Abstract
Modified Vaccinia Ankara (MVA) is a replication-defective strain of vaccinia virus (VV) that is being investigated in humans as an alternative vaccine against smallpox. Understanding the parameters of a MVA vaccine regimen that can effectively enhance protective immunity will be important for clinical development. The present studies utilize cohorts of rhesus monkeys immunized with recombinant MVA (rMVA) or recombinant VV (rVV) vaccine vectors to investigate the magnitude, breadth, and durability of anti-VV immunity elicited by a single or multi-dose vaccine regimen. These data demonstrate that a single immunization with rMVA elicits weaker cellular and humoral immunity compared to a single inoculation with rVV. However, vaccine-elicited antibody responses, but not T cell responses, are significantly enhanced with repeated immunizations of rMVA. Importantly, only monkeys receiving up to four inoculations with rMVA generated neutralizing antibody (NAb) responses that were comparable in magnitude and durability to those elicited in monkeys receiving two inoculations with rVV. These data also show that the breadth of antibody responses against protein antigens associated with two antigenically distinct forms of infectious VV are similar in rMVA and rVV immunized monkeys. Together, these studies suggest that a multi-dose vaccine regimen utilizing up to four inoculations of MVA generates robust and durable antibody-mediated immunity comparable to that elicited by replication-competent VV.
Keywords: MVA, vaccinia virus, vaccine, neutralizing antibody, rhesus monkey
1. Introduction
Smallpox (variola major) was declared eradicated by the World Health Organization in 1979 following a successful worldwide vaccination program [1]. As routine vaccination against smallpox was largely halted in the 1970's, it is believed that a majority of the world's population today may lack effective immunity [2]. The concern that smallpox remains a bioterrorism threat recently led the U.S. to begin stockpiling existing stores of vaccine and to briefly re-initiate immunization of healthcare workers and first responders [2]. The current FDA licensed vaccines for smallpox consist of live replication-competent vaccinia virus (VV), a closely related orthopoxvirus. While highly effective at eliciting cross-protective immunity against variola, VV is also associated with infrequent but severe adverse reactions, especially in certain high-risk populations (e.g., individuals with histories of eczema, atopic dermatitis, heart disease, or immunosuppression) [3]. Thus there is an urgent need for the development of new and safer smallpox vaccines for potential large-scale use in the general population.
One candidate that has been extensively studied as an alternative or supplemental vaccine against smallpox is Modified Vaccinia Ankara (MVA). MVA is an attenuated virus that was developed by > 570 serial passages of VV in chick embryo fibroblasts during which it acquired multiple large genomic deletions and mutations [4,5]. It is replication-incompetent in most mammalian cells due to a block in the late stages of viral assembly, but is still capable of expressing early and late gene products [6,7]. Importantly, numerous vaccine studies in mice and non-human primates have shown that MVA is immunogenic [8-10], and also well tolerated when used in models of immunodeficiency [11-13]. It has also demonstrated a superior safety profile compared to traditional VV-based vaccines when tested in humans [14-17].
Vaccine studies utilizing animal challenge models have proven insightful to the protective efficacy of MVA. In particular, recent publications have demonstrated that monkeys immunized with MVA are protected from an intravenous or respiratory challenge with a lethal dose of monkeypox virus [18-20]. While much remains to be elucidated about the nature and specificity of immunity generated by MVA, it is likely that vaccine-induced antibodies will play an essential role in protection. In vivo depletion of B cells in rhesus monkeys, but not CD4+ or CD8+ T-lymphocytes, has been shown to abrogate vaccinia-elicited protection against a lethal monkeypox challenge [21]. Additional evidence suggests that optimal protection is achieved when antibody responses target two structurally and antigenically distinct forms of infectious virus. The intracellular mature virion (IMV) is thought to be primarily responsible for virus transmission between hosts, whereas the extracellular enveloped virion (EEV) has been implicated in cell-to-cell dissemination of virus within a host [22]. Thus the breadth of antibody-mediated immunity generated by MVA vaccination will also be an important determinant of protective efficacy.
While MVA is being actively pursued as a candidate vaccine in phase I human clinical trials, it remains to be determined what dose, route, or number of inoculations is required for generating optimal immunity. Animal models suggest that multiple immunizations with MVA will be required to achieve levels of humoral and cellular immunity that are comparable to those elicited by a single immunization with replication-competent VV [10,18,19]. While vaccine studies in animals and humans have primarily utilized up to two inoculations of MVA in a prime/boost setting, it has not been thoroughly investigated whether additional immunizations can further enhance the magnitude and durability of anti-viral immunity. Therefore, understanding the optimal dosing schedule will be important for maximizing the effectiveness of an MVA smallpox vaccine. The present studies were initiated to investigate the magnitude, breadth, and durability of anti-viral immunity elicited by MVA in comparison to replication-competent VV following a single or multi-dose vaccine regimen.
2. Materials and Methods
2.1 Cell Lines
HeLa and CV-1 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The chick embryo fibroblast cell line DF-1 was a generous gift from Dr. Mark Feinberg (Emory Vaccine Center). All cell lines were maintained in D-MEM growth medium (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum (FBS), 25 mM HEPES, and 50 μg/ml gentamicin, and grown at 37° C in humidified air containing 5% CO2. Cells were harvested using Trypsin/EDTA solution (Invitrogen).
2.2 Viruses
VV:WR was obtained from ATCC. The recombinant strain of VV:WR containing a luciferase reporter gene (VV:Luc) was a generous gift from Dr. David Bartlett (University of Pittsburgh). The vaccinia virus strain IHD-J was a generous gift from Dr. Bernard Moss (NIAID/NIH). All stocks of vaccinia virus were grown on HeLa cells and purified by sucrose centrifugation. Vaccinia virus titers were determined by plaque assay on CV-1 cells. The ACAM3000 strain of Modified Vaccinia Ankara was obtained through the Biodefense & Emerging Infections Research Resources Repository (BEI Resources, Manassas, VA). The recombinant strain of MVA containing a luciferase reporter gene (MVA:Luc) was a generous gift from Dr. Mariano Esteban (Centro National de Biotechnologia, Madrid, Spain). All stocks of MVA were grown on DF-1 cells and purified by sucrose centrifugation. Virus titers were determined using an immunostaining assay on DF-1 cells as previously described [23].
