Whilst the benefits of prophylactic replacement therapy for children with hemophilia and of immune tolerance for those with inhibitors are both generally accepted, venous access can be a limiting problem in their delivery. In this paper, Mancuso and coworkers report on their extensive experience using arterio-venous fistulae to deal with this problem and suggest they could be more widely adopted.
Keywords: arteriovenous fistula, hemophilia, inhibitor, prophylaxis, venous access
Abstract
Background
An easy and stable venous access is essential in hemophilic children who receive regular prophylaxis or immune tolerance induction treatment. Central venous access devices improve treatment feasibility, but their use is complicated by infection and/or thrombosis. Arteriovenous fistula (AVF) has been evaluated as an alternative to central venous access devices in hemophilic children since 1999.
Design and Methods
This study provides results obtained in a large series after seven years of follow-up.
Results
From 1999 to 2008, 43 procedures were performed in 38 children (median age: 2.7 years). Thirty-five AVFs (81%) achieved maturation after a median of 58 days and were used for a median of five years (range: 0.4–8.5). A brachial artery caliber larger than 1.2 mm was associated with successful maturation (p<0.05). Complications with some impact on arteriovenous fistula use or duration were observed in 14/43 procedures (32%) and in 13/38 children (34%). Age at arteriovenous fistula creation was younger in children who lost arteriovenous fistula patency (p<0.05) and aneurysms were more frequent in children who were on daily treatment regimen and thus had a greater cumulative number of arteriovenous fistula accesses (p<0.05). At the end of the follow-up period, 22 AVFs were still in use and 9 had been surgically dismantled. Arteriovenous fistula use allowed long-term prophylaxis (up to 8.5 years) in 11 children and the completion of immune tolerance induction without interruptions in 18 children.
Conclusions
This study confirms the feasibility of arteriovenous fistula with an acceptable rate of complications and suggests that its use is particularly favorable in children with inhibitors in whom it should be considered as first-choice venous access.
Introduction
Children with hemophilia need an easy and stable venous access to receive factor concentrates on a regular basis for primary prophylaxis or immune tolerance induction (ITI) regimens. Even though peripheral veins are the first choice, central venous access devices (CVADs) are often necessary to improve treatment feasibility,1 but their use is complicated by infections and/or thrombosis.2 Arteriovenous fistula (AVF) is the preferred access for children on hemodialysis, because it is a long-lasting autologous access that carries a very low risk of infections.3–6 Since 1999, AVF has been evaluated as a suitable alternative to CVADs in hemophilic children. Our preliminary experience7 showed that AVF was well accepted by children and parents because it is easy to use in the home setting, does not limit the child’s activities and is associated with a low rate of complications.7 However, a prolonged follow-up was warranted to establish the safety of this approach. This study provides data on the long-term use of AVF in hemophilic children and evaluates the impact of this mode of venous access on the feasibility of treatment in children with or without factor VIII inhibitors.
Design and Methods
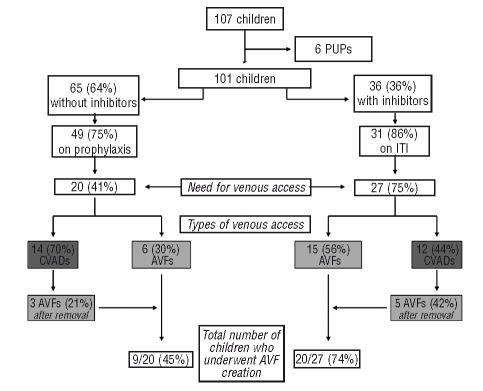
All children included in the study were prospectively followed-up from fistula creation and data collection was approved by the Institutional Review Board of the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center of Milan. The need and types of venous accesses were evaluated in the unselected cohort of children with severe or moderately severe hemophilia, aged < 12 years (the age of the eldest patient who underwent AVF creation) regularly followed-up in Milan (Figure 1), while complications related to AVF creation and long-term outcome were also analyzed including procedures performed in children referred from other Italian centers.
Figure 1.
Prescribed treatment regimens, need for artificial venous access and types of venous accesses in the cohort of children regularly followed-up at the Hemophilia Center of Milan between 1987 and 2008. (PUPs denotes previously untreated patients; AVF: arteriovenous fistula; ITI: immune tolerance induction; CVAD: central venous access device).
Patients
Children who needed an artificial venous access to allow home treatment because they lacked suitable peripheral veins were included. Concomitant cardiac or vascular diseases represented exclusion criteria. Risks and benefits associated with the use of AVFs and CVADs were discussed with parents or guardians and a written informed consent for the surgical procedure was obtained.
