Abstract
The growth factor proepithelin functions as an important regulator of proliferation and motility. Proepithelin is overexpressed in a great variety of cancer cell lines and clinical specimens of breast, ovarian and renal cancer, as well as glioblastomas. Using recombinant proepithelin on 5637 transitional cell carcinoma-derived cells, we have shown previously that proepithelin plays a critical role in bladder cancer by promoting motility of bladder cancer cells. In this study, we used the ONCOMINE database and gene microarray analysis tool to analyze proepithelin expression in several bladder cancer microarray studies. We found a statistically significant increase in proepithelin messenger RNA expression in bladder cancers vis-à-vis non-neoplastic tissues, and this was associated with pathologic and prognostic parameters. Targeted downregulation of proepithelin in T24 transitional carcinoma cells with small hairpin RNA inhibited both Akt and mitogen-activated protein kinase pathways, severely reduced the ability of T24 cells to proliferate in the absence of serum and inhibited migration, invasion and wound healing. In support of these in vitro results, we discovered that proepithelin expression was significantly upregulated in invasive bladder cancer tissues compared with normal urothelium. In addition, proepithelin was secreted in the urine, where it was detectable by immunoblotting and enzyme-linked immunosorbent assay. Collectively, these results support the hypothesis that proepithelin may play a critical role as an autocrine growth factor in the establishment and progression of bladder cancer and suggest that proepithelin may prove a novel biomarker for the diagnosis and prognosis of bladder neoplasms.
Introduction
Proepithelin, also known as PC cell-derived growth factor, granulin–epithelin precursor, GP88, progranulin or acrogranin, is a growth factor that plays a critical role in development, cell cycle progression, cell motility and tumorigenesis (1).
Proepithelin, originally isolated from different sources by several independent laboratories (2–5), is translated as a 593 amino acid protein with a predicted molecular weight of ∼68 kDa, but due to its high degree of glycosylation, it is typically secreted as a ∼90 kDa protein (4,6). The precursor protein has a secretory signal peptide and 7½ cystein-rich 6 kDa tandem repeats (1).
Proepithelin undergoes elastase-mediated proteolytic processing (4) with the liberation of small, ∼6 kDa peptides, which retain biological activity but usually exert opposite biological function compared with the precursor protein (7). The secretory leukocyte protease inhibitor counteracts this proteolysis by either direct binding to elastase or by sequestering epithelin peptides from the enzyme (4).
A vast body of evidence has now established that proepithelin plays a critical role in tumorigenesis (1). In several breast cancer cell lines, proepithelin expression correlates with an aggressive phenotype (8,9) and immunoneutralization of proepithelin inhibits estrogen-mediated proliferation of MCF-7 cells (10). Block of proepithelin expression by antisense strategy inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468 (9). In SW13 carcinoma cells, proepithelin-dependent activation of the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase (MAPK) pathways protects cells from anoikis, confers anchorage-independent growth and promotes tumor formation in nude mice (11).
Moreover, high proepithelin expression plays also a significant role in adipocytic teratoma, glioblastomas, multiple myeloma and renal cell, gastric and ovarian carcinomas (1,12–17).
Recently, our laboratory has shown that recombinant proepithelin promotes migration, wound healing and invasion of 5637 bladder cancer cells, supporting the evidence that proepithelin may play as well a critical role in bladder cancer (6). Proepithelin is expressed in bladder cancer cells suggesting that endogenous proepithelin might also contribute in an autocrine fashion to these processes.
In this study, we used the ONCOMINE database and gene microarray analysis tool to analyze proepithelin expression in available bladder cancer microarray studies. In several independent data sets, we found a statistically significant increase in proepithelin messenger RNA (mRNA) expression in bladder cancers compared with non-neoplastic tissues. Significantly, proepithelin overexpression correlated with pathologic and prognostic parameters.
We confirmed that endogenous proepithelin expression contributes to the transforming phenotype of bladder cancer cells by targeting endogenous proepithelin in T24 transitional cell carcinoma-derived cells by shRNA approaches. Stable proepithelin depletion was associated with a severe reduction of the activation of the Akt and MAPK pathways, inhibited the ability of T24 cells to proliferate in the absence of serum and impaired motility of these cells.
Immunohistochemical staining revealed that proepithelin expression was significantly higher in invasive bladder tumors as compared with normal bladder tissues. Furthermore, proepithelin was detectable in the urine by both immunoblotting and enzyme-linked immunosorbent assay (ELISA).
Collectively, these results support the hypothesis that proepithelin may play a critical role as an autocrine growth factor in the establishment and progression of bladder cancer and it may prove a novel clinical biomarker for diagnosis and prognosis in bladder tumors.
