Abstract
Sulfobromophthalein (BSP) is used to study hepatobiliary excretory function. BSP is conjugated with glutathione (GSH), whereas its dibrominated analog disulfobromophthalein (DBSP) is not conjugated with GSH prior to biliary excretion. In addition, both BSP and DBSP are transported into hepatocytes via organic anion–transporting polypeptides and excreted into bile via multidrug resistance–associated protein 2 (Mrp2). Nuclear factor erythroid 2–related factor 2 (Nrf2) is a transcription factor that under basal conditions is targeted for proteasomal degradation in the cytosol by kelch-like ECH–associated protein 1 (Keap1). Electrophilic and oxidative stress facilitate Nrf2 nuclear translocation and subsequent induction of cytoprotective genes, including GSH synthetic enzymes, GSH-S-transferases (Gsts), and Mrp transporters. The current study determined whether varying the amount of Nrf2 activation would effect the elimination of BSP and DBSP. Male wild-type (WT), Nrf2-null, and Keap1-knockdown (Keap1-kd) mice were administered BSP or DBSP. Within 30 min, Nrf2-null mice excreted 25%, WT mice 52%, and Keap1-kd mice 80% of the injected BSP. Liver GSH content was not altered by BSP. The biliary excretion of GSH and messenger RNA (mRNA) expression of major Gsts were directly proportional to the amount of Nrf2. Moreover, BSP-GSH conjugation activity in the liver of Nrf2-null and Keap1-kd mice was 42% and 237% of WT mice, respectively. In contrast to BSP, there were no differences in biliary excretion or plasma disappearance of DBSP among the three genotypes, suggesting that the modest differences in Mrp2 mRNA expression among genotypes do not affect BSP or DBSP biliary excretion. Collectively, these results indicate that increased biliary excretion of BSP, and possibly other compounds, is due to Nrf2-induced Gst mRNA expression and enzyme activity.
Keywords: Nrf2, Gsts, BSP, biliary excretion
Sulfobromophthalein (BSP) is a prototypical compound used to assess hepatobiliary transport, biotransformation, and excretory mechanisms. BSP is transported into hepatocytes via the organic anion–transporting polypeptide (Oatp) family of uptake transporters (Cattori et al., 2001; Hagenbuch et al., 2000; Kanai et al., 1996; van Montfoort et al., 2002). BSP is conjugated with glutathione (GSH) in hepatocytes by glutathione-S-transferases (Gsts) (Alin et al., 1985; Brauer and Pessotti, 1949; Combes, 1965; Combes and Stakelum, 1960, 1961; Grodsky et al., 1959; Krebs and Brauer, 1958; Tahir et al., 1985; Yalcin et al., 1983) and then excreted from hepatocytes into the bile via the efflux transporter, multidrug resistance–associated protein 2 (Mrp2) (Cui et al., 2001; Tanaka et al., 2003). The dibrominated analog of BSP, disulfobromophthalein (DBSP), is also used to assess hepatobiliary function. Similar to BSP, DBSP is transported into the liver via Oatps and exported by Mrp2 (Johnson and Klaassen, 2002). In contrast to BSP, however, DBSP is not conjugated with GSH before biliary excretion and therefore is excreted into bile as the parent compound (Javitt, 1964).
The nuclear factor erythroid 2–related factor 2 (Nrf2)-kelch-like ECH–associated protein 1 (Keap1) pathway has been characterized over the past decade as an important endogenous cellular mechanism for coping with oxidative stress. Nrf2 is a transcription factor that promotes transcription of a battery of cytoprotective genes via antioxidant response elements (AREs) in promoter regions, thus restoring the intracellular balance between oxidants and antioxidants. Under conditions when oxidative stress is low, Keap1 sequesters Nrf2 in the cytosol by acting as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2 (Tong et al., 2006). Upon increased oxidative stress within the cell, Nrf2 circumvents Keap1-mediated proteasomal degradation and translocates into the nucleus (Bloom and Jaiswal, 2003). Once in the nucleus, Nrf2 heterodimerizes with a small musculo-aponeurotic fibrosarcoma protein and thereby promotes transcription of various cytoprotective genes through direct ARE binding (Itoh et al., 1997). Genes upregulated by Nrf2 include, but are not limited to, Mrp2, which effluxes BSP and DBSP into bile; glutamate-cysteine ligase catalytic and modifier subunits, which are responsible for the rate-limiting step in GSH synthesis and Gsts (Maher et al., 2007; Ramos-Gomez et al., 2001; Solis et al., 2002).