Perioperative treatment
To create AVF, patients with hemophilia A or B without inhibitors were treated with factor VIII or IX (FVIII or FIX) concentrates for 5–6 days.7 Patients with high-responding inhibitors were treated with recombinant activated FVII (rFVIIa), administered by bolus injection or by continuous infusion (CI) for 5–6 days.7
Arteriovenous fistula creation
Surgical eligibility was evaluated by the same vascular surgeon (LB) and the most suitable vascular site and the configuration of AVF were decided according to age, vessel size and blood flow,7 the non-dominant upper limb being preferred if possible. Caregivers were recommended not to access the chosen vessels until AVF creation. AVFs were created under general anesthesia. Broad-spectrum intravenous antibiotic prophylaxis was given 30 min before surgery.
AVFs were created proximally in the forearm at the elbow crease by an anastomosis between the brachial artery and a nearby vein by end-to-side or side-to-side techniques.8,9 Peripheral pulses were checked by palpation and Doppler ultrasound (US) examination performed after the procedure was completed. Sutures were removed after 15–20 days.
Follow-up evaluation
The flow through AVF was evaluated by physical and Doppler US examination prior to discharge, at suture removal and monthly until AVF maturation. Successful maturation was defined as arterialization and dilatation of the vein adequate to allow factor concentrate infusion. Caregivers were trained to access AVF by conventional venepuncture technique at the Hemophilia Center and continued the prescribed treatment regimen at home. Afterwards, patients were clinically evaluated as outpatients at least every three months in order to undergo comparative physical examination of the upper limbs. Doppler US and echocardiography were carried out at least once a year. Caregivers were advised to report promptly any sign or symptom referable to the creation of the AVF. AVFs were dismantled as soon as peripheral veins became suitable for regular access.
Statistical analysis
Continuous variables, expressed as median values and ranges, were compared by the Student t-test or the Mann-Whitney U-test. Categorical variables, expressed as frequencies and percentage values, were compared by χ2 or Fisher’s exact test. Correlation coefficients were calculated by Pearson’s or Spearman’s rho test. The association between complications and age at AVF creation, AVF configuration, brachial artery caliber, inhibitor status, cumulative number of AVF accesses, number of accesses per month and daily treatment regimen was evaluated. The small number of complicated cases did not allow a multivariate analysis to be performed. The duration of AVF use was calculated from the first access onwards, if not otherwise specified. All reported p values are two-sided and values <0.05 were considered significant. All analyses were performed by using SPSS software (release 16.0, SPSS Inc.).
Results
Patients’ characteristics
Between January 1987 and June 2008, an unselected cohort of 107 hemophilic children with severe or moderately severe hemophilia (106 boys with FVIII/IX ≤ 2 IU/dL and one girl with factor VII <1 IU/dL) aged ≤ 12 years was regularly followed-up at the Hemophilia Center. The prescribed treatment regimens, the need for a venous access and the types of venous accesses provided to these children are shown in Figure 1. Since the ‘90s the use of CVADs in hemophiliac children has been implemented at our Center,10 however due to the high rate of infectious complications, AVF was evaluated as a candidate option.7 The first AVFs were created from 1999 in children who had their CVADs removed because of infection; the use of AVF was then gradually introduced as the first option in patients who needed a long-lasting venous access. For this reason CVADs were mainly used at our center until 1999 (only 5 implanted after 2000) and AVFs were preferred afterwards.
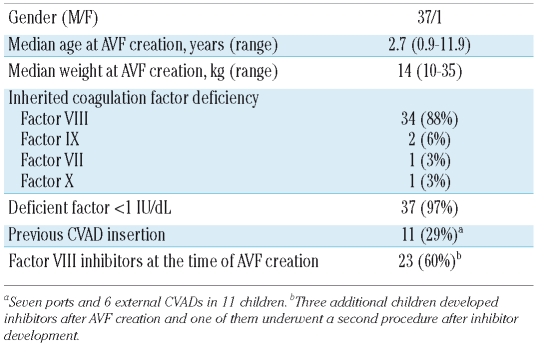
Nine additional children (6 hemophiliacs with inhibitors, 2 without and one factor X deficient boy) were referred to us for AVF creation from other Italian Centers, so that in all 38 children underwent AVF creation between 1999 and 2008. Patients’ characteristics are shown in Table 1.
Table 1.
Characteristics of the 38 children with arteriovenous fistula.
Surgical procedure and perioperative hemostatic treatment
Over eight years, 43 internal AVFs were created in 38 children because 5 underwent a second procedure at the opposite limb after a median of seven months (range: 4–7). The type of anastomosis was radio-cephalic in one (2%), brachio-cephalic in 7 (17%), brachio-basilic in 4 (9%), brachio-median-cephalic in 4 (9%) and brachio-median-basilic in 27 (63%). The median caliber of the brachial artery prior to AVF creation was 1.2 mm (range 0.8–2.1).