Materials and methods
Complementary DNA microarray analysis
The ONCOMINE database and gene microarray analysis tool, a repository for published complementary DNA microarray data (http://www.oncomine.org) (18,19), were explored (15 September 2008) for mRNA expression of proepithelin (progranulin or PC cell-derived growth factor) in non-neoplastic and bladder cancers. Statistical analysis of the differences in proepithelin expression between the aforementioned tissues was accomplished through use of ONCOMINE algorithms, which allow for multiple comparisons among different studies (18–20). Only studies with analysis results with P < 0.05 were considered.
Cell lines
Transitional cell carcinoma-derived human T24 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in RPMI medium supplemented with 10% fetal bovine serum. Serum-free medium (SFM) is Dulbecco’s modified Eagle’s medium supplemented with 0.1% bovine serum albumin and 50 μg/ml of transferrin (Sigma–Aldrich, St. Louis, MO).
T24 cells with the stable depletion of endogenous proepithelin and controls were generated by transfecting the pRS vector, the pRS-shRNA GFP and pRS shPEP plasmids (OriGene Technologies, Rockville, MD). The sequence of the proepithelin-specific 29mer shRNA (TI350373) is ‘GAGTAAGTGCCTCTCCAAGGAGAACGCTA’.
T24 cells were transfected using the Amaxa Nucleofector system (Amaxa, Lonza Group, Walkersville, MD). Ten microliters of cell suspension (2 × 106) was mixed with 2 μg of plasmid DNA and 100 μl of Nucleofector Solution R using program T-030. Cells were then plated onto 100 mm plates and selected in medium supplemented with 1 μg/ml of puromycin. After selection, the pool of proepithelin-depleted T24 cells was tested for proepithelin expression levels in cell lysates and media by immunoblot using anti-proepithelin polyclonal antibodies (USB Biologicals, MA). Proepithelin levels in conditioned media (CM) from proepithelin-depleted, vector-transfected, parental T24 cells and urine samples were quantified using a proepithelin-specific ELISA (ALPCO Diagnostics, Salem, NH).
Cell growth and migration assays
Various T24 cells were plated in triplicate at a density of 3 × 104 cells per 35 mm plates in serum-supplemented medium. After 24 h, cells were washed three times in Dulbecco’s modified Eagle’s medium and transferred to SFM. Cells were counted after 24 and 48 h with a hemocytometer.
HTS FluoroBloks™ inserts (Becton Dickinson, Franklin Lakes, NJ) were saturated for 2 h at room temperature with phosphate-buffered saline–1% bovine serum albumin. After 24 h of starvation in SFM, cells were labeled with DiI (Molecular Probes, Invitrogen Corporation, Carlsbad, CA) for 20 min at 37°C and then seeded in the HTS FluoroBloks™ upper chamber and incubated at 37°C for 24 h. Migration was blocked by fixing the HTS FluoroBloks™ membranes in 4% paraformaldehyde. The HTS FluoroBloks™ membranes were mounted on a slide and migrated cells were counted and photographed with a Zeiss Axiovert 200M cell live microscope.
Wound healing and invasion assays
T24 cells were seeded onto 35 mm plates in serum-containing medium until subconfluence and then transferred to SFM. After 24 h, the plates were scratched with a thin disposable tip to generate a wound (500 μM) in the cells’ monolayer (6). Cells were analyzed and photographed after 3 and 6 h with a Zeiss Axiovert 200M cell live microscope using the Metamorph Image Acquisition and Analysis software (Universal Imaging, Ypsilanti, MI) at the Kimmel Cancer Center Confocal Microscopy Core Facility. Cell invasion through a three-dimensional (3D) extracellular matrix was assessed by a Matrigel invasion assay using BD Matrigel™ Invasion Chambers (BD Biocoat) with 8.0 μm filter membranes. Cells (5 × 104) in 200 μl of SFM were plated onto each filter and 750 μl of SFM placed in the lower chamber. After 24 h, filters were washed, fixed and stained with Coomassie brilliant blue. Cells on the upper surface of the filters were removed with cotton swabs. Cells that had invaded to the lower surface of the filter were counted under the microscope.
For 3D evasion assay (21), cells (5000 per drop) were included in Matrigel drops (6 mg/ml, Becton Dickinson). The drops were incubated for 3, 4 and 5 days in SFM or complete medium as control. Cell motility was analyzed by microscopy. Images were collected using a digital camera (Nikon Instruments Inc., Melville, NY) and cells outside each drop (five drops per cell line per experiment) were counted. The evasion ability was also determined by measuring the distance covered by the cells from the drop edges, after 5 days from inclusion. To perform this analysis, cells were stained with crystal violet and then pictures were taken. The distance covered was calculated by converting pixel to millimeters.
Immunoblot detection of activated signaling pathways
CM from T24, T24/pRS and T24/shPEP was prepared as described previously (22). Serum-starved T24 cells were then stimulated with the CM for 10 and 30 min. The activation of p90RSK, Akt, extracellular signal-regulated kinase 1/2 (ERK1/2) and S6 ribosomal protein was analyzed by western immunoblot using the PathScan Multiplex Western Cocktail I (Cell Signaling Technology, Inc., Danvers, MA) that provides a mix of phospho-specific antibodies for different activated protein. ElF4E protein is the control to monitor the loading of the samples.