Because activation of the Nrf2-Keap1 pathway results in induction of Mrp2 as well as genes responsible for both the synthesis and conjugation of GSH, the present study investigated whether changes in the amount of Nrf2 in vivo (Nrf2-null, wild-type [WT], and Keap1-knockdown [Keap1-kd] mice) would alter the pharmacokinetics of BSP and DBSP. Nrf2-null mice, which have no functional Nrf2 protein, are highly susceptible to tissue injury due to a decreased capability to induce cytoprotective genes upon oxidative stress (Aoki et al., 2001; Enomoto et al., 2001; Kraft et al., 2004; Ramos-Gomez et al., 2001). Keap1-kd mice have decreased functional Keap1 protein and therefore, have increased Nrf2 in the nucleus, resulting in constitutively expressed, higher levels of cytoprotective genes (Okada et al., 2008; Reisman et al., 2009). The current study investigates the functional importance of Nrf2 on the pharmacokinetics of BSP and DBSP in WT, Nrf2-null, and Keap1-kd mice.
MATERIALS AND METHODS
Materials.
BSP was purchased from Sigma-Aldrich (St. Louis, MO). DBSP was purchased from SERB Laboratories (Paris, France). All other chemicals were purchased from Sigma-Aldrich.
Animals and husbandry.
Eight-week–old male C57BL/6 mice were purchased from Charles River Laboratories Inc. (Wilmington, MA). Nrf2-null mice were obtained from Dr Jefferson Chan (University of California, Irvine, CA) (Chan et al., 1996). Keap1-kd mice were graciously supplied by Dr Masayuki Yamamoto (Tohoku University, Aoba-ku, Sendai, Japan) (Okada et al., 2008).
Both Nrf2-null and Keap1-kd mice were backcrossed into C57BL/6 mice, and >99% congenicity was confirmed by the speed congenics group at Jackson Laboratories (Bar Harbor, ME). Animals were housed in a temperature-, light-, and humidity-controlled environment and had access to Teklad Rodent Diet #8604 (Harlan Laboratories, Madison, WI) and water ad libitum. The housing facility is an American Animal Associations Laboratory Animal Care–accredited facility at the University of Kansas Medical Center, and all procedures were preapproved in accordance with Institutional Animal Care and Use Committee guidelines.
Determination of plasma concentration and biliary excretion of BSP and DBSP.
WT, Nrf2-null, and Keap1-kd mice (n = 5, 8 weeks old) were anesthetized by injection of ketamine/midazolam (100 and 5 mg/kg, respectively, i.p.). Body temperature was maintained at 37°C by rectal probe–controlled heating pads. Subsequently, the right carotid artery was cannulated with PE-10 tubing and the common bile duct cannulated with the shaft of a 30-gauge needle attached to PE-10 tubing through a high abdominal incision. Depth of anesthesia was monitored by pinching the footpad before and throughout surgery, and if necessary, additional anesthetic drugs were administered during sample collection. Bile samples were collected in 15-min periods into preweighed 0.6-ml microcentrifuge tubes for five periods. The tubes into which bile was collected were immersed in ice. After the first bile collection, BSP (80 μmol/kg/10 ml) or DBSP (120 μmol/kg/10 ml) was injected via the carotid cannula. Thirty to 35 μl of blood were collected into heparinized tubes at −2, 7.5, 22.5, 37.5, and 52.5 min after BSP or DBSP injection. The volumes of bile samples were determined gravimetrically, taking 1.0 as specific gravity. Concentrations of BSP and DBSP in bile and plasma were quantified spectrophotometrically at 580 nm after an appropriate dilution of the samples with 0.1M sodium hydroxide.
Determination of hepatic GSH depletion by BSP.
In WT mice, the peak excretion rate of BSP was within 20 min after injection. Therefore, WT mice were surgically prepared as described above, and after a 15-min bile collection, saline or BSP (80 μmol/kg/10 ml) was injected. During these experiments, bile and plasma samples were collected at the same time points described above. The livers were removed 20 min after saline or BSP administration, frozen in liquid nitrogen, and stored at −80°C.