2.3 Immunization of rhesus monkeys
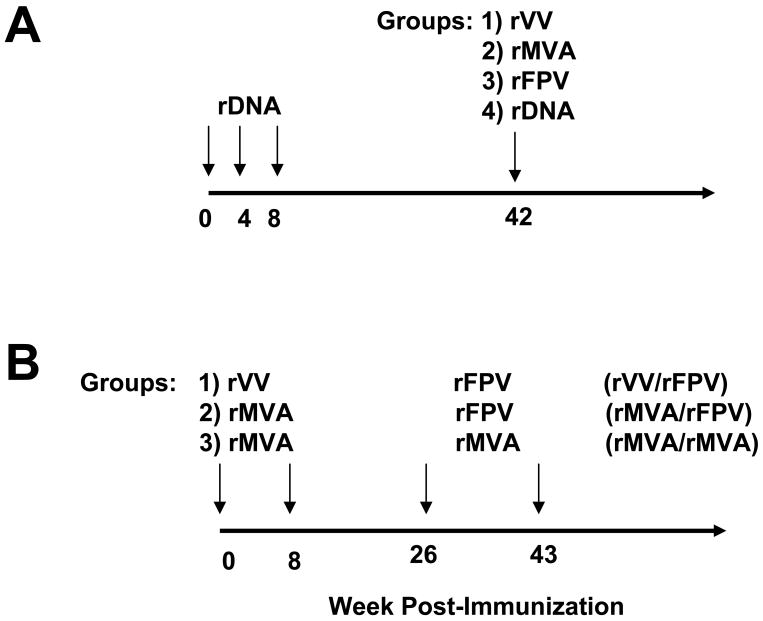
Anti-viral immunity was analyzed in cohorts of Indian-origin rhesus monkeys that participated in two independent vaccine studies investigating the use of recombinant pox virus vectors for delivery of candidate HIV-1 vaccines. In the first study, monkeys were immunized with HIV-1 Envelope and SIV Gag immunogens following a DNA prime/recombinant poxvirus boost regimen [24]. Twenty-eight Indian-origin rhesus monkeys were distributed into four experimental groups, each consisting of seven animals. As part of the HIV-1/AIDS vaccine regimen, three groups of monkeys were primed with plasmid DNA at weeks 0, 4, and 8, and boosted with either 2×109 plaque forming units (pfu) of rVV, rMVA, or rFPV by both intramuscular and intradermal routes at week 42. A fourth group of monkeys that received plasmid DNA at week 42 served as negative controls (Figure 1A). All recombinant viruses were produced by Therion Biologics (Cambridge, MA). The parental strain for generating rVV (TBC-Wy strain) is an isolate from the Wyeth NYCBH vaccine strain (Dryvax).
Figure 1.
Schematic representation of two vaccine studies in rhesus monkeys utilizing recombinant poxvirus vaccine vectors. rDNA, rVV, rMVA, and rFPV are recombinant vectors expressing SIV Gag and HIV-1 Env vaccine inserts. (A) Monkeys (7 per group) received rDNA prime/recombinant poxvirus or rDNA boost vaccinations as described in Materials and Methods. (B) Monkeys (6 per group) received recombinant poxvirus prime/recombinant poxvirus boost vaccinations. All poxvirus immunizations were administered by intramuscular and intradermal injections with 2×109 pfu.
In the second study, groups of monkeys were immunized with the same recombinant poxvirus vectors as in the first study but using a poxvirus prime/poxvirus boost regimen [25]. Eighteen Indian-origin rhesus monkeys were distributed into three experimental groups, each consisting of six animals (Figure 1B). Each group of monkeys received two priming immunizations with recombinant poxvirus vectors at weeks 0 and 8, followed by two boost immunizations at weeks 26 and 43 as either (i) rVV prime/rFPV boost, (ii) rMVA prime/rFPV boost, or (iii) rMVA prime/rMVA boost (a total of 4 rMVA vaccinations).
2.4 Recombinant Protein and Antibody Reagents
Soluble recombinant forms of baculovirus-produced L1R, A27L, B5R, and A33R proteins were obtained from BEI Resources. Human Vaccinia Immune Globulin (VIG, lot #1730206) was obtained from the Centers for Disease Control and Prevention (CDC, Atlanta, GA).
2.5 Neutralization Assays
Neutralizing antibody responses against VV and MVA were measured using a luciferase-based assay in HeLa or DF-1 cells, respectively. This assay measures the reduction in luciferase reporter gene expression in target cells following a single-round of virus infection. Briefly, 3-fold serial dilutions of plasma samples were performed in triplicate (96-well flat bottom plate) in 10% D-MEM growth media (100 μl/well). VV:Luc or MVA:Luc virus (5×104 pfu) was added to each well in a volume of 50 μl and the plates were incubated for 1 hour at 37° C. HeLa (VV:Luc assays) or DF-1 (MVA:Luc assays) cells were then added (5×104/well in 50 μl volume) in 10% D-MEM growth medium to achieve a multiplicity of infection (MOI) of 1:1. Cytosine arabinofuranoside (Sigma, St. Louis, MO) was added at a final concentration of 20 μg/ml to prevent secondary rounds of infection. Assay controls included replicate wells of target cells alone (cell control) and target cells with virus (virus control). Following an overnight incubation at 37° C, 100 μl of assay medium was removed from each well and 100 μl of Bright-Glo luciferase reagent (Promega, Madison, WI) was added. The cells were allowed to lyse for 2 minutes, then 150 μl of the cell lysate was transferred to a 96-well black solid plate and luminescence was measured using a Victor 3 luminometer (Perkin Elmer). The ID50 titer was calculated as the serum dilution that caused a 50% reduction in relative luminescence units (RLU) compared to the virus control wells after subtraction of cell control RLUs. VIG was utilized as a positive control reagent for all neutralization assays performed.
2.6 Enzyme-linked immunosorbent assays (ELISA)
ELISA assays were essentially performed as previously described [26]. Maxisorp ELISA plates (Nunc, Roskilde, Denmark) were coated using 1 μg/ml recombinant protein or 1×107 pfu/ml of sucrose-purified, paraformaldehyde treated ACAM3000 MVA or VV: WR virus as antigen. Serum samples were serially diluted in duplicate wells. A pre-immune negative control plasma sample was tested in parallel with the corresponding post-vaccination plasma samples for each monkey. The endpoint titer for each monkey was established as the last dilution with a corrected optical density >0.1 after subtraction of pre-immune background values.
2.7 Comet-reduction Assay
Confluent monolayers of CV-1 cells in 6-well plates (Costar, Gaithersburg, MD) were infected with VV:IHD-J diluted in 10% D-MEM growth medium to achieve approximately 25 plaques per well. Following 2 hours of rocking at 37° C, the virus containing inoculum was aspirated and replaced with 2 ml fresh 10% D-MEM medium. Pre- and post-immune plasma samples from each monkey were then added to achieve a 1:50 final dilution. The plates were incubated for 2 days at 37° C and then stained with crystal violet. The degree of comet inhibition in wells containing monkey plasma was compared to wells containing virus alone and graded as follows: 0 (no inhibition), + (some inhibition), ++ (moderate inhibition), and +++ (complete inhibition).