Immunohistochemical detection of proepithelin in normal and cancer bladder tissue specimens
Immunohistochemical analysis of proepithelin levels in bladder tissues was performed as described previously (23). Formalin-fixed, paraffin-embedded sections from five primary urothelial cell carcinomas were obtained from the Pathology Tissue Bank of Thomas Jefferson University and used to analyze the expression of proepithelin in normal and cancerous tissues. Informed consent to use excess pathological specimens for research purposes was obtained from all five patients. Slides were incubated overnight at room temperature with 1:100 dilution (100 ng/ml) of an anti-human monoclonal anti-proepithelin (PC cell-derived growth factor/GP88) antibody (A&G Pharmaceutical, Columbia, MD). We have previously tested the specificity of anti-proepithelin antibody in normal bladder stained with primary antibody after affinity depletion with blocking peptide (Santa Cruz Biotechnology, Inc., Santa Curz, CA, data not shown). An additional negative control was used by omitting the primary antibody and replacing it with preimmune serum.
Statistical analysis
Experiments were carried out in triplicate and repeated at least three times. Results are expressed as mean ± SD. All statistical analyses were carried out with SigmaStat for Windows version 3.10 (Systat Software, Port Richmond, CA). Results were compared by using the two-sided Student's t-test. Differences were considered statistically significant at P < 0.05.
Results
Proepithelin is overexpressed in bladder cancer
We have recently shown that human recombinant proepithelin promotes migration, wound healing and invasion of 5637 bladder cancer cells, supporting the evidence that proepithelin may play a critical role in bladder cancer (6). Proepithelin is expressed in bladder cancer cells (data not shown) suggesting that endogenous proepithelin may contribute in an autocrine fashion to the transforming phenotype.
In order to determine whether proepithelin is overexpressed in bladder cancer, we analyzed proepithelin expression in different publicly available bladder cancer microarray studies using the ONCOMINE database and gene microarray data analysis tool (18,19). The analysis evaluated proepithelin mRNA expression levels for each of the individual studies as well as performing a summary statistic, taking into account the significance of the gene expression across the considered studies. In two independent data sets (Figure 1A, classes A and B), there was a statistically significant increase of proepithelin mRNA expression levels in primary bladder cancers compared with non-neoplastic controls (P = 8 × 10−6) (24,25). Significantly, proepithelin overexpression was associated with pathologic and prognostic parameters. Proepithelin mRNA levels were increased in high-grade compared with low-grade bladder cancer (class C) and in patients dead after 5 years of follow-up treatment compared with patients alive after 5 years of follow-up treatment (class D) (26). Proepithelin was also overexpressed in T1 versus Ta stage cancers (class E) (27).
Fig. 1.
Proepithelin mRNA is overexpressed in bladder cancer and is required for cell growth of T24 transitional cell carcinoma-derived cells in the absence of serum. (A) Expression array analysis of multiple bladder cancer microarray data sets was analyzed and statistical significance was calculated. Classes A and B proepithelin expression in normal tissues and bladder cancer; in blue is indicated the expression in normal tissues, whereas in red the expression in bladder cancers. (A) Sanchez-Carbayo et al. (24) (t-test = −7.768; P = 6.3 × 10−12; 52 normal urothelium, 105 bladder tumors: 33 superficial and 72 invasive); (B) Dyrskjot et al. (25) (t-test = −4.98; P = 1.6 × 10−5; 14 normal urothelium, 40 bladder tumors: 27 superficial and 13 invasive). Classes C, D and E: proepithelin expression associated to pathologic and prognostic parameters. (C) Blaveri et al. (26) (t-test = −3.41; P = 0.004); in blue is indicated the expression in low-grade bladder cancers, whereas in red the expression in high-grade cancers. (D) Blaveri et al. (26) (t-test = −2.405; P = 0.025); in blue is indicated the expression in patients alive after 5 years of follow-up after treatment, whereas in red the expression in patients dead in the 5 years of follow-up. (E) Lindgren et al. (27) (t-test = −2.057; P = 0.05); in blue is indicated the expression in Ta stage cancers, whereas in red the expression in T1 stage cancers. (B) The generation of T24/pRS and T24/shPEP has been described in Materials and Methods. Proepithelin expression in lysates and CM (medium) (40 μg) of parental (P), vector-transfected (C) and shRNA proepithelin T24 cells (shPEP) was detected by immunoblot. (C) Cell growth in SFM was determined as described in Materials and Methods. The experiment is the average of four independent experiments run in duplicates ± standard deviation. *P < 0.05 compared with vector-transfected control cells (second and fifth columns). At 48 h, SFM of proepithelin-depleted cells was also supplemented with 400 ng/ml of recombinant proepithelin (shPEP + PEP). ♦P < 0.05 compared with proepithelin-depleted T24 cells in SFM.