Total GSH.
Total GSH was quantified in bile and liver using a commercial GSH assay kit (Sigma). Bile was diluted 60× in 5% 5-sulfosalicylic acid (SSA) solution, whereas liver was homogenized (0.1 g/ml) in 5% SSA and further diluted 10× in 5% SSA. Samples were preincubated in assay buffer, GSH reductase, and 5,5′-dithiobis(2-nitrobenzoic acid) at room temperature prior to adding reduced nicotinamide adenine dinucleotide phosphate (NADPH). In the presence of NADPH, the GSH-dependent production of 5-thio-2-nitrobenzoic acid was quantified spectrophotometrically at 412 nm.
BSP-GSH conjugation.
Optimal assay conditions were described previously (Goldstein and Combes, 1966; Klaassen and Plaa, 1967). The BSP-GSH solution was prepared with 0.1M (pH 8.4) sodium pyrophosphate buffer to achieve a final solution of 226μM BSP, 21.1mM GSH, at pH 8.0. All components were incubated at 37°C, and 546 μl of incubation medium (23 μl BSP + 68 μl GSH + 455 μl pyrophosphate buffer) was added to 1-ml cuvettes. Livers were homogenized (0.4 g/ml) in 250mM sucrose-10mM Tris buffer (pH 7.4) with protease inhibitors. The cytosolic fraction was separated after two consecutive centrifugations (1000 × g for 12 min; 100,000 g for 60 min at 4°C). Cytosols were diluted 50× in sodium pyrophosphate buffer, incubated at 37°C, and 454 μl of each sample was added to the BSP and GSH mixture in 1-ml cuvettes. GSH conjugation of BSP was determined spectrophotometrically at 330 nm and corrected for the amount of BSP per cuvette, cytosolic dilution factor, protein content of sample, time in minutes of conjugation reaction, and nonenzymatic conjugation.
Statistical analysis.
All data were analyzed using one-way ANOVA followed by Duncan's multiple range test (p < 0.05).
RESULTS
Bile Flow and GSH Excretion
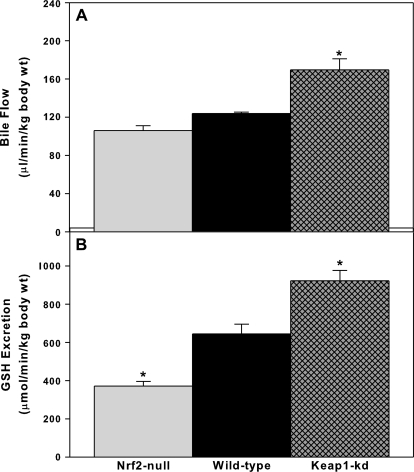
For bile flow and biliary excretion, data were normalized to body weight, as performed previously (Gregus and Klaassen, 1982; Slitt et al., 2003). Mean body weight was not significantly different among 8-week–old WT (26 g), Nrf2-null (24 g), and Keap1-kd (26 g) mice. Bile flow tended to be lower in Nrf2-null mice, whereas it was higher (∼30%) in Keap1-kd mice (Fig. 1A). Nrf2-null mice excreted GSH 30% slower, whereas Keap1-kd mice excreted GSH 30% faster than WT mice (Fig. 1B).
FIG. 1.
Basal bile flow (A) and GSH excretion (B) in WT, Nrf2-null, and Keap1-kd mice (n = 5). Values are expressed as mean ± SEM. *indicate a statistically significant difference from WT mice (p ≤ 0.05).