2.8 ELISPOT assays
Vaccinia-specific cellular immune responses in vaccinated rhesus monkeys were assessed by IFN-γ ELISPOT assays essentially as described previously [26]. Rhesus monkey PBMC (2×105 per well) were incubated with 2 μg/ml Gag peptide pool or vaccinia virus WR strain at a multiplicity of infection (MOI) of 1 in triplicate. SFC per 106 cells were calculated. Media backgrounds were consistently < 15 SFC per 106 cells.
2.9 Protein array ELISA
Protein array assays were performed as detailed in full elsewhere [27]. Briefly, following bioinformatic and bibliographic determination of likely viral protein ectodomains, the cDNA encoding vaccinia proteins (vaccinia Copenhagen nomenclature) indicated in Figure 6 were amplified directly from vaccinia WR genomic DNA excluding signal peptides and transmembrane domains followed by incorporation of putative viral ectodomain cDNA into pcDNA3.1-myc-His-A (Invitrogen) with an artificial start codon inside an optimal Kozak sequence. The constructs were expressed in a reticulocyte lysate-based coupled transcription/translation reaction (Promega) where protein was biosynthetically-labeled with biotin by incorporation of tRNAlys preloaded with lysine ε-amino-labeled with biotin (Promega). Lysate containing biotin-labeled protein was incubated directly on neutravidin-coated 384 well plates (Pierce; approximately 8-10ng labeled protein/well) for 24h. As a positive control, baculovirus-produced L1R, B5R, A27L, and A33 recombinant proteins (BEI Resources) were also used in the array assay at a concentration of 100 ng/well. Following immobilization, unbound protein was washed away and the plates were extensively washed with Tris-buffered saline containing 0.05% Tween 20 (TBS-T) followed by incubation with monkey sera (1:250 in TBS-T + 1% BSA) for 1.5h. After 3 washes in TBS-T, wells were incubated with goat anti-monkey alkaline phosphatase (1:10,000 in TBS-T + 1%BSA; Fitzgerald, Concord MA) for 1h. After 3 additional washes in TBS-T, bound alkaline phosphatase was detected by hydrolysis of pNPP assayed at 405nm. VIG (20 μg/ml) was used as a positive control. Results were plotted as a heatmap generated by the JColorGrid program [28].
Figure 6.
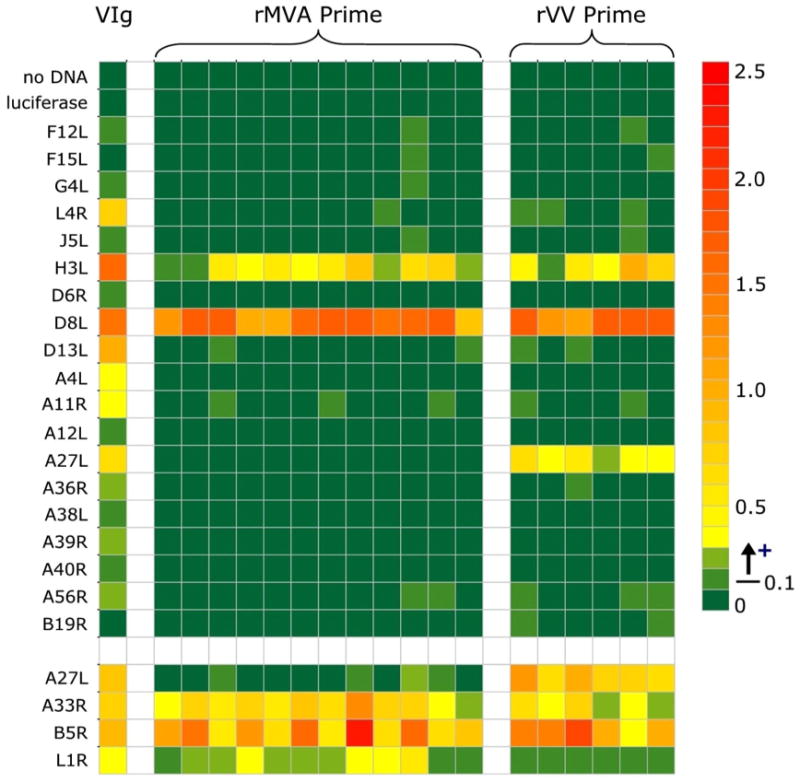
Protein array analysis of antibody responses to a panel of vaccinia antigens. Plasma samples were obtained from vaccinated monkeys at week 13 following priming immunizations with either rVV or rMVA and tested at a 1:250 dilution against the indicated protein antigens by ELISA. VIG was used as a positive control (20 μg/ml). The baculovirus-produced A27L, A33R, B5R, and L1R recombinant proteins used in the standard ELISA assays described in Figure 5 were included as positive controls, and are shown in the last four rows separated from the main array. Data are presented as response at 4 weeks following subtraction of the response from matched pre-immune plasma. Background responses were consistently below 0.04, a response of 0.05-0.1 was considered borderline and a response above 0.1 as positive. Mean replicate variation was 2.1% +/- 2.3%.
2.10 Statistical Analysis
The nonparametric Kruskal-Wallis test was used for multiple group comparisons for neutralizing and endpoint antibody binding titers. Differences between groups were analyzed by Mann-Whitney test. All tests were performed using GraphPad Prism software, version 4.0.