Collectively, these results strongly suggest that proepithelin may play a critical role as an autocrine growth factor in the establishment and progression of bladder cancer.
Because T24 bladder cancer cells produce considerable levels of proepithelin and exhibit the ability to proliferate and migrate in the absence of serum, we determined whether endogenously produced proepithelin could contribute to the transforming phenotype of T24 cells. Therefore, we generated T24 cells stably expressing a 29mer shRNA construct targeting proepithelin. Mock-, vector- and shRNA proepithelin-transfected T24 cells were selected in medium containing puromycin and resistant cells were pooled and tested for proepithelin expression. Immunoblot analysis showed that shRNA proepithelin complementary DNA transfection significantly reduced proepithelin protein expression in T24 cells in both cell lysates and CM (Figure 1B), compared with parental and vector-transfected T24 cells. We also quantified the amount of proepithelin secreted in the CM by an ELISA. Parental and vector-transfected T24 cells produced comparable amount of proepithelin, whereas shRNA-transfected T24 showed a 3-fold decrease in proepithelin secretion (Table I).
Table I.
Proepithelin levels in the various T24 cell lines
| Cell line | Proepithelin (ng/ml) |
| T24 | 360.8 ± 9.4 |
| T24/pRS | 393.1 ± 52.3 |
| T24/shPEP | 127.0 ± 25.8 |
Proepithelin levels were determined by ELISA in CM collected after 72 h incubation in SFM. Volumes were normalized on the number of cells. Data are the average of three independent experiments ± standard deviation.
Proepithelin depletion induced a considerable reduction of cell proliferation in serum-deprived condition of T24 cells compared with parental and vector-transfected cells (Figure 1C). The ability of proepithelin-depleted T24 cells to grow was significantly restored at 48 h by supplementing the SFM with 400 ng/ml of recombinant proepithelin (6) (Figure 1C), confirming that the reduction in cell proliferation of T24/shPEP cells is due to reduced proepithelin secretion.
Collectively, our results strongly indicate that endogenously produced proepithelin contributes to the ability of T24 bladder cancer cells to grow in the absence of serum.
Although the ability of growing in the absence of serum is one critical feature of transformed cells, cancer cells are not completely deprived of growth factors in vivo. We therefore repeated cell growth experiments in the presence of reduced concentration of serum (5% fetal bovine serum). Proepithelin-depleted T24 cells showed a statistically significant reduction of cell proliferation compared with parental and vector-transfected T24 cells (supplemental Figure 1 is available at Carcinogenesis Online) indicating that proepithelin production is a critical component to sustain the ability of T24 cells to grow in reduced serum condition.
Endogenous proepithelin promotes motility of T24 cells
Next, we examined cellular motility in the various T24 cell lines using an in vitro ‘wound healing’ motility assay (6). T24 cells were plated at high density in serum-containing medium. After 24 h starvation in SFM, confluent T24 cells were wounded (Figure 2A, wound) and incubated for 3 and 6 h in SFM. Parental and vector-transfected T24 control cells showed substantial migration of the cells into the denuded area (Figure 2A). In contrast, shRNA proepithelin-transfected T24 cells were severely inhibited in their capacity to fill the wound (Figure 2A).
Fig. 2.
Endogenous proepithelin promotes in vitro closure of a wound and invasion of T24 cells. (A) The in vitro wound healing motility assay in T24 cells in SFM was performed as described in Materials and Methods. Cells were analyzed with a cell live microscope using the Metamorph Image Acquisition and Analysis software (Universal Imaging) (×100). Ten fields per plate were examined. (B) For migration assay, T24 cells were seeded on FluoroBloks and allowed to migrate for 24 h. Top, representative fields of migrated T24 cells (magnification, ×100); bottom, quantification of migrated T24 cells. The data are the average of three independent experiments run in duplicates ± standard deviation. (C) Quantification of invading T24 cells plated on Matrigel-coated FluoroBloks and allowed to invade for 24 h. Data are expressed as invading cells per field. Mean ± SD of three independent experiments done in duplicate. *P < 0.01 versus T24 cells; ♦P < 0.01 versus vector-transfected T24 cells.
Because the ability of T24 cells to close a wound is a very quick response compared with cell proliferation, we can rule out the possibility that the reduction of cell proliferation of proepithelin-depleted cells may contribute to the reduced healing capacity of these cells in a wound healing motility assay. In addition, these results suggest that the ability of proepithelin to promote lateral motility (wound healing) can be separated from the capacity to induce cell proliferation.
We further determined the ability of proepithelin to induce migration of the various T24 bladder cancer cell lines testing migration through Transwells (6). Proepithelin-depleted T24 cells were significantly inhibited in the ability to migrate (Figure 2B) compared with parental and vector-transfected T24 cells.
These results indicate that endogenous production of proepithelin plays an important role in regulating both the directional motility both in monolayer and through Transwells in the absence of serum, one of the distinctive features of transformed cells.
