Abstract
c-Abl is a widely expressed Src family protein tyrosine kinase that is activated by chromosomal translocation in certain human leukemias. While shown in various experimental systems to regulate cell division and stress responses, its biological functions remain poorly understood. Although expressed at similar levels throughout B cell development, we found that the fraction of phosphorylated, active c-Abl peaks at the pro-B stage. We went on to perform a detailed analysis of B cell development in c-Abl-deficient mice. We confirmed a striking but variable decrease in pro- and pre-B cell numbers, a decrease in pre-B cell growth and an increase in pre-B cell apoptosis. This phenotype was not rescued by transgenic expression of a functional IgHC transgene and only partially rescued by the anti-apoptosis gene Bcl-x. Unlike their wild-type counterparts, c-Abl-deficient pre-B cells show a defect in Ca2+ flux upon cross-linking of CD19, a co-receptor known to be involved in pre-B cell receptor signaling and failed to express CD25 on the cell surface. Despite these pre-B cell-signaling defects, selection for in-frame heavy-chain rearrangements was intact in the mutant mice. Remarkably, we were able to rescue the proliferative defect by culturing cells in vitro with large amounts of rIL-7. We conclude that c-Abl is required for normal B cell differentiation and survival.
Keywords: apoptosis, B cell development, c-Abl, CD19, pre-BCR
Introduction
c-Abl and its homolog ARG comprise a unique subgroup of the Src family of non-receptor protein tyrosine kinases (1). c-Abl has been implicated in growth factor responses (2), the regulation of cell proliferation and survival, cytoskeletal organization, cell migration and responses to oxidative stress and DNA damage (1). Alteration of c-Abl structure and function as a consequence of chromosomal translocation (Bcr-Abl, Tel-Abl) results in the production of fusion proteins that bypass the usual stringent regulation of its kinase activity and accounts for a large majority of cases of human chronic myelogenous leukemia and a fraction of acute lymphocytic leukemia (3).
Abl-family kinases are highly conserved in structure and function. They share conserved kinase, actin-binding and DNA-binding domains and proline-rich sequences (4–8). However, c-Abl contains nuclear localization and exports signals in its C-terminus. This distinction suggests critical differences in function involving the shuttling of c-Abl between cytoplasm and nucleus (7, 9). Mice with null mutations in both c-Abl and ARG display embryonic lethality due to high levels of apoptosis and hemorrhage in many tissues (10). However, homozygous disruption of either gene alone results in viable animals, albeit far fewer for c-Abl-deficient mice (10–12). c-Abl-deficient mice are runted, lymphopenic and exhibit thymic and splenic atrophy due to reduced B and T cell populations. c-Abl deficiency also results in abnormal osteoblast maturation, increased susceptibility to infection and post-natal lethality that appears to be partially background strain dependent (13).
Despite being the focus of many studies, the precise physiologic functions of c-Abl remain poorly understood. We have chosen to approach this issue by characterizing the role of c-Abl in the development of B cells in the bone marrow of young adult mice. Developing B cells depend upon a series of growth and survival-promoting membrane receptors while they undergo V(D)J recombination and maturation in the bone marrow. IL-7 is a necessary growth and survival factor for early-B cell development (14, 15). Developing pro-B cells first assemble an Igμ heavy-chain gene by recombination of V, D and J gene segments (16). Productive (in-frame) joins encode clonotypic Igμ heavy chains, which are assembled into the pre-B cell receptor (BCR). In conjunction with the IL-7 receptor, the pre-BCR drives proliferation, survival and maturation of these cells to the small pre-B cell stage (17). It is at this stage where Ig light-chain gene assembly (κ and λ) occurs, leading to IL-7 independence and eventually allowing the BCR to be expressed on immature and mature B cells. CD19, a co-receptor for the pre-BCR and BCR, modulates signaling thresholds at multiple stages of development (18). The BCR remains necessary for B cell survival throughout the subsequent stages of development.
There have been a number of observations implicating a role for c-Abl in lymphocyte function. Transformation of pro-B cells by the v-Abl oncogene results in cells that proliferate indefinitely without growth factor (IL-7) stimulation, but remain arrested in a late-pro-B/early-pre-B-like stage of development. Recently, we demonstrated that inhibition of v-Abl by the small molecule inhibitor, STI-571 (Gleevec), drives partial maturation of these cells to a small pre-B cell-like stage in which RAG expression is up-regulated and Ig light-chain gene rearrangement occurs (19). c-Abl is also phosphorylated downstream of BCR/CD19 and TCR/LAT signaling and loss of c-Abl hampers optimal antigen receptor signaling (20, 21). Previous studies of B cell development in the c-Abl null mouse showed a significant but highly variable decrease of cells at the pro-B and pre-B stages, increased rates of apoptosis in vitro and in vivo and a potential defect in IgHC gene assembly (22–24). This phenotype was shown to be cell autonomous since adoptive transfer of adult bone marrow from mutant mice into irradiated wild-type mice recapitulated the defect in B cell development (23). Interestingly, transfer of fetal liver progenitors in a similar experiment failed to show any defect.
Here, we confirm that c-Abl is required for normal progression to the pre-B cell stage of development. Although we find that c-Abl protein is expressed at similar levels throughout B cell development, kinase activity is maximal at the pro-B cell stage. Using pharmacologic inhibition and c-Abl-deficient mice, we observed dramatic reduction of the small pre-BII cell population in vivo. These included far fewer intracellular μ+ pre-B cells. Transgenic expression of an IgHC fails to rescue this defect, however. Early- (large) and late-pre-B cells exhibited reduced fractions of IL-7 receptor α (IL-7Rα)-expressing cells and absent CD25 expression. c-Abl-deficient pre-B cells display reduced proliferation and increased levels of apoptotic cells in vivo. However, when c-Abl-deficient cells are cultured in vitro with exogenous IL-7, they hyperproliferate and become large in size. We argue based on our results that mutation of c-Abl interferes with multiple overlapping receptor signaling pathways that might account for many aspects of the mutant phenotype.
Methods
Mice
c-Abl m1/m1 mice (B6/129 background) (11) were the kind gift of Anthony Koleske (Yale University). Human Igμ transgenic (25) mice were from Michel Nussenzweig and p53−/− mice (26) were the gift of David Raulet. Mice were used at 6–10 weeks of age and were housed in the sterile barrier facility at University of California, Berkeley. All animal experiments were done in accordance with the University of California, Berkeley Animal Care and Use Committee guidelines.