3. Results
3.1 Anti-viral immunity elicited by a single inoculation with rMVA, rVV, or rFPV
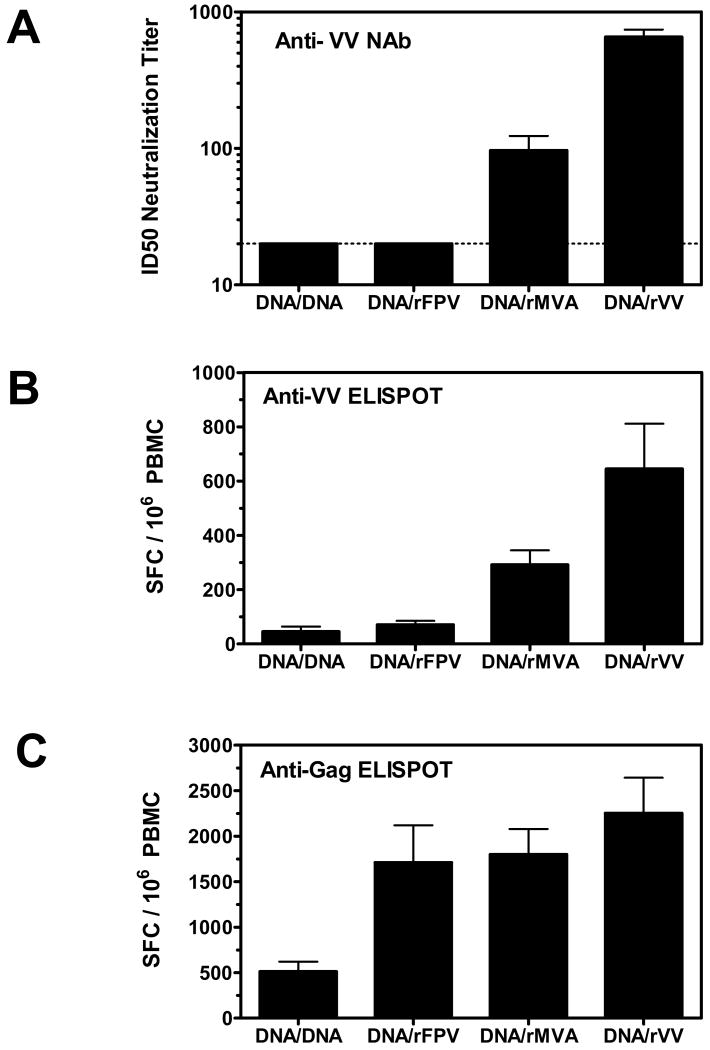
The ability of rMVA and rVV to elicit cross-reactive humoral and cellular immunity against the pathogenic vaccinia virus-Western Reserve strain (VV:WR) following a single inoculation in rhesus monkeys was assessed (Figure 1A). We included an additional group of monkeys vaccinated with recombinant fowlpox virus (rFPV), a distantly related avipoxvirus. The cohort of animals used for these studies were part of a previously described HIV-1/AIDS vaccine study investigating the immunogenicity of DNA prime/recombinant poxvirus boost-based vaccine regimens [24]. Monkeys receiving plasmid DNA prime/DNA boost vaccines with no exposure to orthopox or fowlpox virus served as a negative control group for the studies described here. We first sought to examine the magnitude of cross-reactive NAb responses against VV:WR four weeks following recombinant poxvirus immunization. All monkeys receiving a single inoculation of rVV generated a robust NAb response against VV:Luc (Figure 2A). Monkeys immunized with rMVA also had detectable NAb activity against VV:Luc, although responses were significantly lower than those observed in rVV immunized monkeys (mean 50% inhibitory dose (ID50) titers of 90 and 620, respectively, p < 0.0006). In contrast, monkeys immunized with either rFPV or plasmid DNA (negative control group) had no detectable NAb activity against VV:Luc.
Figure 2.
Anti-VV NAb and cellular immune responses elicited by rVV, rMVA, and rFPV vaccine vectors. Plasma and PBMC samples were obtained from vaccinated monkeys 4 weeks following boost immunizations with either plasmid DNA, rFPV, rMVA or rVV. (A) Serial dilutions of plasma samples were tested for NAb activity against VV:Luc. Data are presented as the mean ID50 neutralization titer from seven monkeys per group +/- SEM. The dashed line represents the assay limit of detection (ID50 titer >20). PBMC were assessed for IFN-γ ELISPOT responses against VV:WR (B) or the vaccine insert, HIV-1 Gag (C). Data are presented as the mean number of antigen-specific SFC per 106 PBMC +/- SEM from seven monkeys per group.
We further assessed the magnitude of cross-reactive cellular immune responses against VV:WR in this cohort of poxvirus-immunized monkeys. As shown in Figure 2B, all monkeys immunized with rVV and rMVA demonstrated VV:WR-specific ELISPOT responses four weeks following immunization, although responses were higher in the latter group of monkeys (645 and 292 mean spot forming cells (SFC)/106 PBMC, respectively, p = 0.05). PBMC from rFPV immunized monkeys generated ELISPOT responses that were similar to those measured in monkeys immunized with plasmid DNA alone, suggesting a lack of cross-reactive T cell immunity against VV: WR. As all recombinant vectors used in these studies encoded for the SIV Gag protein, we also measured Gag-specific cellular immune responses by pooled peptide IFN-γ ELISPOT assay to demonstrate that the differences observed in anti-VV: WR-specific cellular immunity were not attributed to infectivity differences among these poxvirus vectors. In fact, the magnitude of Gag-specific cellular immune responses was similar in monkeys immunized with either rVV, rMVA, or rFPV (Figure 2C).
3.2 Vaccine-elicited antibody responses generated by rMVA prime/boost vaccinations
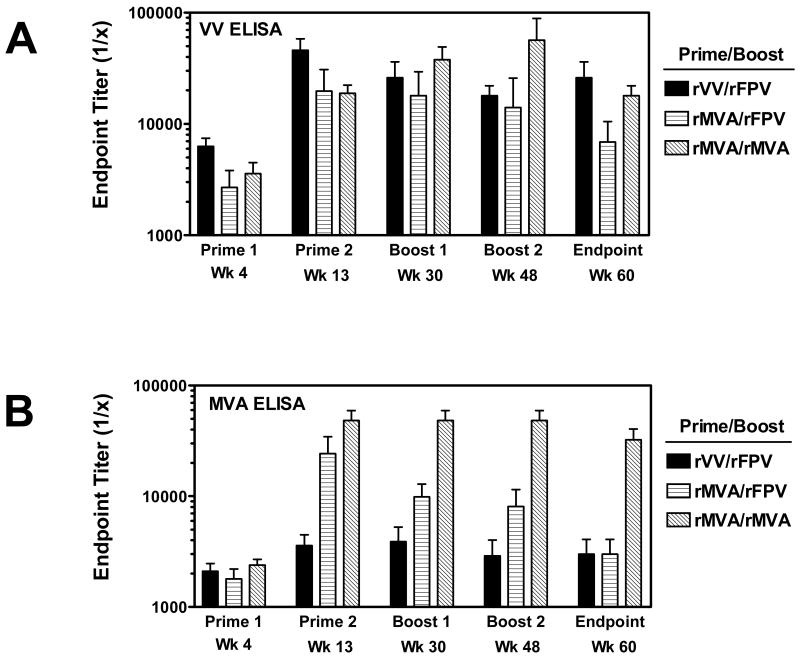
We next assessed the magnitude and durability of VV-specific humoral and cellular immunity elicited by rMVA in the setting of multiple prime/boost vaccinations. To this end, we utilized a second cohort of rhesus monkeys that were part of a previously described HIV-1/AIDS vaccine study investigating the immunogenicity of recombinant poxvirus-based prime/boost vaccine regimens [25] (Figure 1B). We first assessed humoral immunity elicited by these prime/boost vaccinations by ELISA using whole inactivated VV:WR or MVA as antigen. As shown in Figure 3A, plasma antibody binding titers to VV:WR were detected in all monkeys four weeks following a single inoculation with either rVV or rMVA (mean endpoint titers of 6,300 and 3,150 (combined rMVA primed groups), respectively, p = 0.052). An approximate 6-fold increase in endpoint titer was observed in all groups of animals following the second homologous priming immunization. No further increase in anti-VV:WR-specific antibody titers were observed in groups of monkeys primed with either rVV or rMVA and boosted twice with rFPV. In contrast, monkeys in the rMVA / rMVA group demonstrated incremental increases in anti-VV:WR antibody binding titers with each subsequent boost immunization. At week 60 (17 weeks following the final boost immunization) mean endpoint titers were higher in the rVV/rFPV and rMVA/rMVA groups (26,100 and 18,000, respectively) compared to monkeys in the rMVA/rFPV group (mean titer 6,900). Thus, monkeys receiving a total of four rMVA immunizations had higher anti-VV:WR antibody binding titers compared to monkeys receiving two rMVA immunizations (p < 0.05 at week 48), and furthermore had titers comparable to or higher than those measured in monkeys receiving two immunizations with rVV. Plasma antibody binding titers to whole VV:WR and MVA antigen were found to be similar in rMVA immunized monkeys, suggesting a high-level of antibody cross-reactivity against the VV:WR virus (Figure 3B). Interestingly, rVV immunized monkeys demonstrated lower antibody titers against MVA antigen compared to VV:WR antigen.