Proepithelin enhances invasion and evasion of bladder cancer cells
The acquisition by cancer cells of an invasive phenotype is a critical step for tumor progression (28). Matrigel-coated filters are widely used to examine invasive migration through a 3D extracellular matrix (1,29). Proepithelin-depleted T24 cells showed a marked decrease in the ability to traverse Matrigel-coated filters after 48 h in the absence of serum as compared with parental and vector-transfected cells (Figure 2C).
We also determined the ability of T24 cells to evade from the extracellular matrix by measuring evasion from Matrigel drops (21). Downregulation of endogenous proepithelin significantly decreased the number of cells able to evade from the drop (Figure 3A and B). Moreover, the migration distance covered by T24/shPEP cells was markedly suppressed as compared with parental and V-control T24 cells (Figure 3C).
Fig. 3.
Endogenous proepithelin stimulates the ability of T24 cells to evade from Matrigel drops. (A) T24, T24/pRS and T24/shPEP cells were included in a Matrigel drop and allowed to evade for 2, 3 and 5 days. The data are the average of three independent experiments in quintuplicate (±standard deviation). *, **, ***P < 0.05 compared with vector-transfected control cells. (B) A typical image from three independent experiments performed in quintuplicate is shown. The dotted line indicates the edge of Matrigel drops. (C) Quantification of the migration distance covered by the various cell lines over a 5 days period. The distance covered was calculated by converting pixel to millimeters. The data are the average of three independent experiments in quintuplicate (±SD). *P < 0.05 compared with vector-transfected control cells.
Collectively, our results suggest that endogenously produced proepithelin not only stimulates the migratory ability of urothelial cancer cells but also the cells’ ability to migrate through and from a complex 3D matrix such as Matrigel.
Proepithelin depletion inhibits the activation of the MAPK- and Akt-signaling pathways
Previous studies from our laboratory have shown that recombinant proepithelin induced the activation of the MAPK pathway in 5637 bladder cancer cells to stimulate cell motility but not proliferation of these cells (6). T24 cell proliferation and motility in the absence of serum are inhibited by proepithelin depletion, suggesting that the production of endogenous proepithelin may contribute in T24 cells to the activation of signaling pathways able to sustain both motility and cell proliferation.
In order to establish the signaling induced by endogenous proepithelin, we tested media conditioned by parental, pRS-transfected and shPEP-transfected T24 cells for their ability to activate Akt and MAPK pathways. We employed the PathScan Multiplex Western Cocktail I (Cell Signaling) that allows the testing of multiple pathways in one single blot. Serum-starved T24 cells were stimulated with the CM for 10 and 30 min. The medium conditioned from T24 and T24/pRS cells induced a prolonged activation of ERK1/2, with a concurrent, albeit less marked activation of p90RSK (the activation is more evident after longer exposures), one of ERK1/2 downstream effectors (6,30) (Figure 4A). Akt was activated as well, with the downstream activation of S6K (Figure 4A) as compared with unstimulated T24 cells. Significantly, CM from proepithelin-depleted T24 cells was severely impaired in the capacity of activating both the MAPK and Akt pathways (Figure 4A).
Fig. 4.
Proepithelin depletion severely reduces the activation of the Akt and MAPK pathways. (A) CM from T24, T24/pRS and T24/shPEP cells was prepared as described (22). T24 cells were serum starved for 24 h and then stimulated with the CM for 10 and 30 min. The activation of p90RSK, Akt, ERK1/2 and S6 ribosomal protein was analyzed by western immunoblot using the PathScan Multiplex Western Cocktail I (Cell Signaling Technology). ElF4E protein is the control to monitor the loading of the samples. Blot is representative of three independent experiments. (B) Recombinant proepithelin restores Akt and ERK1/2 activation in T24/shPEP. T24/shPEP cells were serum starved for 24 h and then stimulated with 400 ng/ml of recombinant proepithelin for 10 and 30 min. The activation of Akt and ERK1/2 was analyzed by western immunoblot using the PathScan Multiplex Western Cocktail I (Cell Signaling Technology). Blot is representative of two independent experiments.
To confirm that the reduction in Akt and ERK1/2 activation in proepithelin-depleted T24 cells was specifically due to the loss of proepithelin, we stimulated T24/shPEP with recombinant proepithelin, which restored the ability of these cells to promote the activation of Akt and ERK1/2 (Figure 4B).
Together, these results strongly indicate that endogenous proepithelin contributes to the activation of both the Akt and MAPK pathways thereby promoting cell proliferation and motility.