Reagents
rIL-7 was obtained from the culture supernatant of an IL-7 cDNA-transfected B lymphoma cell line (a gift from Barbara Kee). Cells were cultured for 5 days at high density then removed by centrifugation. Culture supernatants were filtered using a 0.2-μM filter and this IL-7 supernatant was used at between 1:10 and 1:500 dilutions. The c-Abl inhibitor, STI-571 (Gleevec) (Novartis, East Hanover, NJ, USA), was prepared as a 10 mM stock solution by dissolving 5 mg of STI-571 per ml of PBS and filtering through a 0.2-μM filter for sterilization. Control and p53−/− mice were injected intraperitoneally with 45 mg kg−1 STI-571 twice per day for 7 days.
PCR fragment length polymorphism assay
Pro-B and pre-B cells were sorted from IgM-depleted wild-type and c-Abl-deficient bone marrow using a MoFlo (Dako, Baar, Switzerland) cell sorter. Genomic DNA was isolated and two sequential PCRs were performed using primers hybridizing to VH558 family gene segments and JH2. The first round PCR used primers VH558-FR1 and JHA. One microliter of the first round reaction was used in a second round reaction with internal primers VH558-FR3 and JHB3 which was 32P labeled. Cycling conditions were 94°C for 1 min, 66°C for 2.5 min (20 cycles) and 72°C for 10 min. Primer sequences include the following: JHA 5′-TGCAGACTTCAAGCTTCAGTTCTGG-3′, VH558-FR1 5′-ARGCCTGGGRCTTCAGTGAAG-3′, VH558-FR3 5′-CTGACWTCTGAGRACTCYGCRGTCYATT-3′ and JHB3 5′-ACACACATTTCCCCCCCAACAAA-3′. Genomic DNA from wild-type whole spleen was used as a control.
Flow cytometry
Bone marrow was harvested from the femurs and tibiae of c-Abl m1/m1 and control mice. Red blood cells were removed by ACK lysis and cells were re-suspended in staining buffer (1% BSA, 1× PBS). Monoclonal antibodies B220-PE, Cyc (RA3-6B2), B220-PETR (RM217), IgM–FITC (II/41), IgM-PE (1B4B1), CD43-biotin (S7), 5-bromo-2-deoxyuridine (BrdU)–FITC, CD25-PE, IL-7Rα-PE (SB/14), c-kit-TriColor, Sca-1-FITC (D7), HSA (M1/69) Lineage panel biotin [B220, Ly-6G (GR-1), CD3e, CD11b (MAC-1), TER-119/Ly-76], AA4.1-FITC, SA-Cyc, SA-613, CD19-PE, CD19 F(ab)2 and E47-PE were from BD Biosciences and Caltag. Expression on progenitor populations was monitored by Coulter XL FACs machine and analyzed using FlowJo software. Phosphorylated c-Abl was detected using anti-phospho-cAbl (Y245 and Y412) antibodies (Cell Signaling Technologies) with an anti-rabbit IgG F(ab′)2-PE secondary. Bone marrow cells were harvested and immediately re-suspended in sodium vandate and sodium fluoride to inhibit phosphatase activity. Cells were then fixed in 2% PFA, permeabilized in 0.05% saponin and then stained with appropriate antibodies or controls (normal rabbit serum).
In vitro culture
Short-term bone marrow culture.
Control and c-Abl m1/m1 bone marrow cells were isolated as above from four hind leg and two front leg bones. Red blood cells were removed by ACK lysis, and cells were then cultured in RPMI supplemented with 10% FCS, antibiotics, 50 μM β-ME, IL-7 supernatant (1:50) for 3–4 days. Cells were then stained for developmentally regulated markers and anti-BrdU or phosphatidylinositol (PI) for proliferation and cell cycle analysis.
Ca2+ flux
Bone marrow cells were harvested as above. IgM+ B cells were depleted by using anti-IgM-PE and anti-PE paramagnetic beads (Miltenyi). Cells were then loaded with Indo-1 dye (Molecular Probes, Invitrogen) and then stained with anti-B220, anti-CD43 and anti-CD19 [F(ab′)2] biotin. Following 2–3 min of baseline fluorescence readings, cells were removed from the Coulter Flow Cytometer and CD19 was cross-linked using streptavidin (10 μg ml−1) or cells were treated with ionomycin (1 μM) before replacing the cells in the cytometer and monitoring them for a total of 10–15 min. Calcium flux was then analyzed using FlowJo software by gating on the pre-B cell population in each sample (B220+ CD43−).
Apoptosis assays
Bone marrow was harvested as above. Cells were stained using the cell-permeable APO LOGIX Carboxyfluorescein caspase-3 detection kit (FAM-DEVD-FMK) from Cell Technology (Mountain View, CA, USA). Cell populations were detected using the cell surface marker reagents outlined above.
Proliferation assays and cell cycle analysis
In vivo proliferation was monitored by peritoneal injection of BrdU (1 mg). Bone marrow cells were harvested 4 h later and prepared for flow cytometry. Briefly, cells were fixed in 2% PFA, permeabilized in 0.25% Tween 20/1× PBS and then treated with DNaseI (50 U ml−1) in DNaseI buffer [40 mM Tris–HCl (pH 7.9), 10 mM NaCl, 6 mM MgCl2 and 10 mM CaCl2]. Cells were then stained with anti-BrdU–FITC (BD Biosciences) and anti-B220, CD43 and IgM antibodies (outlined above). In vitro proliferation was monitored by treating cultured cells with 10 μg ml−1 BrdU for 10 min on day 4 of IL-7 culture and then cells were prepared as described above. For cell cycle analysis, cultured cells were fixed and permeabilized as above and then stained with PI (10 μg ml−1) to determine DNA content after 4 days in culture with IL-7.
Results
c-Abl expression and activity during early-B cell development
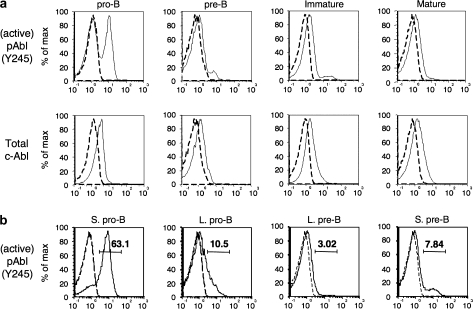
c-Abl is ubiquitously expressed but its kinase activity is tightly regulated by phosphorylation. As a first approach to understanding c-Abl's role in lymphopoiesis, we used multiparameter flow cytometry to measure c-Abl expression and activity at various stages of B cell development. While c-Abl protein is expressed at similar low levels throughout B cell development, we observed high levels of c-Abl Y245 phosphorylation in small pro-B cells that are actively performing V-to-DJ rearrangement (Fig. 1). Phosphorylation at Y245 was shown to be required for full activation of c-Abl kinase activity (27). Phosphorylation decreased to levels just above control in all later stages of development when c-Abl is thought to be responsive to Ig and growth factor signaling (20).