Figure 3.
Antibody responses elicited by poxvirus prime/boost immunizations. Plasma samples were obtained from vaccinated monkeys at various timepoints following prime/boost immunizations with rVV, rMVA, and rFPV as indicated. Serial dilutions of samples were tested for antibody binding activity against VV:WR (A) or MVA (B) by ELISA. Data are presented as the mean endpoint titer from six monkeys per group +/- SEM.
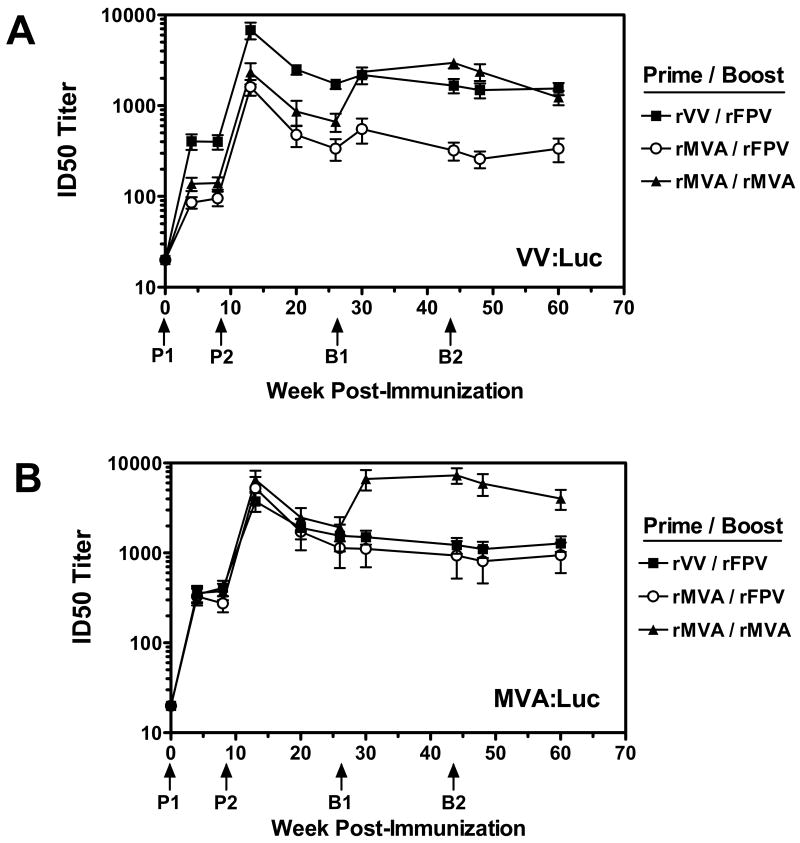
NAb responses elicited by these poxvirus prime/boost vaccine regimens were assessed using VV:Luc as well as a recombinant MVA virus expressing firefly luciferase (MVA:Luc). As previously observed, a single inoculation of rVV was more effective at eliciting NAb responses against VV:Luc than a single inoculation of rMVA (Figure 4A, mean ID50 titers of 404 for rVV prime and 112 for the rMVA prime groups combined, p <0.005). An approximate 18-fold increase in NAb titers was observed in rMVA immunized monkeys two weeks following a second rMVA priming immunization (mean titer for rMVA prime groups combined measuring 1,967), however, these responses were still significantly lower than those measured in monkeys receiving two priming immunizations with rVV (mean titer of 6,799, p = 0.008). No additional increase in NAb titers were observed in monkeys primed with either rVV or rMVA and boosted twice with rFPV. In contrast, monkeys primed with rMVA and boosted with rMVA had an approximate two fold increase in anti-VV:WR NAb titers at week 30 that were significantly higher than those in monkeys primed with rMVA and boosted with rFPV (2,350 and 552, respectively, p = 0.002). These titers remained stable through week 60, at which time mean ID50 titers in rMVA/rMVA immunized monkeys were similar to those in rVV/rFPV immunized monkeys (1,237 and 1,546, respectively), and remained significantly higher than those measured in rMVA/rFPV immunized monkeys (337, p = 0.002). Together, these data show that multiple prime/boost vaccinations with rMVA can enhance humoral immune responses against VV:WR, and demonstrate that monkeys receiving four rMVA immunization have significantly higher NAb titers than monkeys receiving only two rMVA immunizations. NAb assays performed with MVA:Luc demonstrated a similar profile in prime/boost responses, although plasma from rMVA immunized monkeys neutralized MVA:Luc more efficiently than VV:Luc (Figure 4B).
Figure 4.
Neutralizing antibody responses elicited by poxvirus prime/boost immunizations. Plasma samples were obtained from vaccinated monkeys at various timepoints following prime/boost immunizations with rVV, rMVA, and rFPV as indicated. Serial dilutions of samples were tested for NAb activity against VV:Luc (A) or MVA:Luc (B). The timing of prime and boost immunizations are indicated by arrows. Data are presented as the mean ID50 NAb titer +/- SEM from six monkeys per group.
3.3 NAb responses against vaccinia EEV
The NAb and ELISA assays previously described measure antibody responses against the IMV form of VV. We further assessed the ability of rMVA prime/boost immunizations to elicit NAb responses against the EEV form of vaccinia virus by comet-reduction assay. This assay measures the inhibition of satellite plaque formation caused by the release of EEV from the IHD-J strain of vaccinia virus (a derivative of the New York City Board of Health strain). Four weeks following the first priming immunization, plasma from monkeys immunized with rVV exhibited a higher level of comet-inhibition activity than plasma from monkeys immunized with rMVA (Table I). However, a marked increase in EEV-neutralizing activity was observed in rMVA monkeys five weeks following the second priming immunization (week 13). While anti-comet activity appeared to wane in rMVA primed monkeys boosted with rFPV, monkeys boosted with rMVA continued to exhibit high levels of comet inhibition that were comparable to those measured in rVV/rFPV immunized monkeys. Together with our previous NAb and ELISA results, these data demonstrate that additional boost immunizations with rMVA effectively enhance the magnitude and durability of neutralizing antibodies against both the IMV and EEV forms of infectious vaccinia virus.