Proepithelin is overexpressed in bladder cancer tissues and is secreted in the urine
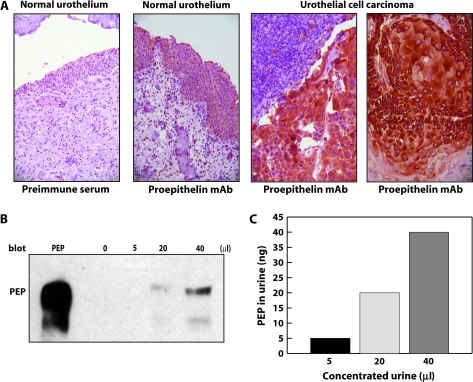
Because proepithelin is overexpressed in several cancers (1) and the ONCOMINE analysis revealed that a statistically significant increase in proepithelin expression in primary bladder cancers compared with non-neoplastic controls (Figure 1), we determined by immunohistochemical analysis the level of proepithelin expression in normal and bladder cancer sections. First, we established that proepithelin is present, although at low levels, in normal urothelial tissue (Figure 5A). Second, we discovered that proepithelin was expressed at considerably higher level in all five tumor bladder tissues analyzed (Figure 5A).
Fig. 5.
Proepithelin detection in bladder cancer tissue specimens and urine samples. (A) Formalin-fixed, paraffin-embedded sections of non-malignant human bladder and bladder cancer tissue were deparaffinated in preparation for antigen retrieval by heating in a microwave oven for 10 min. Slides were cooled, rinsed and immunostained using a monoclonal antibody raised against human proepithelin (A&G Pharmaceutical) and visualized with an avidin–biotin method. From left to right: negative control normal urothelium stained with preimmune serum (×200), normal urothelium expressing proepithelin (×200), high levels of proepithelin are detected in invasive urothelial cancer (×400) and in area of squamous differentiation (×400). (B) Random and unidentified urine samples from normal individuals were pooled and then concentrated (10 ml ⇒ 200 μl) using Amicon Ultra Centrifugal Filter Devices at 4000 r.p.m. for 20 min (Millipore's Corporate Headquarters, Billerica, MA, 10 K). Proepithelin levels were determined by immunoblot using anti-proepithelin polyclonal antibodies (Zymed Laboratories, Inc., South San Francisco, CA). Recombinant human proepithelin (300 ng) was loaded as control (PEP). Blot is representative of two independent experiments. (C) The amount of proepithelin present in urine was calculated by ELISA using a proepithelin ELISA kit (ALPCO Diagnostics).
Proepithelin is present in the urine
The results presented above correlate well with our central hypothesis on the role of proepithelin in urinary pathology and further suggest that proepithelin might be secreted in the urine either through active secretion or after cell death, which often occurs in bladder cancer. Indeed, bladder cancers have a tendency to recur, necessitating close and regular follow-up. Therefore, there is a need for additional non-invasive and simple diagnostic tools with a high sensitivity and specificity for the detection of this cancer. Moreover, urothelial neoplasms may be related to the concentration of some growth factors such as the epidermal growth factor (31).
To evaluate proepithelin as a potential clinical marker of bladder cancer and correlate in future studies’ proepithelin urinary levels to disease severity, we needed to first determine whether proepithelin could be detected in the urine. We could detect proepithelin by immunoblotting in samples from concentrated urines obtained from non-cancerous patients, confirming the feasibility of the proposed approach (Figure 5B). Furthermore, we quantified proepithelin levels in the urine by ELISA (Figure 5C).
These results indicate that the combined analysis of proepithelin expression levels in urothelial tissues and urines may serve as a novel diagnostic and prognostic tool for bladder tumors.
Discussion
Although bladder cancer is one of the most common malignancies in the USA (32), very little is known about the molecular mechanisms that determine transformation in the urothelium lining of the bladder wall. Most bladder cancers are characterized by frequent recurrences and often progress into an invasive phenotype, regardless of treatment with surgery, chemotherapy or immunotherapy. The present study provides the first evidence that the growth factor proepithelin contributes in an ‘autocrine’ fashion to bladder tumor formation and progression by promoting cell growth, migration and invasion of bladder cancer cells. Proepithelin may also prove as a novel clinical marker for the diagnosis and prognosis of bladder tumors.
Our results can be summarized as follows. (i) Using the ONCOMINE data analysis tool, we determined that proepithelin mRNA levels are increased in bladder cancer compared with normal tissues. (ii) Proepithelin mRNA levels correlate with prognostic parameters. (iii) Targeting endogenous proepithelin by shRNA approaches severely reduces the ability of T24 bladder cancer cells to proliferate in serum-deprived condition. (iv) Proepithelin depletion inhibits motility of T24 cells as determined by wound healing, migration, invasion and evasion assays. (v) The reduction of proepithelin expression inhibits the activation of the Akt and MAPK pathways. (vi) Proepithelin expression is significantly increased in bladder cancer specimens compared with normal tissue controls. (vii) Proepithelin is detectable by immunoblot analysis and can be measured by ELISA in urine samples.