Fig. 1.
c-Abl expression and phosphorylation state during early-B cell development. (A) Intracellular flow cytometric analysis of active (Y245, upper) and total (lower) c-Abl in bone marrow pro- (B220+ CD43+ sIg−), pre- (B220+ CD43− sIg−), immature (IgMhi IgDlo) and mature (IgDhi IgMlo) bone marrow B cells. The dashed line in each histogram represents control antibody staining. (B) Staining for active c-Abl (Y245) gated on small pro-B (B220+ CD43+ FSlo), large pro-B (B220+ CD43+ FShi), large pre-B (B220+ CD43− FShi) and small pre-B cells (B220+ CD43− FSlo). Numbers indicate percentages of cells above the background established by control antibody staining in each case (dashed lines). This data are representative of at least three experiments on a total of six mice.
Early-lymphoid commitment and the pro-B to pre-B transition are impaired in the bone marrow of c-Abl m1/m1 mice
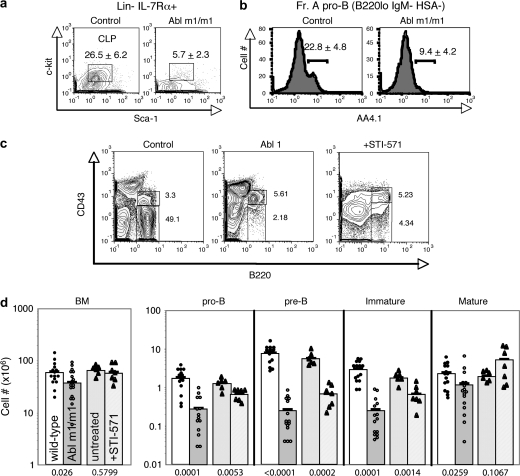
In an attempt to identify specific processes dependent upon c-Abl, we used flow cytometry to compare bone marrow B cell progenitor populations in wild-type and c-Abl m1/m1 mice. This targeted mutant expresses a small amount of a truncated version of c-Abl (11). Common lymphoid progenitors (CLPs) and AA4.1+ Fraction A pro-B cells are two of the earliest progenitor fractions preceding classic B cell development as defined by the rearrangement of heavy- and light-chain Ig loci (28). We observed a dramatic reduction in CLP (Fig. 2A, Supplementary Figure 1C is available at International Immunology Online) and AA4.1+ Fraction A pro-B cells (Fig. 2B, Supplementary Figure 1D is available at International Immunology Online) in c-Abl-deficient mice. A similar defect in Fraction A cells defined using different markers was reported previously (23). These results suggest that c-Abl plays an important role in the division, survival or differentiation of these cell populations early in development. Hematopoietic stem cell levels were relatively normal (Supplementary Figure 1A and 1B is available at International Immunology Online).
Fig. 2.
Flow cytometric analysis of B cell development in c-Abl m1/m1 mice. (a and b) The indicated reagents and gates were used to compare the numbers of CLPs (Lin−, IL7Rα+, c-kitlo, sca-1lo) and Fraction A pro-B cells (B220lo, IgM−, HSA−, AA4.1+) in wild-type and c-Abl m1/m1 mouse bone marrow. The numbers indicate average percentage and standard deviation of displayed cells from sets of eight mice. (c) Analysis of pro- and pre-B cells in bone marrow cells from wild-type and c-Abl m1/m1 mice and a wild-type mouse treated with STI-571 for 7 days prior to analysis. Data were gated on forward and side scatter and sIgM+ cells were excluded. Numbers indicate percentage of cells in pro-B (B220+ CD43+) and pre-B (B220+ CD43−) fractions. (d) Total number of bone marrow cells in the indicated populations from wild-type and c-Abl m1/m1 mice and from wild-type mice treated for 7 days with STI-571. Each symbol represents data from an individual mouse and the numbers below each pair indicate P-values of the differences in each set (WT versus c-Abl m1/m1 or WT versus STI-571 treated).
Previous analyses of c-Abl-deficient mice noted defects in pro-B and pre-B cell numbers (11, 12, 23). Pro-B and pre-B cells are most clearly distinguished by the expression of CD43 and intracellular Igμ heavy-chain (icμ). As pro-B cells (B220+ IgM− CD43+ icμ−) successfully rearrange the Ig heavy-chain locus, heavy-chain μ protein is expressed in a complex with the surrogate light chain on the cell surface as the pre-BCR (17). Subsequent signaling from this receptor in part drives proliferation and differentiation to the pre-B cell stage (B220+ IgM− CD43− icμ+).
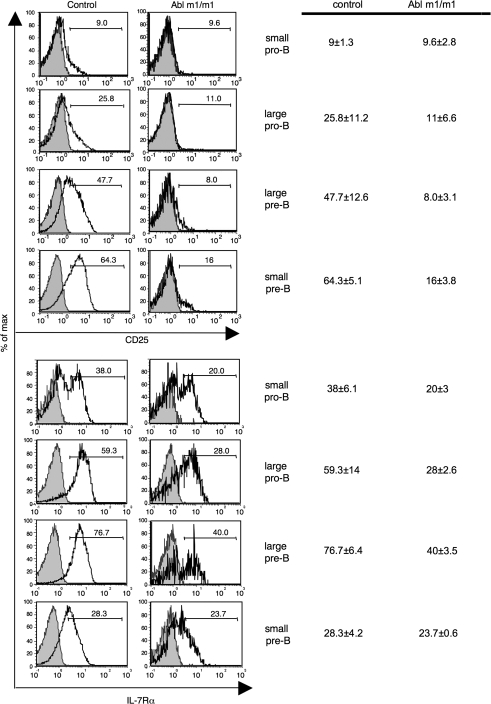
In agreement with earlier studies, we found that c-Abl m1/m1 and STI-571-treated wild-type bone marrow exhibit reduced pre-B cellularity (Fig. 2C and D; Supplementary Figure 2 is available at International Immunology Online). This deficiency is most pronounced at the late small pre-B cells stage (B220+ CD43−icμ+ FSClo) (Fig. 2C, lower) but is highly variable for unknown reasons (Fig. 2D). Although a few pre-B cells (CD43− icμ+) do emerge, they are abnormal in that they do not up-regulate CD25 (Fig. 3, top), a marker of pre-B cell maturation (29). In addition, fewer c-Abl m1/m1 cells express high levels of IL-7Rα at each stage across the pro-to-pre-B cell transition (Fig. 3, bottom). Thus, there are both quantitative and qualitative defects in the pro-to-pre-B cell transition.
Fig. 3.
CD25 (upper) or IL-7Rα (lower) expression on the surface of wild-type and c-Abl m1/m1 bone marrow pro- and pre-B cells (defined as in Fig. 1B). CD25 or IL-7Rα, unfilled; isotype control, gray. Numbers denote the percentage of cells in the indicated gate. The chart to the right indicates the mean and standard deviation of a set of three to four mice of each genotype.