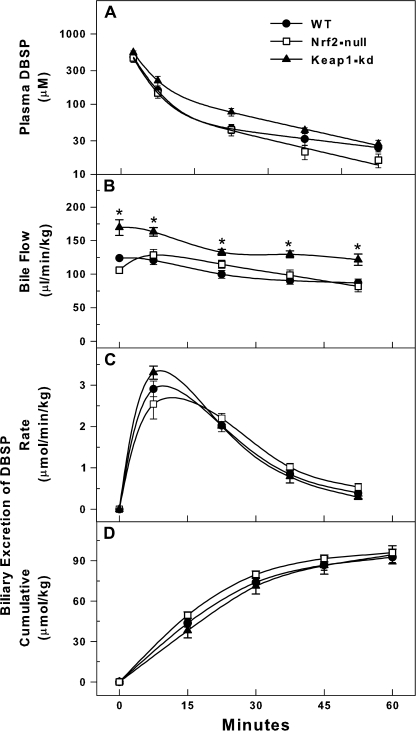
Plasma Concentration and Biliary Excretion of BSP
Nrf2-null mice had slower plasma disappearance of BSP than WT mice, whereas there was not a statistical difference in the plasma disappearance of BSP between Keap1-kd and WT mice (Fig. 2A). After BSP injection, Nrf2-null mice continued to have a slower bile flow, whereas Keap1-kd mice had a higher bile flow than WT mice (Fig. 2B). Nrf2-null mice had slower biliary excretion of BSP and lower cumulative biliary excretion of BSP than WT mice (Fig. 2C). In contrast, Keap1-kd mice had faster biliary excretion of BSP and higher cumulative biliary excretion of BSP than WT mice (Fig. 2D). Thirty minutes after BSP injection, Nrf2-null mice excreted 25%, WT mice 52%, and Keap1-kd mice 80% of the injected BSP.
FIG. 2.
Plasma disappearance (A), bile flow (B), biliary excretion (C), and cumulative biliary excretion (D) of BSP (80 μmol/kg) in WT, Nrf2-null, and Keap1-kd mice (n = 5). Values are expressed as mean ± SEM. *indicate a statistically significant difference from WT mice (p ≤ 0.05).
Plasma Concentration and Biliary Excretion of DBSP
There were no differences in plasma disappearance, biliary excretion, or cumulative biliary excretion of DBSP in WT, Nrf2-null, and Keap1-kd mice (Figs. 3A, C, and D). However, after DBSP administration, Keap1-kd mice maintained higher bile flow (Fig. 3B). It should be noted that Keap1-kd mice also had a higher bile flow rate than WT mice before injection of DBSP (Fig. 1A).
FIG. 3.
Plasma disappearance (A), bile flow (B), biliary excretion (C), and cumulative biliary excretion (D) of DBSP (120 μmol/kg) in WT, Nrf2-null, and Keap1-kd mice (n = 5). Values are expressed as mean ± SEM. *indicate a statistically significant difference from WT mice (p ≤ 0.05).
Effect of BSP Administration on Hepatic GSH Concentration
Twenty minutes after BSP (80 μmol/kg) administration, GSH concentrations were quantified in WT mice to determine whether GSH depletion after BSP administration might play a role in biliary excretion of BSP. GSH concentrations in liver were not decreased by BSP (Fig. 4).
FIG. 4.
Liver GSH concentration in WT mice 20 min after intravascular injection of saline or BSP (80 μmol/kg, n = 5). No differences were observed between treated and untreated groups.
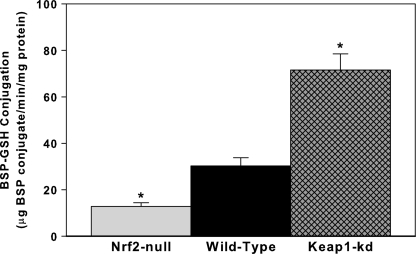
BSP-GSH Conjugation Activity
BSP-GSH conjugation activity was determined in hepatic cytosol fractions. Consistent with the biliary excretion of BSP, conjugation of BSP with GSH is 42% less in Nrf2-null mice than in WT mice, whereas Keap1-kd mice have 137% higher conjugation activity than WT mice (Fig. 5).
FIG. 5.
BSP-GSH conjugation activity in WT, Nrf2-null, and Keap1-kd mice (n = 5). *indicate a statistically significant difference from WT mice (p ≤ 0.05).
DISCUSSION
BSP has been used for decades as a model compound to assess hepatobiliary function, both clinically and experimentally. In the present study, BSP was used to elucidate the role of Nrf2 in hepatobiliary function. Nrf2 is a transcription factor that upon activation induces a wide variety of cytoprotective genes, such as GSH synthesis enzymes, Gsts, and Mrps; all of which play a role in biliary excretion of xenobiotics.