We have recently established a role for proepithelin in bladder cancer by showing that recombinant proepithelin can effectively promote migration and invasion of 5637 cells at nanomolar amounts (6). Proepithelin was instead not strongly mitogenic for 5637 cells (6), suggesting that proepithelin in some cell systems may play a more critical role in promoting a stimulus for migration and invasion than for proliferation (11,33). Furthermore, in 5637 cells, proepithelin did not promote the activation of Akt, suggesting that the phosphatidylinositol 3′-kinase pathway does not play a role in migration and invasion in these cells. Because endogenously produced proepithelin in T24 cells activates both the Akt and MAPK pathways and promotes cell growth and motility, we conclude that proepithelin-evoked activation of these two signaling pathways might be required to promote not only cell motility but also cell proliferation of bladder cancer cells.
5637 cells responded in migration and invasion to the purified proepithelin precursor (6). Furthermore, we were able to detect only the 88 kDa proepithelin precursor by immunoblot on medium of T24 (Figure 1) and 5637 cancer cells (data not shown). These results would indicate that these cells secrete the proepithelin precursor protein, which is probably the bioactive form on bladder cancer cells. However, we cannot totally rule out the possibility that some intracellular processing into epithelin peptides may occur and contribute to the biological response in 5637 and T24 cells.
The ability of cancer cells to migrate and invade through the extracellular matrix is a critical step for tumor metastasis (28). Our results suggest that proepithelin, by promoting migration and invasion of bladder cancer cells, could help the transition from a non-invasive to an invasive phenotype in bladder cancers and perhaps in other solid tumors, where the role of proepithelin in promoting invasion and migration has been reported as well (11,29,34).
Cell migration is also a fundamental aspect in numerous normal processes (28). Proepithelin expression is detectable in normal bladder urothelium (Figure 5) and promotes migration of primary cultures of normal urothelial cells (data not shown). Thus, our results would strongly suggest that proepithelin is not only important for migration of bladder cancer cells but may also play a physiological role in regulating cell migration of normal urothelial cells.
Because all the in vitro studies and the ONCOMINE analysis from several independent data sets pointed out the critical role of proepithelin as an autocrine growth factor for bladder cancer cells, we examined proepithelin expression in bladder cancer specimens by immunohistochemistry and determined that proepithelin is significantly overexpressed in invasive urothelial carcinomas compared with normal tissue. Furthermore, we have established that proepithelin as a precursor protein is detectable in urine samples where it can be quantified by ELISA.
Our present results together with previously published observations studies (6,35) suggest that the combined analysis of proepithelin levels in bladder cancer tissues and in urine from bladder cancer patients may have a diagnostic and prognostic value, especially in potentially identifying patients with increased risk of recurrence or aggressive behavior.
Taking into account the biological properties of proepithelin, it has been hypothesized that it could act through a ‘classic’ membrane receptor, as for the other known growth factors. However, to date a functional receptor has not been identified, although proepithelin (36) and epithelins (37) bind specifically to membrane proteins. Data from competitive binding experiments indicate that a long list of known growth factors and cytokines are unable to displace radiolabeled epithelin binding to its putative receptor (37) suggesting that the receptor for proepithelin is not a known tyrosine kinase receptor.
We are aware of the limitation of our previous and present studies based on cell lines and on a limited number of bladder cancer samples. Further, in vitro and in vivo experiments are required to fully elucidate proepithelin function in bladder cancer.
In conclusion, the identification of the growth factor proepithelin as a novel autocrine regulator of tumor cell motility and invasion could represent a novel molecular target for bladder cancer. Further characterization of proepithelin signaling could provide new leads for developing novel tumor biomarkers and for improved therapeutic approaches against bladder cancer.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
Benjamin Perkins Bladder Cancer Fund; Martin Greitzer Fund; National Institutes of Health (RO1 DK068419 to A.M., RO1 CA39481 and RO1 CA47282 to R.V.I.).