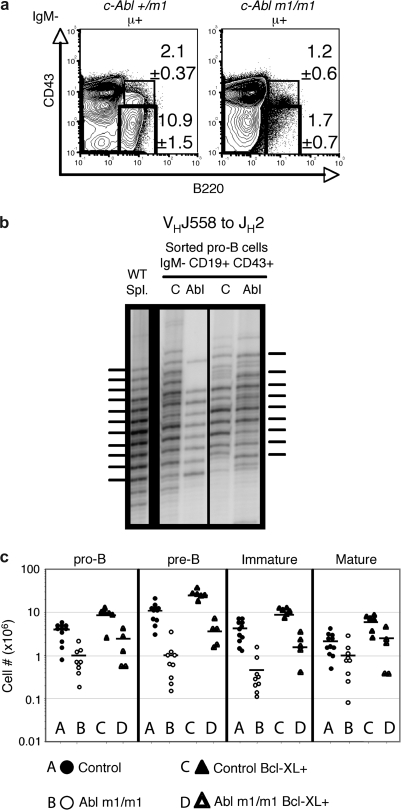
A recent report suggested that IgHC V-to-DJ rearrangement may be defective in c-Abl null mice (24). To address the possibility that the c-Abl m1/m1 phenotype is due solely to inefficient IgHC gene assembly, we crossed an Igμ transgene onto the c-Abl m1/m1 background (25). We observed the same pre-B cell deficit even in the setting of heavy-chain transgene expression (Fig. 4A). Therefore, the primary defect in c-Abl-deficient mice occurs irrespective of Ig heavy-chain expression. To determine whether c-Abl is required for normal pre-BCR-dependent Ig heavy-chain gene selection, we isolated genomic DNA from sorted pro- and early-pre-B cells (IgM− CD19+ CD43+) and performed a PCR CDR3 fragment length polymorphism assay using primers hybridizing to VH558-family gene segments and JH2. If this population has undergone selection for IgHC expression, pre-B cell proliferation or selective survival enriches the population for in-frame alleles (over-representation of every third nucleotide in the distribution of CDR3 lengths) as shown by the splenic B cell DNA control (Fig. 4B, left). Enrichment for in-frame heavy-chain alleles was observed in both control and c-Abl m1/m1 pro-B/early-pre-B cell populations suggesting that the c-Abl defect does not interfere with the ability of pre-B cells to assemble a functional pre-BCR or for the pre-BCR to promote either proliferation or survival.
Fig. 4.
Partially defective pre-BCR function in c-Abl m1/m1 mice. (a) Flow cytometric comparison of pro- (B220+ CD43+ sIgM−) and pre-B (B220+ CD43− sIgM−) cell populations in c-Abl +/m1 hμ transgenic and m1/m1 hμ transgenic mice. The numbers indicate percentages and standard errors of total IgM− cells in the indicated gates from a set of four mice of each genotype. (b) PCR fragment length polymorphism assay. Analysis of reading frame selection in VH558 to JH2 rearrangements in sorted pro-/early-pre-B cells (IgM− CD43+ CD19+) from two control (c) and two c-Abl m1/m1 (Abl) mice. Wild-type spleen is shown for comparison. A phosphorimage of a denaturing polyacrylamide gel is shown with the horizontal lines indicating positions of in-frame V(D)J junctions. (c) Numbers of pro- (B220+ CD43+ sIg−), pre- (B220+ CD43− sIg−), immature (IgMhi IgDlo) and mature (IgDhi IgMlo) bone marrow B cells in groups of individual mice of the indicated genotypes. A, control; B, c-Abl m1/m1; C, Control Bcl-xL transgenic and D, c-Abl m1/m1 Bcl-xL transgenic.
In addition to activating early-pre-B cell proliferation, assembly of a pre-BCR increases the expression of anti-apoptosis factors such as Bcl-xL (30). To determine whether decreased pre-B cell numbers might be due to failure to activate anti-apoptosis gene expression, we bred a Bcl-xL transgene onto the c-Abl m1/m1 genetic background (30). As shown in Fig. 4(C), transgenic Bcl-xL expression partially rescues the quantitative defect in B cell development expressed by c-Abl m1/m1 mice. Since the numbers of wild-type developing B cells are also increased due to forced Bcl-xL over-expression, the partial rescue of cell numbers observed in c-Abl m1/m1 mice might not reflect an aspect of the mutant phenotype.
c-Abl m1/m1 progenitor B cells exhibit proliferative and apoptotic defects in vivo
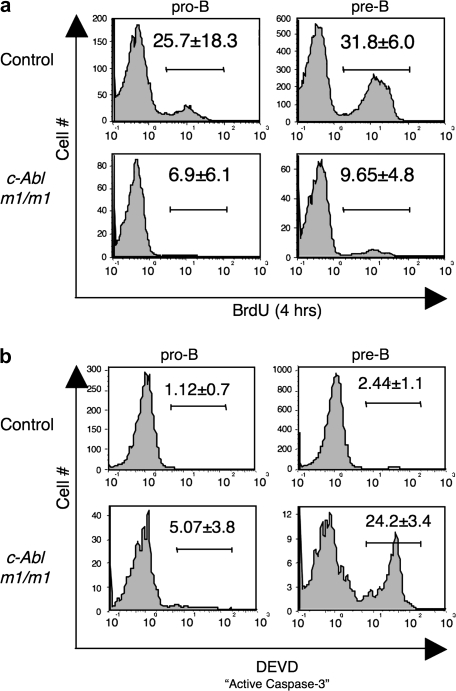
To test whether c-Abl m1/m1 mice have a defect in pre-BCR-mediated proliferation, we injected mice with BrdU and harvested bone marrow 4 h later to monitor the extent of proliferation at the pro-to-pre-B cell transition in vivo by flow cytometry. Wild-type pro-B (B220+ IgM− CD43+) and pre-B (B220+ IgM− CD43−) cells incorporated BrdU to expected levels; however, BrdU incorporation in c-Abl-deficient pro-B and pre-B cells was dramatically reduced (Fig. 5A). Since we observed normal heavy-chain selection in c-Abl m1/m1 mutants, it remains possible that significant proliferation is occurring in vivo in c-Abl m1/m1 mice, but BrdU+ cells might not be observed if dividing cells are frequently undergoing apoptosis, as apoptotic cells are cleared quickly from the bone marrow microenvironment. Alternatively, heavy-chain selection may be due to an intact survival signal in c-Abl m1/m1 mice.