Basal bile flow tends to be lower in Nrf2-null mice, whereas it is higher in Keap1-kd mice. These trends in bile flow correlate with the decreased and increased biliary excretion of GSH in Nrf2-null and Keap1-kd mice, respectively (Fig. 1B). GSH provides the osmotic force that drives bile acid–independent bile flow (Ballatori and Truong, 1989, 1992). In addition, there were no differences in the biliary excretion of total bile acids among the three genotypes (p > 0.05, data not shown), as quantified colorimetrically (Bioquant, San Diego, CA).
Among WT, Nrf2-null, and Keap1-kd mice, Nrf2-null mice have the slowest disappearance of BSP from the plasma, slowest bile flow after BSP, slowest biliary excretion of BSP, and lowest cumulative excretion of BSP (Fig. 2). The opposite is true when Nrf2 is more highly activated (Keap1-kd mice), thus there is a direct correlation with the amount of activated Nrf2 and plasma disappearance of BSP, bile flow, and biliary excretion of BSP.
Clearly, Nrf2 influences bile flow and GSH biliary excretion; however, because Nrf2 affects multiple pathways that could affect BSP biliary excretion, additional experiments were performed to elucidate how Nrf2 alters BSP disposition. To investigate the possible role of transporters, the dibrominated analog of BSP, namely DBSP, was used in experiments similar to those performed with BSP. DBSP is transported into hepatocytes and effluxed into bile by the same transporters (Oatps and Mrp2) as BSP, but unlike BSP, DBSP is not conjugated with GSH. Therefore, if BSP biliary excretion differences are due to a transporter-mediated phenomenon rather than a biotransformation phenomenon, then there should be differences in the biliary excretion of DBSP among the three genotypes. However, no such differences in plasma disappearance and biliary excretion of DBSP were observed among the three genotypes, suggesting that transporter activity (at the dose of BSP administered) is not responsible for the changes observed in the biliary excretion of BSP (Fig. 3). Further evidence for a lack of transporter involvement in the differences observed in the biliary excretion of BSP among the three genotypes was reported previously, where only minor differences, if any, were observed in the messenger RNA (mRNA) expression of Oatps and Mrp2 among WT, Nrf2-null, and Keap1-kd mice (Reisman et al., 2009).
After parallel studies with DBSP failed to show differences in the biliary excretion among the genotypes, BSP metabolism was considered in more detail. Because BSP is conjugated with GSH before biliary excretion, and GSH excretion is a determinant of bile acid–independent flow, experiments were conducted to determine whether the differences in biliary excretion of BSP were due to depletion of GSH after BSP administration. The lack of decrease in GSH concentrations 20 min following BSP administration demonstrates that differences in biliary excretion of BSP are not due to depletion of the cosubstrate, GSH (Fig. 4).
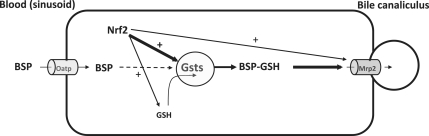
Because the aforementioned studies suggested Nrf2-dependent differences in BSP biliary excretion might be due to GSH conjugation, Gst activity and mRNA expression were quantified. Interestingly, Nrf2-null mice have 42% less and Keap1-kd mice have 137% more enzyme activity to conjugate BSP with GSH, than do WT mice. Furthermore, the mRNA expression of eight Gst isoforms, namely Gsta1, a4, m1, m2, m3, m4, m6, and p2 were 40–85% lower in Nrf2-null mice and 20–58.5% higher in Keap1-kd mice, as summarized in Table 1 (Reisman et al., 2009). The differences in mRNA expression of Gsts correlates with the differences in the activity of liver to conjugate BSP with GSH and the biliary excretion of BSP in WT, Nrf2-null, and Keap1-kd mice. A proposed mechanism by which Nrf2 modulates BSP biliary excretion is summarized in Figure 6.
TABLE 1.