Table 1.
Assessment of anti-EEV NAb responses by comet-reduction assay.
| Group | Monkey | Comet Reduction Scorea | ||||
|---|---|---|---|---|---|---|
| Wk 0 | Wk 4 | Wk 13 | Wk 30 | Wk 48 | ||
| rVV / rFPV | 88M | 0 | + + | + + + | + + + | + + + |
| 90M | 0 | + | + + + | + + + | + + + | |
| 91M | 0 | + + | + + + | + + + | + + + | |
| 92M | 0 | + + | + + + | + + | + + | |
| 95M | 0 | + | + + | + + | + + | |
| 96M | 0 | + | + + + | + + + | + + | |
| rMVA / rFPV | 80M | 0 | 0 | + + | + | + |
| 81M | 0 | + | + + + | + + | + + | |
| 82M | 0 | 0 | + + | + | + | |
| 83M | 0 | 0 | + + | + + | + | |
| 84M | 0 | 0 | + + | + + | + | |
| 87M | 0 | + | + + + | + + | + + | |
| rMVA / rMVA | 001M | 0 | + | + + + | + + + | + + + |
| 002M | 0 | + | + + + | + + + | + + + | |
| 003M | 0 | + | + + | + + + | + + + | |
| 77M | 0 | 0 | + + | + + + | + + + | |
| 78M | 0 | + | + + | + + + | + + | |
| 996L | 0 | + | + + | + + + | + + | |
| VIGb | Control | + + + | - | - | - | - |
Plasma samples from individual monkeys were isolated following Prime 1 (Wk 4), Prime 2 (Wk 13), Boost 1 (Wk 30), or Boost 2 (Wk 48) and tested in a comet reduction assay at a 1:50 dilution. Matched pre-immune plasma (Wk 0) served as a negative control. The degree of comet reduction was scored as follows: 0, no reduction; +, some; ++, intermediate; +++, complete.
VIG was used as a positive control at 1:500 dilution.
3.4 Breadth of antibody recognition against IMV and EEV-associated antigens
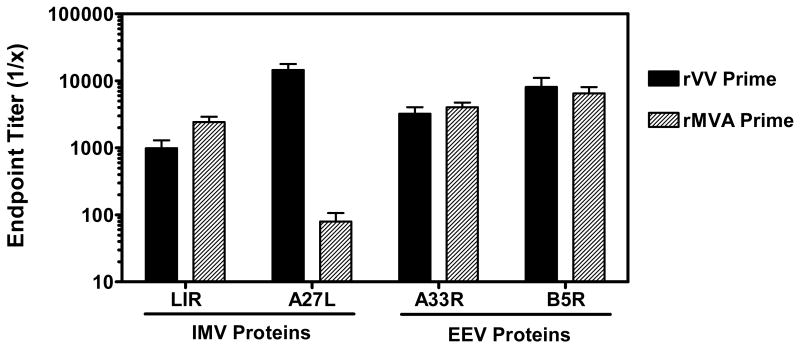
We investigated the breadth of antibody responses against IMV and EEV-associated antigens in monkeys immunized with either rVV or rMVA. Antigen-specific ELISA assays were performed using two IMV-associated proteins (L1R and A27L) and two EEV-associated proteins (A33R and B5R). Plasma endpoint antibody titers were measured in individual monkeys at week 13, five weeks following the second priming immunization. For these analyses, data from monkeys in the rMVA/rMVA and rMVA/rFPV groups were combined, as both received rMVA for the priming immunizations. As shown in Figure 5, similar magnitude antibody responses against L1R, A33R, and B5R antigens were observed in monkeys primed with either rMVA or rVV. In contrast, monkeys immunized with rVV had antibody binding titers to A27L that were approximately 2-logs higher than those observed in monkeys immunized with rMVA (mean titers of 14,580 and 80, respectively, p < 0.001). We did not observe significant enhancement of antibody titers against these antigens in any of the vaccine groups following boost immunizations (data not shown).
Figure 5.
Antibody responses to vaccinia IMV- and EEV-associated proteins following immunization with either rVV or rMVA. Plasma samples were obtained from vaccinated monkeys at week 13 following priming immunizations with either rVV or rMVA. Serial dilutions of samples were tested by ELISA for antibody binding activity against the IMV-associated proteins L1R and A27L, and the EEV-associated proteins A33R and B5R. Data are presented as the mean endpoint titer +/- SEM from either 6 (rVV prime) or 12 (rMVA prime) monkeys per group.
We further assessed the breadth of antibody responses against an additional nineteen IMV- and EEV-associated VV antigens by protein array ELISA (Figure 6). Both rMVA and rVV primed monkeys demonstrated dominant antibody responses against the IMV-associated antigens H3L and D8L, both of which are glycosaminoglycan receptors with a likely role in virus-cell adhesion and entry [29,30]. These two antigens were also strongly recognized by human Vaccinia Immune Globulin (VIG). Importantly, the lack of an anti-A27L antibody response in rMVA immunized monkeys was also confirmed in the protein array format. It should be noted that the recombinant proteins utilized in this array were produced using an in vitro transcription/translation system which may result in improper folding for some of the target antigens [27]. However, the antigenicity of the cell lysate-produced proteins was confirmed using human vaccinia hyperimmune antibody (VIG). Furthermore, the four recombinant baculovirus-produced proteins used in the standard ELISA assays described above were included in the protein array format as positive controls and concordant reactivities were detected. Together, these data suggest that rMVA immunization can elicit antibody responses against IMV and EEV-associated antigens that are similar in breadth and magnitude as those generated by rVV immunization, although responses against other certain key antigens may be absent.
3.5 Cellular immune responses elicited by rMVA prime/boost immunizations
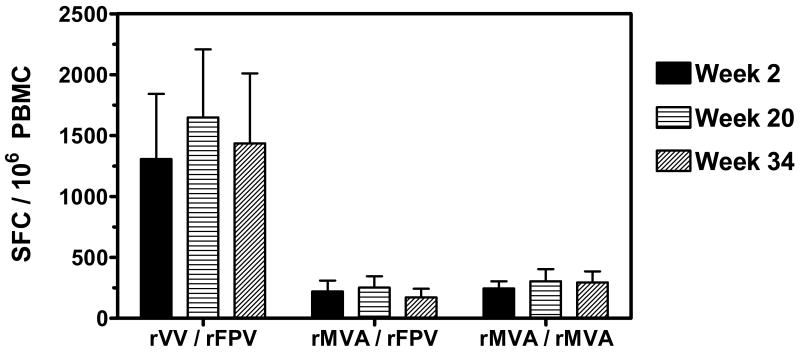
Anti-VV cellular immune responses elicited by poxvirus prime/boost immunizations were assessed by IFN-γ ELISPOT assay. Due to the limited availability of frozen PBMC from this study, we were only able to assess cellular responses two weeks following the first priming immunization (Week 2), twelve weeks following the second priming immunization (Week 20), and eight weeks following the first boost immunization (Week 34). Monkeys in the rVV/rFPV group demonstrated robust cellular immune responses against VV:WR at all timepoints tested (Figure 7). In contrast, monkeys receiving rMVA/rFPV and rMVA/rMVA immunizations demonstrated equivalent low magnitude VV-specific immune responses following both prime and boost immunizations. Thus we could not detect evidence of significant enhancement in anti-VV cellular immunity following multiple immunizations with rMVA.