Fig. 5.
c-Abl m1/m1 pro-B and pre-B cells exhibit diminished proliferation and increased apoptosis in vivo. (a) Mice were injected with BrdU and bone marrow cells were harvested 4 h later. Pro-B and pre-B cell populations (defined as in Fig. 1A) were analyzed for BrdU incorporation. A representative analysis is shown and numbers represent the average percentage of cells that have incorporated BrdU and the standard deviation from a set of six or seven mice of each genotype. (b) Bone marrow cells were stained with cell surface markers and caspase-3 detection reagent (DEVD-FITC) to assess the percentage of pro- and pre-B cells (defined as in Fig. 1A) undergoing apoptosis. The data are representative of three mice of each genotype with the numbers representing average percentage of cells in the indicated gate and standard deviation.
We assayed apoptosis occurring in vivo by analyzing pro- and pre-B cells immediately ex vivo for their levels of active caspase-3 using a fluorogenic substrate. c-Abl m1/m1 mice displayed a modest increase in apoptosis at the pro-B stage and a striking increase at the pre-B cell stage where the primary defect is observed (Fig. 5B). Since c-Abl has been implicated in the cellular response to DNA damage and pre-B cells contain double-stranded DNA (dsDNA) breaks in rearranging IgLC genes (31), we went on to ask whether pre-B cell apoptosis in the absence of c-Abl depends upon p53 activity. Since c-Abl m1/m1 p53−/− mice exhibit embryonic lethality (32), we treated p53−/− mice with STI-571. Interestingly, we found that the c-Abl null phenotype was not dependent on p53 (Supplementary Figure 3 is available at International Immunology Online).
c-Abl m1/m1 pre-B cells fail to flux calcium in response to anti-CD19 cross-linking
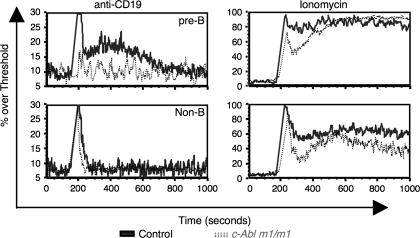
c-Abl has been shown to interact with and phosphorylate CD19 after BCR cross-linking (21). Cross-linking of CD19 induces calcium flux in pre-B and mature B cells and is thought to contribute to the magnitude of pre-BCR/BCR signaling (33, 34). To investigate the role of c-Abl in pre-B cell signaling, we cross-linked CD19 on the surface of bone marrow pre-B cells and examined the ability of cells to flux calcium. Both control and c-Abl m1/m1 cells responded similarly to ionomycin demonstrating equal loading and capacity to flux calcium (Fig. 6, right). As expected, non-B cells (CD19−) did not respond to anti-CD19 cross-linking (Fig. 6, bottom left) while a significant percentage of wild-type pre-B cells (B220+ IgM− CD43−) cells responded with a prolonged calcium flux. c-Abl-deficient pre-B cells, however, did not respond to CD19 cross-linking (Fig. 6, top left). The inability to flux calcium was not due to the absence of CD19 expression since CD19 was detected on c-Abl-deficient pro-B and pre-B cells (data not shown). Interestingly, CD19 expression was generally higher in c-Abl-deficient small pre-B cells than control. We have observed that CD19 is down-regulated following pre-BCR signaling; however, this was often not the case in the absence of c-Abl (data not shown) (33).
Fig. 6.
c-Abl m1/m1 pre-B cells fail to flux calcium in response to anti-CD19 cross-linking. (a) IgM-depleted bone marrow was stained with anti-B220, anti-CD43 and biotinylated anti-CD19 antibody [F(ab′)2 fragment]. Cells were then stimulated with streptavidin and calcium flux was monitored for up to 15 min within the population of B220+ CD43− pre-B cells. Ionomycin treatment was used as a control for Indo-1 loading and overall responsiveness of the cells. Wild-type, solid line; c-Abl m1/m1, dotted lines. Data are representative of three independent experiments on individual pairs of mice.
IL-7 causes hyperproliferation, partial differentiation and increased apoptosis in cultured c-Abl-deficient B cell progenitors
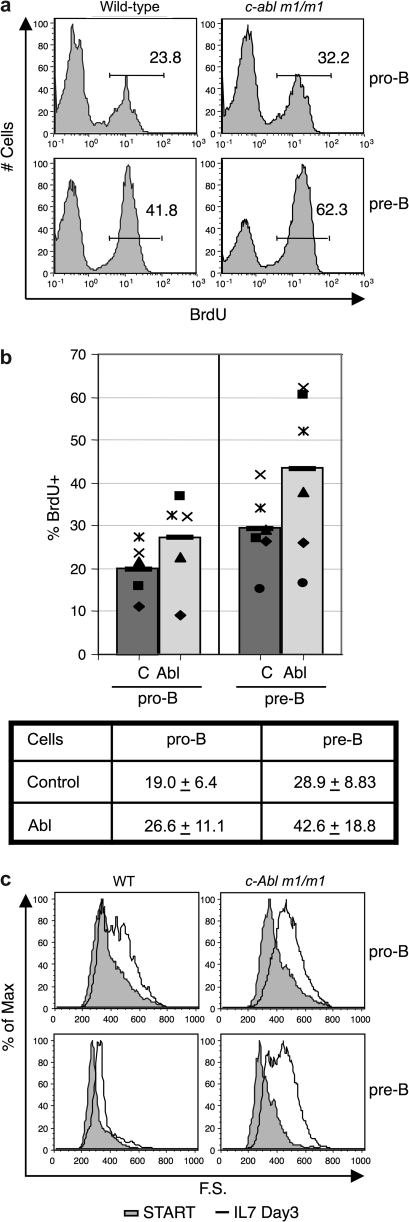
To assess the extent to which saturating amounts of IL-7 might rescue the c-Abl m1/m1 phenotype, we used a short-term primary cell culture system in which wild-type and c-Abl-deficient bone marrow cells were cultured with rIL-7 supernatant (35). Using a slightly different culture system, a previous study showed a highly variable response of mutant cells to rIL-7 (23). We found that c-Abl m1/m1 progenitor B cells proliferated to a significantly greater extent than wild-type progenitors in vitro as assessed by BrdU incorporation after 3–4 days in culture with rIL-7 (Fig. 7A and B). c-Abl deficiency may relieve a certain level of control on pre-B cell-cycle progression resulting in hyperproliferation in response to mitogenic stimuli such as IL-7. Early-pre-B cells become large in response to pre-BCR signaling correlating with cell proliferation. Interestingly, c-Abl-deficient pre-B cells were significantly larger than the control after 3–4 days in IL-7 culture (Fig. 7C) suggesting a defect in the regulation of cell growth or cell cycle exit. In similar experiments, we observed a subtle hyperproliferative response of mature B cells to anti-IgM stimulation, which was also accompanied by higher levels of cell death in the absence of c-Abl (data not shown). Although the c-Abl m1/m1 phenotype is variable, consistent hyperproliferation was observed in five independent experiments. Thus, IL-7 receptor signaling is intact in c-Abl null pro- and pre-B cells but is abnormal.