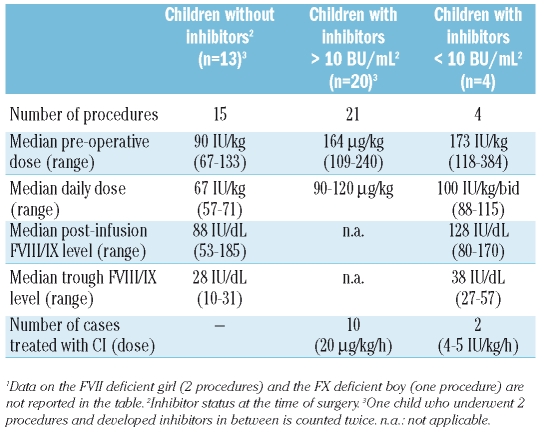
The perioperative hemostatic treatment is shown in Table 2. Children without inhibitors were treated with recombinant FVIII or FIX products (rFVIII/FIX) for 5–6 days. The factor X deficient boy and the factor VII deficient girl (who underwent AVF creation twice) received a prothrombin complex concentrate and plasma-derived factor VII concentrate.7 Children with inhibitors were treated with rFVIIa when inhibitor titer exceeded 10 BU/mL (Table 2). rFVIIa was administered every 2–3 h for 24–48 h, every four hours till post-operative day 4 and every six hours for an additional 48 hours when given by bolus. Of the 4 children treated with high-dose rFVIII, 2 received CI (Table 2) and 2 received twice daily infusions for 5–6 days. Of these children, 2 continued to receive FVIII according to ITI regimens through a CVAD until AVF maturation, one with a persistent low-titer inhibitor started ITI after AVF maturation and one underwent regular prophylaxis because the inhibitor was transient (see below).
Table 2.
Perioperative hemostatic treatment provided to 36 hemophilic children for 40 procedures.1
Follow-up and complications
The median follow-up from AVF creation was 7.0 years (range: 3.0–8.8). Of 43 AVFs created, 35 (81%) achieved maturation. Time to AVF maturation ranged between 21 and 135 days (median: 58) and it was neither correlated with age at AVF creation or brachial artery caliber (r=−0.005 and r=−0.04, respectively; p=ns), nor associated with AVF configuration. Mature AVFs were used for a median of 5.0 years (range: 0.4–8.5). The number of AVF accesses per patient/month ranged between 9 and 48 (median: 16), accounting for a cumulative number of accesses per patient between 166 and 2,036 (median: 1052). Twenty-nine children (76%) used AVF according to the initial indication (10 for prophylaxis and 19 for ITI), while 6 did not, because 2 developed inhibitors after surgery and underwent ITI, one had a transient inhibitor and started prophylaxis, and 3 were non-compliant to ITI regimens. The latter 3 children, referred from other centers, ultimately used AVFs to be treated on demand with by-passing agents for a median of 6.8 years (range: 4.2–7.5). The details of AVF use according to prophylaxis or ITI regimens are shown in Table 3.
Table 3.
Details on the use of mature AVFs in 32 children according to prophylaxis and ITI regimens.
Inadequate maturation of AVF was observed after 8/43 procedures (19%) in 7/38 children (18%), however only 3 (8%) could not benefit from AVF use, because 5 underwent a second procedure that was successful in 4. Among all the variables analyzed, the brachial artery caliber was associated with the likelihood of AVF maturation, being larger in patients who achieved AVF maturation (median: 1.3 mm, range: 0.8–2.1 vs. 0.9 mm, range: 0.8–1.2 in children who did not achieve maturation; p<0.01). No linear correlation was found between brachial artery caliber and age at AVF creation (r=0.09; p=ns).
Post-operative local hematoma occurred after 8 procedures (19%) in 8 children (21%). Six had inhibitors and received rFVIIa by CI (n=2) or repeated bolus (n=4). Bleeding was controlled by adding rFVIIa bolus to CI or switching rFVIIa administration from CI to repeated bolus in the former, and by shortening the interval between each bolus in the latter. The remaining 2 patients had no detectable inhibitors at surgery, but low-titer inhibitors were detected post-operatively in both, so that the hematomas were managed by increasing the frequency of FVIII infusions as previously described.7 The presence of inhibitors was the only factor associated with post-operative bleeding (8/27, 30% vs. 0/16 in non-inhibitor children; p<0.05).