Figure 7.
Anti-VV cellular immune responses elicited by poxvirus prime/boost immunizations. PBMC were isolated from vaccinated monkeys at weeks 2 (post-prime 1), 20 (post-prime 2), and 34 (post-boost 1) following immunization. Cellular immune responses against VV:WR were assessed by IFN-γ ELISPOT assays. The data are presented as the mean number of antigen-specific SFC per 106 PBMC +/- SEM from six monkeys per group.
4. Discussion
As MVA is being actively pursued as a supplemental or alternative vaccine against smallpox, it will be important to determine an immunization regimen by which this replication defective virus can elicit the highest magnitude and most durable protective immunity. Recent studies in humans have primarily focused on comparing the dose and route of MVA immunization in a two inoculation prime/boost setting [14,16]. The question of whether additional inoculations may further enhance vaccine-elicited immunity has not been adequately addressed. Here, we have utilized a cohort of rhesus monkeys receiving multiple prime/boost immunizations to demonstrate that up to four inoculations with rMVA is more effective at eliciting durable high-titer anti-VV antibody responses than is two inoculations. Importantly, only monkeys receiving a total of four rMVA vaccinations had NAb responses that were similar in magnitude and durability to those observed in monkeys receiving two vaccinations with replication-competent rVV. It should be noted that the contribution of the fourth rMVA immunization is not well defined in this study, as we did not have a group of animals receiving a total of three rMVA immunizations for comparison. While we did not observe an increase in the magnitude of NAb following the fourth immunization, it is possible that this vaccination contributed to the enhanced durability of the response.
It should be noted that the studies described here utilized animals that were part of studies designed to analyze the utility of poxvirus vectors for delivery of candidate HIV-1 vaccines, and were not designed to be representative of smallpox vaccinations in humans. The dose of rVV administered in these monkeys (2×109 pfu) is higher than that delivered by the standard regimen of multi-puncture scarification with Dryvax (approximately 2×105 pfu). Thus it is possible that our results may actually underestimate the comparative immunogenicity of multi-dose MVA versus VV in humans. While the magnitude and durability of rMVA elicited immunity was assessed for 60 weeks following vaccination, humans receive single immunizations with Dryvax that are separated by years. Whether MVA elicited antibody and T cell immunity demonstrate comparable durability to that observed with Dryvax immunization has not been well defined [23]. Nonetheless, these data suggest that increasing the number of vaccinations may be an important parameter for optimizing MVA-elicited antibody responses in humans. A recent phase I study of MVA in vaccinia-naïve humans attempted to compare the immunogenicity of a single versus multi-dose vaccine regimen [15]. Unfortunately the data were inconclusive, as the dose of MVA administered to volunteers (∼106 pfu) was found to be suboptimal and did not elicit a detectable NAb response even in participants receiving three inoculations. As MVA vaccinations with 107 or 108 pfu have been demonstrated to be safe and immunogenic in humans [14,16], a single versus multi-inoculation comparison within this dose range would be informative.
Our NAb and comet-reduction assays demonstrate that multiple immunizations with rMVA can effectively generate protective antibody responses against both the IMV and EEV forms of infectious VV. Furthermore, we have utilized an array of IMV and EEV associated proteins to demonstrate that immune plasma from rMVA and rVV immunized monkeys exhibit very similar profiles of antigen recognition. Predominant antibody binding was observed against the IMV-associated proteins L1R, D8L, and H3L, as well as the EEV-associated proteins A33R and B5R. Importantly, recent studies have suggested that antibody responses against these particular antigens are effective in conferring protection against orthopoxvirus infection [26, 31-35]. However, we also observed that rVV-vaccinated monkeys generated high-titer antibody responses against A27L, whereas rMVA-vaccinated monkeys did not. A27L is an IMV-associated membrane protein that has previously been described as a target antigen for neutralizing antibodies [36]. Thus, the lack of A27L-specific antibodies in rMVA monkeys demonstrates that certain key elements of a protective immune response may be lacking in the setting of MVA vaccination. While the gene encoding A27L is present in MVA, it remains possible that the protein is not efficiently expressed or presented in the proper context to elicit a robust antibody response.
We have also assessed the efficiency of antigen cross-recognition in rMVA vaccinated monkeys by utilizing NAb and ELISA assays that incorporate both VV and MVA as target IMV antigens. Antibody binding titers against MVA and VV were found to be nearly equivalent in plasma from rMVA immunized monkeys, suggesting a high degree of antigen cross-reactivity. Interestingly, vaccine-elicited antibody responses in rMVA immunized monkeys were slightly more effective at neutralizing MVA:Luc than VV:Luc. This could not be attributed to MVA:Luc exhibiting a more neutralization-sensitive phenotype, as both viruses demonstrated similar sensitivity to neutralization by VIG (data not shown). As stocks of MVA virus are grown in chick embryo fibroblast cells, it remains possible that antibody responses targeting other host cell associated antigens incorporated into the viral membrane may be contributing to neutralization. We also observed that immune plasma from rVV immunized monkeys demonstrated lower levels of ELISA antibody binding activity against MVA than against homologous VV antigen. This may indicate that MVA IMV lack particular membrane-associated proteins that are normally present on VV IMV which are targets for vaccine-elicited antibodies. In fact, earlier reports have indicated that MVA lacks the ability to express certain protein antigens, such as the A-type inclusion body protein, that are recognized by VIG [37]. Whether these results may be explained in part by a lack of A27L expression on MVA IMV also warrants additional analysis.
We have also investigated the ability of rFPV, an avipoxvirus, to elicit cross-reactive humoral or cellular immunity against VV. Our results demonstrate that monkeys receiving a single inoculation of rFPV do not exhibit any cross-reactive NAb or T cell responses against VV. This result may be expected considering the low degree of protein similarity between these two distant members of the Poxviridae family (approximately 35%, www.poxvirus.org). Therefore in the groups of monkeys receiving rVV/rFPV or rMVA/rFPV vaccine regimens, it is unlikely that the two boost inoculations with rFPV significantly contributed to the overall antibody or T cell responses measured.