Fig. 7.
c-Abl m1/m1 pro-B/pre-B cells hyperproliferate and fail to exit the cell cycle when cultured with rIL-7 in vitro. (a) Representative data from analysis of pro-B and pre-B proliferation by BrdU incorporation. At the end of 3–4 days in culture, BrdU was added to the culture of either wild-type or c-Abl m1/m1 bone marrow for 10 min prior to cell harvest and analysis. (b) Variability of BrdU incorporation. Data are plotted from the analysis in A of a series of six individual control and c-Abl m1/m1 mice. Bars indicate mean values and corresponding symbols denote individual littermate mice. Averages and standard deviations are shown from data derived from six pairs of mutant and wild-type mice. (c) Size of pro-B and pre-B cells before (START) and following culture in IL-7 for 3 days as monitored by forward scatter. Wild-type, left panels; c-Abl m1/m1, right panels. Pro-B cells were B220+ CD43+ IgM− and pre-B cells were B220+ CD43− IgM−. The data are representative of six pairs of mice.
Discussion
Despite a large number of studies, a clear delineation of the physiological roles of the proto-oncogene c-Abl remains elusive. c-Abl can either increase or inhibit cell proliferation depending upon the stimulus and cellular context (1). Over-expression of c-Abl in fibroblasts induces cell cycle arrest, an effect that requires its kinase activity, nuclear localization signal, p53 and Rb (8, 36, 37). Over-expression of kinase-dead c-Abl (K290R) results in increased cell cycle re-entry with addition of serum to the culture (37), while over-expression of wild-type c-Abl inhibited S-phase entry (38). These results suggest that in some contexts, c-Abl acts as a negative regulator of growth and proliferation. However, other studies suggest that c-Abl enhances cell proliferation. Inhibition of c-Abl via knockout or anti-sense oligonucleotides resulted in diminished numbers of hematopoietic cells in S phase (39), a 4-h delay in S-phase entry in response to platelet-derived growth factor (PDGF) (2) and a reduced growth rate of c-Abl anti-sense-treated NIH-3T3 cells (40). Of particular interest with regard to B cell development, c-Abl also plays a role in the cellular response to DNA damage (38, 41).
c-Abl is expressed widely in hematopoietic, neural and many other cell types. Initial characterization of targeted c-Abl mutations in mice revealed runting, shortened lifespan, splenic atrophy and variable degrees of hematopoietic pancytopenia (11–13, 23). In the B lineage, defects were noted in the numbers and viability of pre-B cells and earlier progenitors but the phenotype was of variable penetrance (23). The B cell defect was found to be independent of any effects the mutation might have on stromal elements since c-Abl null bone marrow transplanted into wild-type mice recapitulated the mutant B cell phenotype (23).
c-Abl has been reported to play a role in signaling by both the TCR and the BCR. In T cells, c-Abl activity was shown to be required for tonic TCR signaling which was necessary to maintain the inactivity of RAG gene expression (20) and for pre-TCR signaling required for developmental progression from the double-negative to the double-positive stage (42). In B cells, c-Abl is required for normal proliferation following BCR cross-linking, perhaps due to its ability to bind and phosphorylate the cytoplasmic tail of the BCR co-receptor CD19 (21).
We detected a previously unreported defect in c-Abl mutant mice at the earliest stages of B cell development. Mutant mice showed decreased numbers of CLPs in addition to Fraction A pro-B cells that had been reported previously [Fig. 2 and (23)]. This is noteworthy also because our analysis of c-Abl kinase activity in wild-type mice (assessed by staining with a phosphorylation state-specific anti-c-Abl antibody) showed the greatest fraction of active Abl kinase at the early-pro-B stage of development (Fig. 1). It is possible that c-Abl may function downstream of either the IL-7 receptor or some other growth factor receptor necessary to support the growth or viability of pro-B cells. Previous studies have shown that c-Abl is activated during epidermal growth factor and PDGF signaling (2). Perhaps, c-Abl is downstream of a similar growth factor receptor in CLPs or pro-B cells accounting for this early-lineage defect. It was shown previously that RAG expression and V(D)J recombination occur in cells during the Go/G1 phase of the cell cycle (31, 43). As noted above, active c-Abl can prevent cell division. Thus, it is possible that c-Abl plays a role in suppressing the induction of cell division by IL-7 while enhancing its anti-apoptotic activity during the period of IgHC gene assembly.
We found that c-Abl mutant pre-B cells undergo less proliferation and more apoptosis than their wild-type counterparts, particularly at the pre-B cell stage (Fig. 4). This was previously observed by other groups (23, 24, 44). Developing pro-B and early-pre-B cells depend in large part on IL-7 for their viability and normal numbers (15). Upon successful IgHC gene assembly, early-pre-B cells express a clonotypic IgHC along with surrogate lightchains in a signaling complex known as the pre-BCR. Pre-BCR signaling results in both a burst of proliferation and a diminished requirement for IL-7 (15). This is accompanied by a decreased level of IL-7Rα expression at the small pre-B cell stage. CD19 also plays an important role during the pro-to-pre-B cell transition presumably through its interaction with the pre-BCR (34).
We suggest that the defect in pre-B cells in c-Abl mutant mice may be a consequence of diminished or defective pre-BCR signaling due at least in part to a defect in CD19 function. As shown in Fig. 6, we found that while anti-CD19 cross-linking results in robust Ca2+ mobilization in wild-type pre-B cells, there is little or no Ca2+ response to cross-linking in c-Abl mutant pre-B cells. This CD19 signaling defect may be related to c-Abl's ability to bind to and phosphorylate CD19’s cytoplasmic domain (21) or perhaps to compete with other factors for binding. It is interesting to note that both CD19 knockout and c-Abl-deficient B cells have been reported to exhibit reduced BCR responses in vitro (18, 21). Pre-BCR function is not completely absent in the setting of c-Abl mutation since we observed selection for in-frame IgHC V(D)J rearrangements (Fig. 4) but we failed to observe the activation of CD25 expression normally dependent on pre-BCR expression [Fig. 3; (29)]. CD25 expression in T lineage cells is regulated by NF-AT, NF-κB, HMG-I(Y) and STAT5 among other factors (45). Experiments are currently underway to test whether these factors are appropriately regulated in pre-B cells in the absence of c-Abl. We are also using gene expression microarrays to probe the breadth of the disruption in the pre-B cell transcriptional program in the setting of c-Abl deficiency.