Supplementary Material
Acknowledgments
We would like to thank Judy Verdone for her skillful assistance with cell culture.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CM
conditioned medium
- 3D
three-dimensional
- ELISA
enzyme-linked immunosorbent assay
- ERK1/2
extracellular signal-regulated kinase 1/2
- MAPK
mitogen-activated protein kinase
- mRNA
messenger RNA
- SFM
serum-free medium
- shRNA
small hairpin RNA
References
- 1.He Z, et al. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J. Mol. Med. 2003;81:600–612. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- 2.Plowman GD, et al. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J. Biol. Chem. 1992;267:13073–13078. [PubMed] [Google Scholar]
- 3.Xu SQ, et al. The granulin/epithelin precursor abrogates the requirement for the insulin-like growth factor 1 receptor for growth in vitro. J. Biol. Chem. 1998;273:20078–20083. doi: 10.1074/jbc.273.32.20078. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 5.Baba T, et al. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol. Reprod. Dev. 1993;34:233–243. doi: 10.1002/mrd.1080340302. [DOI] [PubMed] [Google Scholar]
- 6.Monami G, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- 7.Bateman A, et al. Granulins, a novel class of peptide from leukocytes. Biochem. Biophys. Res. Commun. 1990;173:1161–1168. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- 8.Lu R, et al. Stimulation of PC cell-derived growth factor (epithelin/granulin precursor) expression by estradiol in human breast cancer cells. Biochem. Biophys. Res. Commun. 1999;256:204–207. doi: 10.1006/bbrc.1999.0253. [DOI] [PubMed] [Google Scholar]
- 9.Lu R, et al. Inhibition of PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) expression by antisense PCDGF cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468. Proc. Natl Acad. Sci. USA. 2000;97:3993–3998. doi: 10.1073/pnas.97.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu R, et al. Mediation of estrogen mitogenic effect in human breast cancer MCF-7 cells by PC-cell-derived growth factor (PCDGF/granulin precursor) Proc. Natl Acad. Sci. USA. 2001;98:142–147. doi: 10.1073/pnas.011525198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Z, et al. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res. 2002;62:5590–5596. [PubMed] [Google Scholar]
- 12.Zhang H, et al. Inhibition of tumorigenicity of the teratoma PC cell line by transfection with antisense cDNA for PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) Proc. Natl Acad. Sci. USA. 1998;95:14202–14207. doi: 10.1073/pnas.95.24.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones MB, et al. The granulin-epithelin precursor: a putative new growth factor for ovarian cancer. Gynecol. Oncol. 2003;88:S136–S139. doi: 10.1006/gyno.2002.6704. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, et al. PC cell-derived growth factor (granulin precursor) expression and action in human multiple myeloma. Clin. Cancer Res. 2003;9:2221–2228. [PubMed] [Google Scholar]
- 15.Wang W, et al. PC cell-derived growth factor confers resistance to dexamethasone and promotes tumorigenesis in human multiple myeloma. Clin. Cancer Res. 2006;12:49–56. doi: 10.1158/1078-0432.CCR-05-0929. [DOI] [PubMed] [Google Scholar]
- 16.Davidson B, et al. Granulin-epithelin precursor is a novel prognostic marker in epithelial ovarian carcinoma. Cancer. 2004;100:2139–2147. doi: 10.1002/cncr.20219. [DOI] [PubMed] [Google Scholar]
- 17.Pan CX, et al. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clin. Cancer Res. 2004;10:1333–1337. doi: 10.1158/1078-0432.ccr-1123-03. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes DR, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes DR, et al. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc. Natl Acad. Sci. USA. 2004;101:9309–9314. doi: 10.1073/pnas.0401994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin W, et al. Dysregulation of the annexin family protein family is associated with prostate cancer progression. Am. J. Pathol. 2003;162:255–261. doi: 10.1016/S0002-9440(10)63816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belletti B, et al. Stathmin activity influences sarcoma cell shape, motility, and metastatic potential. Mol. Biol. Cell. 2008;19:2003–2013. doi: 10.1091/mbc.E07-09-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanocco-Marani T, et al. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–5340. [PubMed] [Google Scholar]
- 23.Gonzalez EM, et al. A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth. J. Biol. Chem. 2003;278:38113–38116. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Carbayo M, et al. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 25.Dyrskjot L, et al. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64:4040–4048. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- 26.Blaveri E, et al. Bladder cancer outcome and subtype classification by gene expression. Clin. Cancer Res. 2005;11:4044–4055. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]
- 27.Lindgren D, et al. Molecular characterization of early-stage bladder carcinomas by expression profiles, FGFR3 mutation status, and loss of 9q. Oncogene. 2006;25:2685–2696. doi: 10.1038/sj.onc.1209249. [DOI] [PubMed] [Google Scholar]
- 28.Guo W, et al. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 29.Tangkeangsirisin W, et al. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis. 2004;25:1587–1592. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 30.Morrione A, et al. The role of the insulin receptor substrate-1 in the differentiation of rat hippocampal neuronal cells. Oncogene. 2001;20:4842–4852. doi: 10.1038/sj.onc.1204649. [DOI] [PubMed] [Google Scholar]
- 31.Saika T, et al. Epidermal growth factor in urine from patients with bladder cancer. Urol. Res. 2000;28:230–234. doi: 10.1007/s002400000102. [DOI] [PubMed] [Google Scholar]
- 32.Jemal A, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 33.He Z, et al. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 1999;59:3222–3229. [PubMed] [Google Scholar]
- 34.Chen HY, et al. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol. Cell. Biol. 2004;24:10558–10572. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparro G, et al. Isolation and N-terminal sequence of multiple forms of granulins in human urine. Protein Expr. Purif. 1997;10:169–174. doi: 10.1006/prep.1997.0726. [DOI] [PubMed] [Google Scholar]
- 36.Xia X, et al. Identification of cell surface binding sites for PC-cell-derived growth factor, PCDGF, (epithelin/granulin precursor) on epithelial cells and fibroblasts. Biochem. Biophys. Res. Commun. 1998;245:539–543. doi: 10.1006/bbrc.1998.8498. [DOI] [PubMed] [Google Scholar]
- 37.Culouscou JM, et al. Biochemical analysis of the epithelin receptor. J. Biol. Chem. 1993;268:10458–10462. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.