Abstract
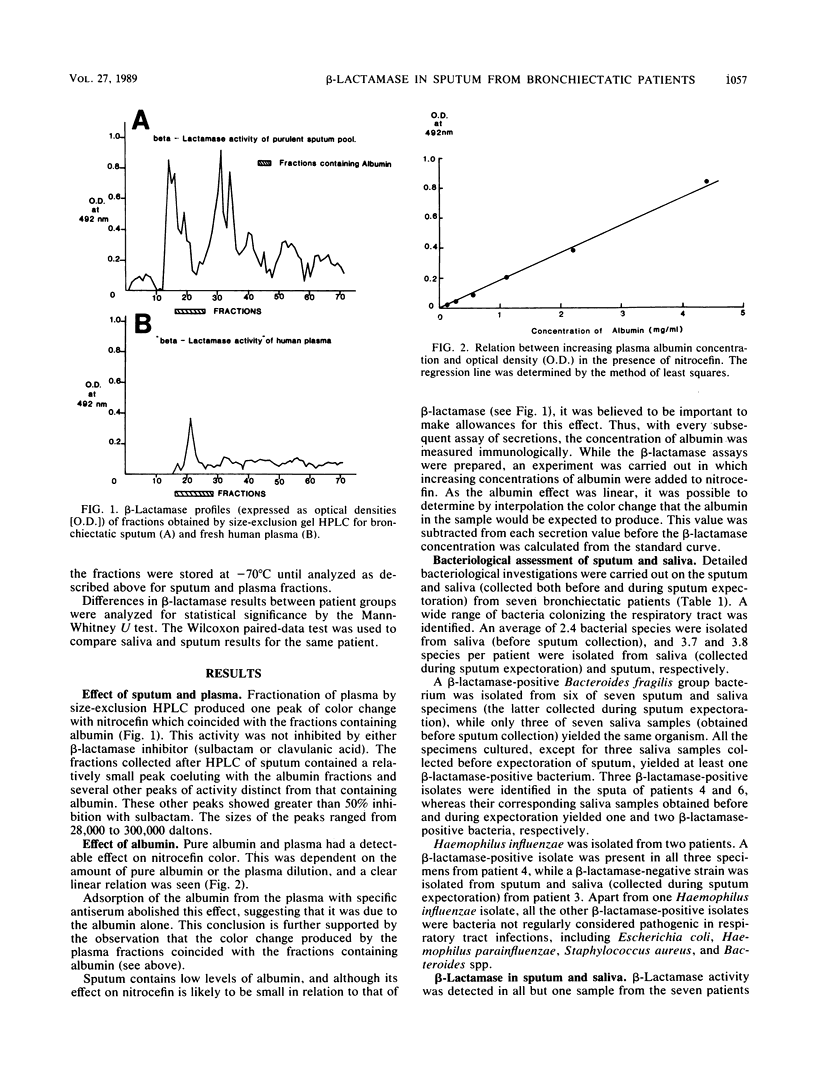
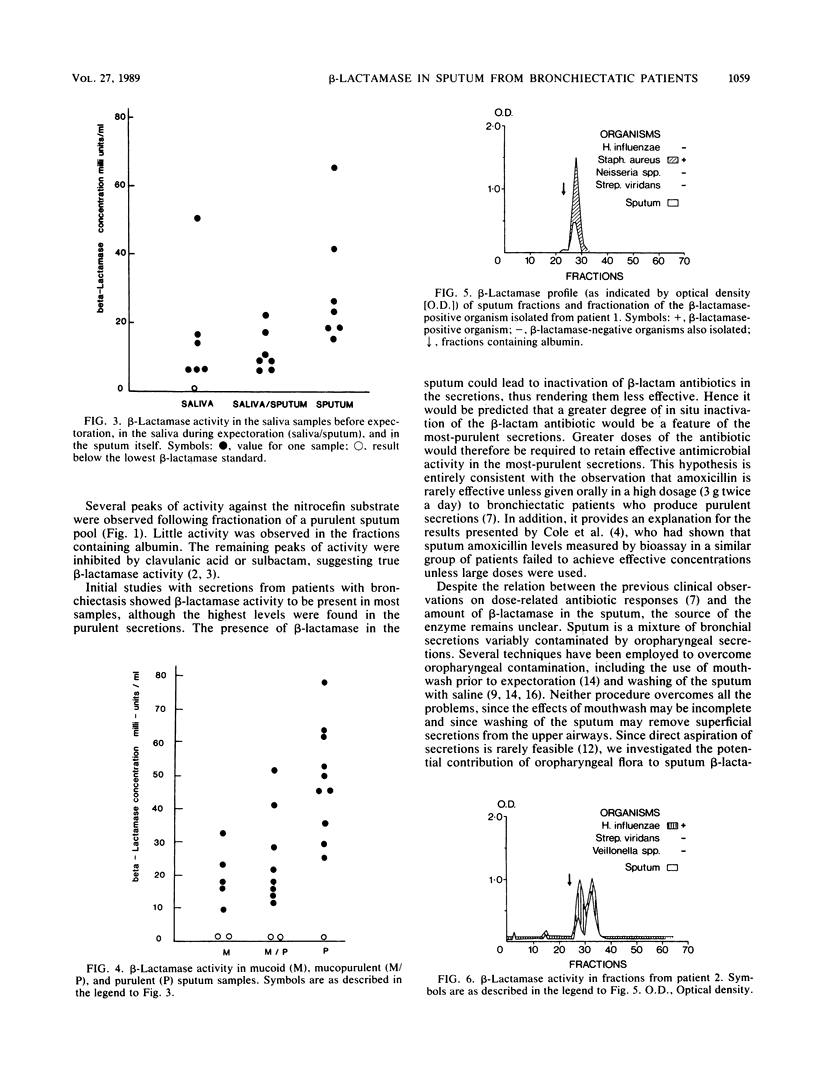
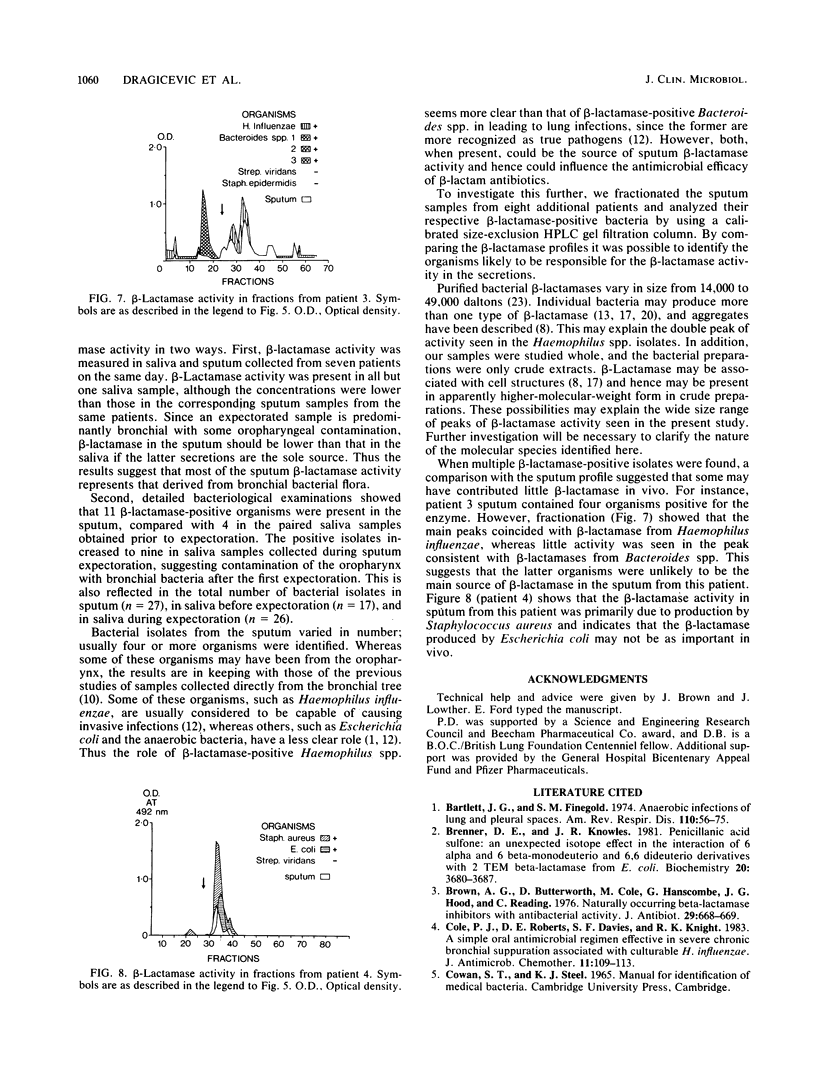
beta-Lactamase activity was measured in secretions from patients with bronchiectasis. Of 28 sputum samples, 23 contained measurable amounts of activity; values were significantly higher (P less than 0.01) in purulent samples than in mucoid or mucopurulent samples. beta-Lactamase activity was usually present in saliva collected before and between sputum expectorations, although values for sputum were higher than for either group of saliva samples (P less than 0.025 and P less than 0.005, respectively). This difference suggests that at least part of sputum beta-lactamase activity originates in the bronchial tree. Detailed microbiological study of a further eight specimens (seven were beta-lactamase positive) led to the isolation of Haemophilus influenzae from six, although only two of these isolates were beta-lactamase positive. Several other beta-lactamase-producing organisms were also isolated, including Staphylococcus aureus (n = 3), Escherichia coli (n = 1), Proteus spp. (n = 1), and Bacteroides spp. (n = 3). Size-exclusion high-performance liquid chromatography of the sputum showed several peaks of beta-lactamase activity which usually coeluted in fractions similar to those of their beta-lactamase-positive isolates. Therefore, sources of sputum beta-lactamases are often bacteria not considered truly pathogenic or not isolated during routine bacteriological assessment. These observations should be considered when embarking on antimicrobial therapy in bronchiectatic patients and suggest that increased dosages of penicillins are indicated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Finegold S. M. Anaerobic infections of the lung and pleural space. Am Rev Respir Dis. 1974 Jul;110(1):56–77. doi: 10.1164/arrd.1974.110.1.56. [DOI] [PubMed] [Google Scholar]