Summary of Gst Liver mRNA Expression
| Gene | Nrf2-null mice | Keap1-kd mice |
| Gsta1 | ↓ | ↑ |
| Gsta4 | ↓ | ↑ |
| Gstm1 | ↓ | ↑ |
| Gstm2 | ↓ | ↑ |
| Gstm3 | ↓ | ↑ |
| Gstm4 | ↓ | ↑ |
| Gstm6 | ↓ | ↔ |
| Gstp2 | ↔ | ↑ |
The hepatic Gst mRNA expression from a previous report is summarized (Reisman et al., 2009). Down arrows (↓) represent a statistically significant decrease (p < 0.05) in mRNA expression, as compared to WT mice. Up arrows (↑) represent a statistically significant increase (p < 0.05) in mRNA expression, as compared to WT mice. Double-sided arrows (↔) represent no difference (p > 0.05) from WT mice.
FIG. 6.
Summary of Nrf2-dependent BSP excretion. Enhanced Nrf2 activation increases BSP biliary excretion by increasing the mRNA expression and activity of Gsts. BSP excretion is not affected by uptake transporters (Oatps), the efflux transporter Mrp2, or GSH content at the dose of BSP administered in this model.
Numerous studies have shown that Nrf2 protects against toxicity, which is frequently attributed to decreased toxicodynamic effects, as exemplified by increased expression of cytoprotective genes, such as NAD(P)H:quinone oxidoreductase 1 (Nqo1), which contribute to protection against oxidative or electrophilic stress (Aleksunes and Manautou, 2007). The present study emphasizes the importance of the Nrf2-Keap1 pathway on the toxicokinetics of chemicals. Whereas activation of Nrf2 protects cells from electrophiles through induction of cytoprotective genes, Nrf2 also increases biliary excretion of potentially toxic xenobiotics, as exemplified by increased GSH conjugation and biliary excretion of BSP, which represents another important defense mechanism.
FUNDING
National Institute of Health ES09716, ES07079, ES013714, ES09649, and RR021940).
Acknowledgments
The authors thank the Klaassen laboratory for technical support and manuscript revision assistance. Nrf2-null mice were graciously provided by Dr Jefferson Chan (University of California, Irvine, CA) and Keap1-kd mice by Dr Masayuki Yamamoto (Tohoku University, Aoba-ku, Sendai, Japan).
References
- Aleksunes LM, Manautou JE. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol. Pathol. 2007;35:459–473. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- Alin P, Jensson H, Guthenberg C, Danielson UH, Tahir MK, Mannervik B. Purification of major basic glutathione transferase isoenzymes from rat liver by use of affinity chromatography and fast protein liquid chromatofocusing. Anal. Biochem. 1985;146:313–320. doi: 10.1016/0003-2697(85)90545-7. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Truong AT. Relation between biliary glutathione excretion and bile acid-independent bile flow. Am. J. Physiol. 1989;256:G22–G30. doi: 10.1152/ajpgi.1989.256.1.G22. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Truong AT. Glutathione as a primary osmotic driving force in hepatic bile formation. Am. J. Physiol. 1992;263:G617–G624. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- Brauer RW, Pessotti RL. The removal of bromsulphthalein from blood plasma by the liver of the rat. J. Pharmacol. Exp. Ther. 1949;97:358–370. [PubMed] [Google Scholar]
- Cattori V, van Montfoort JE, Stieger B, Landmann L, Meijer DK, Winterhalter KH, Meier PJ, Hagenbuch B. Localization of organic anion transporting polypeptide 4 (Oatp4) in rat liver and comparison of its substrate specificity with Oatp1, Oatp2 and Oatp3. Pflugers Arch. 2001;443:188–195. doi: 10.1007/s004240100697. [DOI] [PubMed] [Google Scholar]
- Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes B. The importance of conjugation with glutathione for sulfobromophthalein sodium (Bsp) transfer from blood to bile. J. Clin. Invest. 1965;44:1214–1224. doi: 10.1172/JCI105227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes B, Stakelum GS. Conjugation of sulfobromophthalein sodium with glutathione in thioether linkage by the rat. J. Clin. Invest. 1960;39:1214–1222. doi: 10.1172/JCI104137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes B, Stakelum GS. A liver enzyme that conjugates sulfobromophthalein sodium with glutathione. J. Clin. Invest. 1961;40:981–988. doi: 10.1172/JCI104337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Konig J, Keppler D. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol. Pharmacol. 2001;60:934–943. doi: 10.1124/mol.60.5.934. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Combes B. Spectrophotometric assay of the liver enzyme that catalyzes sulfobromophthalein-glutathione conjugation. J. Lab. Clin. Med. 1966;67:863–872. [PubMed] [Google Scholar]