Vaccine-elicited cellular immunity may also play a protective role against orthopoxvirus infection [8,13]. While a single immunization with rMVA is capable of priming an anti-VV T-cell response, clearly it is not as effective as replication competent rVV. Unfortunately we were not able to optimally assess the effect of multiple prime/boost rMVA immunizations on enhancing the anti-VV cellular immune response due to the limited availability of PBMC from these monkeys. However, we observed that anti-VV ELISPOT responses in monkeys following three rMVA immunizations were similar in magnitude to those observed following one or two immunizations (Figure 7), and significantly lower in magnitude compared to responses measured in rVV-vaccinated monkeys. These results support previous observations that a secondary boost immunization of MVA primed monkeys was more efficient in augmenting virus-specific antibody responses then cellular immune responses [18]. This may reflect the inability of rMVA to elicit a potent inflammatory cytokine environment that can augment T cell immune responses such is observed during infection with replication-competent virus. It also remains possible that the presence of high-titer NAb may significantly blunt the ability of subsequent rMVA inoculations to effectively boost cellular immunity.
The results presented here provide additional support for the use of MVA as an alternative vaccine against smallpox. We have demonstrated that a multi-dose regimen of rMVA vaccination can elicit antibody responses with similar magnitude, durability, and breadth as those observed using replication-competent rVV. These data highlight that the number of inoculations is an additional parameter to be considered for optimizing an MVA vaccine regimen in humans.
Acknowledgments
We thank Sampa Santra for generous assistance and reagents. This work was supported by NIH grants AI057330, AI02042 (E.L.R. and J.S.D.) and NIH contract N01-AI30033 (N.L.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fenner F. The global eradication of smallpox. Med J Aust. 1980;1(10):455–455. doi: 10.5694/j.1326-5377.1980.tb135034.x. [DOI] [PubMed] [Google Scholar]
- 2.Mayr A. Smallpox vaccination and bioterrorism with pox viruses. Comp Immunol Microbiol Infect Dis. 2003;26(56):423–430. doi: 10.1016/S0147-9571(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 3.Slifka MK. The Future of Smallpox Vaccination: is MVA the key? Med Immunol. 2005;4(1):2. doi: 10.1186/1476-9433-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayr A, Stickl H, Muller HK, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl) Zentralbl Bakteriol [B] 1978;167(56):375–390. [PubMed] [Google Scholar]
- 5.Antoine G, Scheiflinger F, Dorner F, Falkner FG. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244(2):365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 6.Drexler I, Heller K, Wahren B, Erfle V, Sutter G. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol. 1998;79(Pt 2):347–352. doi: 10.1099/0022-1317-79-2-347. [DOI] [PubMed] [Google Scholar]
- 7.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A. 1992;89(22):10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belyakov IM, Earl P, Dzutsev A, et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003;100(16):9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulibaly S, Bruhl P, Mayrhofer J, Schmid K, Gerencer M, Falkner FG. The nonreplicating smallpox candidate vaccines defective vaccinia Lister (dVV-L) and modified vaccinia Ankara (MVA) elicit robust long-term protection. Virology. 2005;341(1):91–101. doi: 10.1016/j.virol.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Meseda CA, Garcia AD, Kumar A, et al. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology. 2005;339(2):164–175. doi: 10.1016/j.virol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Edghill-Smith Y, Bray M, Whitehouse CA, et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191(3):372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- 12.Stittelaar KJ, Kuiken T, de Swart RL, et al. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine. 2001;19(27):3700–3709. doi: 10.1016/s0264-410x(01)00075-5. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004;101(13):4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey SE, Newman FK, Kennedy JS, et al. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine. 2007;25(51):8562–8573. doi: 10.1016/j.vaccine.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrino J, McCurdy LH, Larkin BD, et al. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine. 2007;25(8):1513–1525. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vollmar J, Arndtz N, Eckl KM, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24(12):2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, Holzner A. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author's transl) Dtsch Med Wochenschr. 1974;99(47):2386–2392. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- 18.Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 19.Nigam P, Earl PL, Americo JL, et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366(1):73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stittelaar KJ, van Amerongen G, Kondova I, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 22.Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83(Pt 12):2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 23.Staib C, Drexler I, Sutter G. Construction and isolation of recombinant MVA. Methods Mol Biol. 2004;269:77–100. doi: 10.1385/1-59259-789-0:077. [DOI] [PubMed] [Google Scholar]
- 24.Santra S, Barouch DH, Korioth-Schmitz B, et al. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc Natl Acad Sci U S A. 2004;101(30):11088–11093. doi: 10.1073/pnas.0401954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santra S, Sun Y, Parvani JG, et al. Heterologous prime/boost immunization of rhesus monkeys by using diverse poxvirus vectors. J Virol. 2007;81(16):8563–8570. doi: 10.1128/JVI.00744-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman DR, Goudsmit J, Holterman L, et al. Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice. J Virol. 2008;82(14):6829–6837. doi: 10.1128/JVI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duke-Cohan JS, Wollenick K, Witten E, Seaman MS, Dolin R, Reinherz EL. The Heterogeneity of Human Antibody Responses to Orthopox Viruses Revealed Through Use of Focused Protein Arrays. Vaccine. doi: 10.1016/j.vaccine.2008.12.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joachimiak MP, Weisman JL, May B. JColorGrid: software for the visualization of biological measurements. BMC Bioinformatics. 2006;7:225. doi: 10.1186/1471-2105-7-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin CL, Chung CS, Heine HG, Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol. 2000;74(7):3353–3365. doi: 10.1128/jvi.74.7.3353-3365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsiao JC, Chung CS, Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J Virol. 1999;73(10):8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heraud JM, Edghill-Smith Y, Ayala V, et al. Subunit recombinant vaccine protects against monkeypox. J Immunol. 2006;177(4):2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- 32.Hooper JW, Thompson E, Wilhelmsen C, et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78(9):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies DH, McCausland MM, Valdez C, et al. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005;79(18):11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berhanu A, Wilson RL, Kirkwood-Watts DL, et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J Virol. 2008;82(7):3517–3529. doi: 10.1128/JVI.01854-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lustig S, Fogg C, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J Virol. 2005;79(21):13454–13462. doi: 10.1128/JVI.79.21.13454-13462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Y, Manischewitz J, Meseda CA, et al. Antibodies to the A27 protein of vaccinia virus neutralize and protect against infection but represent a minor component of Dryvax vaccine--induced immunity. J Infect Dis. 2007;196(7):1026–1032. doi: 10.1086/520936. [DOI] [PubMed] [Google Scholar]
- 37.Jones-Trower A, Garcia A, Meseda CA, et al. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology. 2005;343(1):128–140. doi: 10.1016/j.virol.2005.08.008. [DOI] [PubMed] [Google Scholar]