In addition, there may be a qualitative or quantitative defect in IL-7 receptor signaling. We suggest this for two reasons. First, we observed increased heterogeneity in IL-7Rα expression levels in the c-Abl mutant cells at the pro- and pre-B cells stages (Fig. 3). Normal IL-7 receptor signaling is known to increase receptor levels (15). Second, we were able to induce enhanced proliferation in c-Abl m1/m1 pro-B cells in an in vitro bone marrow cultures in the presence of high doses of rIL-7 (Fig. 7). Despite a striking increase in pre-B cell numbers, these cells were not normal in that they frequently underwent apoptosis and they may have failed to properly exit the cell cycle based on their large size and increased BrdU incorporation (Fig. 7). Early-pre-B cells are very sensitive to and require low levels of IL-7 for proliferation (46). Pro-B cells unable to assemble a pre-BCR can be rescued by high doses of IL-7, much the same way high doses of rIL-7 can rescue pre-B cell numbers in c-Abl m1/m1 cultures (data not shown). Thus, if c-Abl-deficient pre-B cells show diminished pre-BCR-signaling activity due to a CD19-signaling defect, they might require super-physiological levels of IL-7 for proliferation.
c-Abl has been linked to many well-characterized regulators of B cell development. c-Abl phosphorylates Btk and PLCγ which have documented roles in pre-B cell proliferation and survival (47, 48). Interestingly, Btk−/− BLNK−/− mice have a similar reduction in pre-B cells and a hyperproliferative phenotype (49, 50). It is possible that c-Abl plays an important role in the pathway suppressing inappropriate pro- or pre-B cell proliferation. It is interesting to note, however, that while Btk−/− BLNK−/− mice develop B lymphoid malignancy, no such observation has been made with the c-Abl-deficient mice. The vast majority of c-Abl-deficient mice do not live past 8 weeks; however, so malignancy might not be observed due to early lethality.
We tested two other potential mechanisms for the c-Abl mutant phenotype. First, we asked whether c-Abl, perhaps because of its involvement in the DNA damage response, was required for efficient IgHC gene assembly. Ineffective V(D)J recombination or unrepaired dsDNA breaks introduced by the recombinase might result in increased cell death and thus diminished pre-B cell numbers. We bred an IgHC transgene onto the c-Abl mutant background and found that it failed to rescue the phenotype (Fig. 4). This result is in conflict with a recent report which suggested that ineffective V-to-DJ joining might be involved in the c-Abl mutant phenotype (24). We were unable to reproduce this result (data not shown). This together with the failure of a complete IgHC transgene to complement the c-Abl mutant phenotype makes a V(D)J recombination defect an unlikely explanation for the c-Abl m1/m1 phenotype.
Increased susceptibility to apoptosis as a result of c-Abl deficiency in cell lines is well documented (22, 38). In addition, we observed a striking and rapid decrease in pre-B cell numbers in the bone marrow of mice treated with the Abl kinase inhibitor, STI-571, again indicative of apoptosis (Fig. 2). Therefore, we went on to test the idea that failure to activate Bcl-2 family anti-apoptosis genes might contribute to diminished pre-B cell numbers in c-Abl mutant mice. Since we found that c-Abl mutant pre-B cells failed to activate other developmentally regulated genes such as CD25 (Fig. 3), this was a worthwhile possibility to consider. Bcl-xL is the family member normally expressed at the pro-to-pre-B cell transition (30). Using a transgenic approach, we found that enforced Bcl-xL expression increased pre-B cell numbers in c-Abl mutant as well as wild-type mice (Fig. 4). Thus, it remains uncertain whether anti-apoptosis gene expression is directly related to the c-Abl m1/m1 phenotype.
Therefore, we suggest that c-Abl may exert its effects on developing B cell proliferation, survival and differentiation by interfering with IL-7, CD19 and pre-BCR signaling. A previous study showed that while a wild-type c-Abl transgene could rescue the c-Abl null B cell phenotype, a kinase-dead c-Abl mutant transgene could not (51). Further work is underway to identify the direct targets of c-Abl kinase activity in primary pro- and pre-B cells. In addition, we think it likely that some aspects of c-Abl function during B cell development may be independent of its kinase activity since that activity peaks at the pro-B stage (Fig. 1) while pre-B cells display markedly increased rates of apoptosis.
Supplementary data
Supplementary Figures 1– 3 are available at International Immunology Online.
Funding
Leukemia & Lymphoma Society (NIH T32 CA009179-31); National Institutes of Health (RO1 AI57487).
Supplementary Material
Acknowledgments
We thank Anthony Koleske (Yale) for the c-Abl m1/m1 mice and advice. The manuscript was improved by insightful comments from April Bauer and from the editor and anonymous reviewers.
Glossary
Abbreviations
- BCR
B cell receptor
- BrdU
5-bromo-2-deoxyuridine
- CLP
common lymphoid progenitors
- IL-7Rα
IL-7 receptor α
- PDGF
platelet-derived growth factor
- PI
phosphatidylinositol
References
- 1.Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv. Cancer Res. 2002;85:51. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 2.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heisterkamp N, Stephenson JR, Groffen J, et al. Localization of the c-Ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983;306:239. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- 4.Kipreos ET, Wang JY. Cell cycle-regulated binding of c-Abl tyrosine kinase to DNA. Science. 1992;256:382. doi: 10.1126/science.256.5055.382. [DOI] [PubMed] [Google Scholar]
- 5.McWhirter JR, Wang JY. An actin-binding function contributes to transformation by the Bcr-Abl oncoprotein of Philadelphia chromosome-positive human leukemias. EMBO J. 1993;12:1533. doi: 10.1002/j.1460-2075.1993.tb05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren R, Ye ZS, Baltimore D. Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 1994;8:783. doi: 10.1101/gad.8.7.783. [DOI] [PubMed] [Google Scholar]
- 7.Taagepera S, McDonald D, Loeb JE, et al. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc. Natl Acad. Sci. USA. 1998;95:7457. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen ST, Jackson PK, Van Etten RA. The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 1996;15:1583. [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis JM, Baskaran R, Taagepera S, Schwartz MA, Wang JY. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc. Natl Acad. Sci. USA. 1996;93:15174. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koleske AJ, Gifford AM, Scott ML, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 11.Schwartzberg PL, Stall AM, Hardin JD, et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991;65:1165. doi: 10.1016/0092-8674(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 12.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-Abl proto-oncogene. Cell. 1991;65:1153. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Boast S, de los Santos K, et al. Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nat. Genet. 2000;24:304. doi: 10.1038/73542. [DOI] [PubMed] [Google Scholar]