- Gregus Z, Klaassen CD. Role of ligandin as a binding protein and as an enzyme in the biliary excretion of sulfobromophthalein. J. Pharmacol. Exp. Ther. 1982;221:242–246. [PubMed] [Google Scholar]
- Grodsky GM, Carbone JV, Fanska R. Identification of metabolites of sulfobromophthalein. J. Clin. Invest. 1959;38:1981–1988. doi: 10.1172/JCI103977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Adler ID, Schmid TE. Molecular cloning and functional characterization of the mouse organic-anion-transporting polypeptide 1 (Oatp1) and mapping of the gene to chromosome X. Biochem. J. 2000;345(Pt 1):115–120. [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Javitt NB. Phenol 3, 6 dibromphthalein disulfonate, a new compound For the study of liver disease. Proc. Soc. Exp. Biol. Med. 1964;117:254–257. doi: 10.3181/00379727-117-29550. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Klaassen CD. Role of rat multidrug resistance protein 2 in plasma and biliary disposition of dibromosulfophthalein after microsomal enzyme induction. Toxicol. Appl. Pharmacol. 2002;180:56–63. doi: 10.1006/taap.2002.9375. [DOI] [PubMed] [Google Scholar]
- Kanai N, Lu R, Bao Y, Wolkoff AW, Schuster VL. Transient expression of oatp organic anion transporter in mammalian cells: Identification of candidate substrates. Am. J. Physiol. 1996;270:F319–325. doi: 10.1152/ajprenal.1996.270.2.F319. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Plaa GL. Species variation in metabolism, storage, and excretion of sulfobromophthalein. Am. J. Physiol. 1967;213:1322–1326. doi: 10.1152/ajplegacy.1967.213.5.1322. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs JS, Brauer RW. Metabolism of sulfobromophthalein sodium (BSP) in the rat. Am. J. Physiol. 1958;194:37–43. doi: 10.1152/ajplegacy.1958.194.1.37. [DOI] [PubMed] [Google Scholar]
- Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G735–G747. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman SA, Yeager RL, Yamamoto M, Klaassen CD. Increased Nrf2 Activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol. Sci. 2009;108:35–47. doi: 10.1093/toxsci/kfn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slitt AL, Cherrington NJ, Maher JM, Klaassen CD. Induction of multidrug resistance protein 3 in rat liver is associated with altered vectorial excretion of acetaminophen metabolites. Drug Metab. Dispos. 2003;31:1176–1186. doi: 10.1124/dmd.31.9.1176. [DOI] [PubMed] [Google Scholar]
- Solis WA, Dalton TP, Dieter MZ, Freshwater S, Harrer JM, He L, Shertzer HG, Nebert DW. Glutamate-cysteine ligase modifier subunit: Mouse Gclm gene structure and regulation by agents that cause oxidative stress. Biochem. Pharmacol. 2002;63:1739–1754. doi: 10.1016/s0006-2952(02)00897-3. [DOI] [PubMed] [Google Scholar]
- Tahir MK, Guthenberg C, Mannervik B. Inhibitors for distinction of three types of human glutathione transferase. FEBS Lett. 1985;181:249–252. doi: 10.1016/0014-5793(85)80269-6. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sano N, Takikawa H. Biliary excretion of phenolphthalein sulfate in rats. Pharmacology. 2003;68:177–182. doi: 10.1159/000070456. [DOI] [PubMed] [Google Scholar]
- Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: A hinge and latch mechanism. Biol. Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- van Montfoort JE, Schmid TE, Adler ID, Meier PJ, Hagenbuch B. Functional characterization of the mouse organic-anion-transporting polypeptide 2. Biochim. Biophys. Acta. 2002;1564:183–188. doi: 10.1016/s0005-2736(02)00445-5. [DOI] [PubMed] [Google Scholar]
- Yalcin S, Jensson H, Mannervik B. A set of inhibitors for discrimination between the basic isozymes of glutathione transferase in rat liver. Biochem. Biophys. Res. Commun. 1983;114:829–834. doi: 10.1016/0006-291x(83)90856-2. [DOI] [PubMed] [Google Scholar]