 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z. H., R. M. Luque, R. D. Kineman, and T. Mazzone. 2007. Nutritional regulation of adipose tissue apolipoprotein E expression. Am. J. Physiol. Endocrinol. Metab. 293 E203–E209. [DOI] [PubMed] [Google Scholar]
- 24.Yue L., N. Rasouli, G. Ranganathan, P. A. Kern, and T. Mazzone. 2004. Divergent effects of peroxisome proliferator-activated receptor γ agonist and tumor necrosis factor α on adipocyte apoE expression. J. Biol. Chem. 279 47626–47632. [DOI] [PubMed] [Google Scholar]
- 25.Fantuzzi G., and T. Mazzone. 2007. Adipose tissue and atherosclerosis. Exploring the connection. Arterioscler. Thromb. Vasc. Biol. 27 996–1003. [DOI] [PubMed] [Google Scholar]
- 26.Mazzone T., H. Gump, P. Diller, and G. S. Getz. 1987. Macrophage free cholesterol content regulates apolipoprotein E synthesis. J. Biol. Chem. 25 11657–11662. [PubMed] [Google Scholar]
- 27.Curtiss L. K., and W. A. Boisvert. 2000. Apolipoprotein E and atherosclerosis. Curr. Opin. Lipidol. 11 243–251. [DOI] [PubMed] [Google Scholar]
- 28.Yancey P. G., H. Yu, M. F. Linton, and S. Fazio. 2007. A pathway-dependent on apoE, apoA-I, and ABCA1 determines formation of buoyant high-density lipoprotein by macrophage foam cells. Arterioscler. Thromb. Vasc. Biol. 27 1123–1131. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z. H., M. L. Fitzgerald, and T. Mazzone. 2006. Distinct cellular loci for the ABCA1-dependent and ABCA1-independent lipid efflux mediated by endogenous apolipoprotein E expression. Arterioscler. Thromb. Vasc. Biol. 26 157–162. [DOI] [PubMed] [Google Scholar]
- 30.Arai T., F. Rinninger, L. Varban, V. Fairchild-Huntress, C. P. Liang, W. Chen, T. Seo, R. Deckelbaum, D. Huszar, and A. R. Tall. 1999. Decreased selective uptake of high density lipoprotein cholesteryl esters in apolipoprotein E knock-out mice. Proc. Natl. Acad. Sci. USA. 96 12050–12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasty A. H., M. F. Linton, S. J. Brandt, V. R. Babaev, L. A. Gleaves, and S. Fazio. 1999. Retroviral gene therapy in apoE-deficient mice. ApoE expression in the artery wall reduces early foam cell lesion formation. Circulation. 99 2571–2576. [DOI] [PubMed] [Google Scholar]
- 32.Bellosta S., R. W. Mahley, D. A. Sanan, J. Murata, D. L. Newland, J. M. Taylor, and R. E. Pitas. 1995. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J. Clin. Invest. 96 2170–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorngate F. E., L. L. Rudel, R. L. Walzem, and D. L. Williams. 2000. Low levels of extrahepatic non-macrophage apoE inhibit atherosclerosis without correcting hypercholesterolemia in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 20 1939–1945. [DOI] [PubMed] [Google Scholar]
- 34.Tsukamoto K., R. K. Tangirala, S. Chun, D. Usher, E. Puré, and D. J. Rader. 2000. Hepatic expression of apolipoprotein E inhibits progression of atherosclerosis without reducing cholesterol levels in LDL receptor-deficient mice. Mol. Ther. 1 189–194. [DOI] [PubMed] [Google Scholar]
- 35.Raffai R. L., S. M. Loeb, and K. H. Weisgraber. 2005. Apolipoprotein E promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 25 436–441. [DOI] [PubMed] [Google Scholar]