- 14.Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 1998;391:904. doi: 10.1038/36122. [DOI] [PubMed] [Google Scholar]
- 15.Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521. doi: 10.1016/s1074-7613(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 16.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 17.Geier JK, Schlissel MS. Pre-BCR signals and the control of Ig gene rearrangements. Semin Immunol. 2006;18:31. doi: 10.1016/j.smim.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 19.Muljo SA, Schlissel MS. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat. immunol. 2003;4:31. doi: 10.1038/ni870. [DOI] [PubMed] [Google Scholar]
- 20.Roose JP, Diehn M, Tomlinson MG, et al. T cell receptor-independent basal signaling via Erk and Abl kinases suppresses RAG gene expression. PLoS Biol. 2003;1:E53. doi: 10.1371/journal.pbio.0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zipfel PA, Grove M, Blackburn K, Fujimoto M, Tedder TF, Pendergast AM. The c-Abl tyrosine kinase is regulated downstream of the B cell antigen receptor and interacts with CD19. J. Immunol. 2000;165:6872. doi: 10.4049/jimmunol.165.12.6872. [DOI] [PubMed] [Google Scholar]
- 22.Dorsch M, Goff SP. Increased sensitivity to apoptotic stimuli in c-Abl-deficient progenitor B-cell lines. Proc. Natl Acad. Sci. USA. 1996;93:13131. doi: 10.1073/pnas.93.23.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardin JD, Boast S, Schwartzberg PL, et al. Bone marrow B lymphocyte development in c-abl-deficient mice. Cell. Immunol. 1995;165:44. doi: 10.1006/cimm.1995.1185. [DOI] [PubMed] [Google Scholar]
- 24.Lam QL, Lo CK, Zheng BJ, et al. Impaired V(D)J recombination and increased apoptosis among B cell precursors in the bone marrow of c-Abl-deficient mice. Int. Immunol. 2007;19:267. doi: 10.1093/intimm/dxl143. [DOI] [PubMed] [Google Scholar]
- 25.Nussenzweig MC, Shaw AC, Sinn E, et al. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987;236:816. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 26.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 27.Brasher BB, Van Etten RA. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem. 2000;275:35631. doi: 10.1074/jbc.M005401200. [DOI] [PubMed] [Google Scholar]
- 28.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 29.Rolink A, Grawunder U, Winkler TH, Karasuyama H, Melchers F. IL-2 receptor alpha chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int. Immunol. 1994;6:1257. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 30.Fang W, Mueller DL, Pennell CA, et al. Frequent aberrant immunoglobulin gene rearrangements in pro-B cells revealed by a bcl-xL transgene. Immunity. 1996;4:291. doi: 10.1016/s1074-7613(00)80437-9. [DOI] [PubMed] [Google Scholar]
- 31.Schlissel M, Constantinescu A, Morrow T, Baxter M, Peng A. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5′-phosphorylated, RAG-dependent, and cell cycle regulated. Genes Dev. 1993;7:2520. doi: 10.1101/gad.7.12b.2520. [DOI] [PubMed] [Google Scholar]
- 32.Whang YE, Tran C, Henderson C, et al. c-Abl is required for development and optimal cell proliferation in the context of p53 deficiency. Proc. Natl Acad. Sci. USA. 2000;97:5486. doi: 10.1073/pnas.97.10.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krop I, Shaffer AL, Fearon DT, Schlissel MS. The signaling activity of murine CD19 is regulated during cell development. J. Immunol. 1996;157:48. [PubMed] [Google Scholar]
- 34.Otero DC, Rickert RC. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J. Immunol. 2003;171:5921. doi: 10.4049/jimmunol.171.11.5921. [DOI] [PubMed] [Google Scholar]
- 35.Kee BL, Bain G, Murre C. IL-7Ralpha and E47: independent pathways required for development of multipotent lymphoid progenitors. EMBO J. 2002;21:103. doi: 10.1093/emboj/21.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goga A, Liu X, Hambuch TM, et al. p53 dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene. 1995;11:791. [PubMed] [Google Scholar]
- 37.Sawyers CL, McLaughlin J, Goga A, Havlik M, Witte O. The nuclear tyrosine kinase c-Abl negatively regulates cell growth. Cell. 1994;77:121. doi: 10.1016/0092-8674(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 38.Yuan ZM, Huang Y, Whang Y, et al. Role for c-Abl tyrosine kinase in growth arrest response to DNA damage. Nature. 1996;382:272. doi: 10.1038/382272a0. [DOI] [PubMed] [Google Scholar]
- 39.Rosti V, Bergamaschi G, Lucotti C, et al. Oligodeoxynucleotides antisense to c-abl specifically inhibit entry into S-phase of CD34+ hematopoietic cells and their differentiation to granulocyte-macrophage progenitors. Blood. 1995;86:3387. [PubMed] [Google Scholar]
- 40.Daniel R, Cai Y, Wong PM, Chung SW. Deregulation of c-Abl mediated cell growth after retroviral transfer and expression of antisense sequences. Oncogene. 1995;10:1607. [PubMed] [Google Scholar]
- 41.Kharbanda S, Ren R, Pandey P, et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 42.Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. Defective T cell development and function in the absence of Abelson kinases. J. Immunol. 2007;179:7334. doi: 10.4049/jimmunol.179.11.7334. [DOI] [PubMed] [Google Scholar]
- 43.Grawunder U, Leu TM, Schatz DG, et al. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 44.Lu L, Osmond DG. Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol. Rev. 2000;175:158. [PubMed] [Google Scholar]
- 45.Kim HP, Leonard WJ. The basis for TCR-mediated regulation of the IL-2 receptor alpha chain gene: role of widely separated regulatory elements. EMBO J. 2002;21:3051. doi: 10.1093/emboj/cdf321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J. Immunol. 1998;161:6038. [PubMed] [Google Scholar]
- 47.Backesjo CM, Vargas L, Superti-Furga G, Smith CI. Phosphorylation of Bruton's tyrosine kinase by c-Abl. Biochem. Biophys. Res. Commun. 2002;299:510. doi: 10.1016/s0006-291x(02)02643-8. [DOI] [PubMed] [Google Scholar]
- 48.Plattner R, Irvin BJ, Guo S, et al. A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-gamma1. Nat. Cell Biol. 2003;5:309. doi: 10.1038/ncb949. [DOI] [PubMed] [Google Scholar]
- 49.Jumaa H, Mitterer M, Reth M, Nielsen PJ. The absence of SLP65 and Btk blocks B cell development at the preB cell receptor-positive stage. Eur. J. Immunol. 2001;31:2164. doi: 10.1002/1521-4141(200107)31:7<2164::aid-immu2164>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Kersseboom R, Middendorp S, Dingjan GM, et al. Bruton's tyrosine kinase cooperates with the B cell linker protein SLP-65 as a tumor suppressor in Pre-B cells. J. Exp. Med. 2003;198:91. doi: 10.1084/jem.20030615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardin JD, Boast S, Mendelsohn M, de los Santos K, Goff SP. Transgenes encoding both type I and type IV c-abl proteins rescue the lethality of c-abl mutant mice. Oncogene. 1996;12:2669. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.