- Brenner D. G., Knowles J. R. Penicillanic acid sulfone: an unexpected isotope effect in the interaction of 6 alpha- and 6 beta-monodeuterio and of 6,6-dideuterio derivatives with RTEM beta-lactamase from Escherichia coli. Biochemistry. 1981 Jun 23;20(13):3680–3687. doi: 10.1021/bi00516a003. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Butterworth D., Cole M., Hanscomb G., Hood J. D., Reading C., Rolinson G. N. Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J Antibiot (Tokyo) 1976 Jun;29(6):668–669. doi: 10.7164/antibiotics.29.668. [DOI] [PubMed] [Google Scholar]
- Cole P. J., Roberts D. E., Davies S. F., Knight R. K. A simple oral antimicrobial regimen effective in severe chronic bronchial suppuration associated with culturable Haemophilus influenzae. J Antimicrob Chemother. 1983 Feb;11(2):109–113. doi: 10.1093/jac/11.2.109. [DOI] [PubMed] [Google Scholar]
- Finegold S. M. Pathogenic anaerobes. Arch Intern Med. 1982 Oct 25;142(11):1988–1992. [PubMed] [Google Scholar]
- Hill S. L., Morrison H. M., Burnett D., Stockley R. A. Short term response of patients with bronchiectasis to treatment with amoxycillin given in standard or high doses orally or by inhalation. Thorax. 1986 Jul;41(7):559–565. doi: 10.1136/thx.41.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenot M., Deschaseaux M. L., Michel-Briand Y., Adessi G. L. Beta-lactamase with high molecular weight: evidence for an interaction with neuraminic acid-containing structures. J Antimicrob Chemother. 1984 Nov;14(5):549–552. doi: 10.1093/jac/14.5.549. [DOI] [PubMed] [Google Scholar]
- LAPINSKI E. M., FLAKAS E. D., TAYLOR B. C. AN EVALUATION OF SOME METHODS FOR CULTURING SPUTUM FROM PATIENTS WITH BRONCHITIS AND EMPHYSEMA. Am Rev Respir Dis. 1964 May;89:760–763. doi: 10.1164/arrd.1964.89.5.760. [DOI] [PubMed] [Google Scholar]