- 36.Hui D. Y., and J. E. Basford. 2005. Distinct signaling mechanisms for apoE inhibition of cell migration and proliferation. Neurobiol. Aging. 26 317–323. [DOI] [PubMed] [Google Scholar]
- 37.Wientgen H., F. E. Thorngate, S. Omerhodizic, L. Roinitzky, J. T. Falon, D. L. Williams, and E. A. Fisher. 2004. Subphysiologic apolipoprotein E (apoE) plasma levels inhibit neointimal formation after arterial injury in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 24 1460–1465. [DOI] [PubMed] [Google Scholar]
- 38.Pillarisetti S. 2000. Lipoprotein modulation of subendothelial heparan sulfate proteoglycans (perlecan) and atherogenicity. Trends Cardiovasc. Med. 10 60–65. [DOI] [PubMed] [Google Scholar]
- 39.Kothapalli D., I. Fuki, K. Ali, S. A. Stewart, L. Zhao, R. Yahil, D. Kwaitkowski, E. A. Hawthorne, G. A. FitzGerald, M. C. Phillips, et al. 2004. Antimitogenic effects of HDL and apoE mediated by Cox-2 dependent IP activation. J. Clin. Invest. 113 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali K., S. Lund-Katz, J. Lawson, M. C. Phillips, and D. J. Rader. 2008. Structure-function properties of the apoE-dependent COX-2 pathway in vascular smooth muscle cells. Atherosclerosis. 196 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jofre-Monseny L., A. M. Minihane, and G. Rimbach. 2008. Impact of apoE genotype on oxidative stress, inflammation, and disease risk. Mol. Nutr. Food Res. 52 131–145. [DOI] [PubMed] [Google Scholar]
- 42.Tenger C., and X. Zhou. 2003. Apolipoprotein E modulates immune activation by acting on the antigen presenting cell. Immunology. 109 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Elzen P., S. Garg, L. Leon, M. Brigl, E. A. Leadbetter, J. E. Gumperz, C. C. Dacher, T. Y. Cheng, F. M. Sacks, P. A. Illarionov, et al. 2005. Apolipoprotein-mediated pathways of antigen presentation. Nature. 437 906–910. [DOI] [PubMed] [Google Scholar]
- 44.Ali K., M. Middleton, E. Puré, and D. J. Rader. 2005. Apolipoprotein E suppresses the Type I inflammatory response in vivo. Circ. Res. 97 922–927. [DOI] [PubMed] [Google Scholar]
- 45.Kawamura A., D. Baitsch, R. Telgmann, R. Feuerborn, G. Weissen-Plenz, C. Hagedorn, K. Saku, S. M. Brand-Herrmann, A. von Eckardstein, G. Assmann, et al. 2007. Apolipoprotein E interrupts interleukin-1β signaling in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 27 1610–1617. [DOI] [PubMed] [Google Scholar]
- 46.Miyata M., and J. D. Smith. 1996. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat. Genet. 14 55–61. [DOI] [PubMed] [Google Scholar]
- 47.Pham T., A. Kodvawala, and D. Y. Hui. 2005. The receptor binding domain of apolipoprotein E is responsible for its antioxidant activity. Biochemistry. 44 7577–7582. [DOI] [PubMed] [Google Scholar]
- 48.Forte T. M., G. Subbanagounder, J. A. Berliner, P. J. Blanche, A. O. Clermont, Z. Jia, M. N. Oda, R. M. Krauss, and J. K. Bielicki. 2002. Altered activities of anti-atherogenic enzymes LCAT, paraoxonase, and platelet-activating factor acetylhydrolase in atherosclerosis-susceptible mice. J. Lipid Res. 43 477–485. [PubMed] [Google Scholar]
- 49.Tangirala R. K., D. Praticó, G. A. FitzGerald, S. Chun, K. Tsukamoto, C. Maugeais, D. C. Usher, E. Puré, and D. J. Rader. 2001. Reduction of isoprostanes and regression of advanced atherosclerosis by apolipoprotein E. J. Biol. Chem. 276 261–266. [DOI] [PubMed] [Google Scholar]