- LEES A. W., MCNAUGHT W. Bacteriology of lower-respiratory-tract secretions, sputum, and upper-respiratory-tract secretions in "normals" and chronic bronchitics. Lancet. 1959 Dec 19;2(7112):1112–1115. doi: 10.1016/s0140-6736(59)90099-6. [DOI] [PubMed] [Google Scholar]
- Maddocks J. L., May J. R. "Indirect pathogenicity" of penicillinase-producing enterobacteria in chronic bronchial infections. Lancet. 1969 Apr 19;1(7599):793–795. doi: 10.1016/s0140-6736(69)92063-7. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A. Beta-lactamases. Br Med Bull. 1984 Jan;40(1):18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- Monroe P. W., Muchmore H. G., Felton F. G., Pirtle J. K. Quantitation of microorganisms in sputum. Appl Microbiol. 1969 Aug;18(2):214–220. doi: 10.1128/am.18.2.214-220.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery K., Raymundo L., Jr, Drew W. L. Chromogenic cephalosporin spot test to detect beta-lactamase in clinically significant bacteria. J Clin Microbiol. 1979 Feb;9(2):205–207. doi: 10.1128/jcm.9.2.205-207.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. G., Medeiros A. A., Yolken R. H., Moxon E. R. Ampicillin treatment failure of apparently beta-lactamase-negative Haemophilus influenzae type b meningitis due to novel beta-lactamase. Lancet. 1981 Nov 7;2(8254):1008–1010. doi: 10.1016/s0140-6736(81)91214-9. [DOI] [PubMed] [Google Scholar]
- Stockley R. A. Bronchiectasis--new therapeutic approaches based on pathogenesis. Clin Chest Med. 1987 Sep;8(3):481–494. [PubMed] [Google Scholar]
- Stockley R. A., Hill S. L., Morrison H. M. Effect of antibiotic treatment on sputum elastase in bronchiectatic outpatients in a stable clinical state. Thorax. 1984 Jun;39(6):414–419. doi: 10.1136/thx.39.6.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Tunér K., Nord C. E. Emergence of beta-lactamase producing anaerobic bacteria in the tonsils during penicillin treatment. Eur J Clin Microbiol. 1986 Aug;5(4):399–404. doi: 10.1007/BF02075694. [DOI] [PubMed] [Google Scholar]