Symptoms referable to distal steal syndrome included coldness and tingling of the hand ipsilateral to AVF and were reported in 4/35 children with mature AVF (11%). Symptoms mainly occurred during winter and spontaneously recovered within the first year from AVF creation, never recurring afterwards. In these patients, Doppler US examination showed a slight increase of the flow rates through AVFs that did not require any remedial intervention. No association was found between the occurrence of distal steal syndrome and age at AVF creation, brachial artery caliber or AVF configuration.
Loss of patency was observed only in 4 of 35 children with mature AVF after a median of 1.7 years (range: 0.4–4.1), so that patency rate was 89% at five years. In a 2-year old inhibitor child a symptomatic thrombosis of a venous branch downstream the fistula occurred after nine months of uncomplicated AVF use for ITI and on demand rFVIIa treatment.7 Pain, swelling and warmness of the hand recovered spontaneously within three weeks and AVF was used for 24 additional months for ITI. Overall, treatment was continued through collateral veins in 3 patients, while a port-a-cath was implanted in one. Age at AVF creation was significantly younger in children who lost AVF patency (1.7 years, range: 1.3–2.1 vs. 2.7 years, range: 1.5–11.9 in children who maintained patent AVF; p<0.01); no differences were found with respect to other variables.
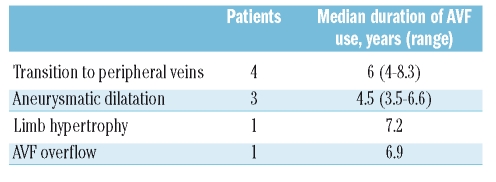
Limb hypertrophy ipsilateral to AVF was observed in a child at the age of 11 years after 5.4 years of thrice-weekly AVF use for regular prophylaxis. AVF was at first surgically remodeled to reduce the caliber of the anastomosis (from 8.0 to 4.5 mm) in order to decrease the blood flow, and then dismantled after 1.7 additional years of use. Echocardiography showed a mild dilatation of the left ventricle that completely recovered after AVF remodeling. The upper limb ipsilateral to AVF remained bigger than the opposite (length 74 vs. 73 cm; arm circumference 32 vs. 27.5 cm and forearm circumference 29 vs. 26 cm).
Aneurysmatic dilatation of the vein developed in 4 children (4/35, 11%) with inhibitors who used the AVF daily, first for ITI and then for a prophylactic regimen for a median of 4.6 years (range: 3–7.5) prior to aneurysm formation. Two patients underwent surgical AVF dismantlement, while one underwent surgical AVF remodeling after five years of use and subsequent dismantlement after an additional 1.6 years. AVF dismantlement and transition to peripheral veins is planned in the remaining patient. Aneurysmatic dilatation was more frequent in children on a daily treatment regimen (4/17, 24% vs. 0/18 in children not treated daily; p<0.05) who underwent a greater cumulative number of AVF accesses (median 1,594, range: 1,054–2,036 vs. 1,020, range: 166–1,965 in children without aneurysm; p<0.05), while no statistically significant difference was found according to the inhibitor status.
Overall, complications with some impact on AVF use or duration (i.e. excluding hematomas and distal steal syndrome) occurred in 14/43 procedures (32%) in 13/38 patients (34%), including AVF overflow (AVF flow rate up to 2,200 mL/min) without US signs of cardiac involvement observed in one child after 6.9 years of AVF use. In this case the AVF was dismantled and treatment continued through peripheral veins.
At the end of the follow-up period, 22 AVFs (51%) were still in use, since 8 did not mature, 4 did not maintain blood flow and 9 were surgically dismantled. Reasons for surgical dismantlement are summarized in Table 4. All patients but one whose AVF lost patency or was surgically dismantled were able to continue the prescribed regimen through peripheral veins.
Table 4.
Reasons for surgical dismantlement of 9 AVF.
Treatment feasibility through arteriovenous fistula
AVF creation allowed long-term prophylaxis (FVIII: 25–40 IU/kg thrice or twice weekly; FIX: 40–50 IU/kg twice weekly) in 11 children but one who lost patency after 4.1 years required port placement. AVF is still in use in 7 children (64%) on regular prophylaxis. ITI regimens (ranging from 50 IU/kg thrice weekly to 200 IU/kg/day) were administered through AVF in 21/22 inhibitor children compliant to treatment (95%, one did not achieve AVF maturation). Daily ITI regimens (100–200 IU/kg once a day) were given to 18/21 patients (86%); the median ITI duration was 15 months (range: 5–51). A second ITI course was administered through AVF in 4 children who previously failed or relapsed (median duration of the second course: 16 months, range: 6–29).
At the end of the follow-up period, at least one ITI course was completed in 18/21 children (86%, ITI ongoing in the remaining 3). All 13 children who achieved tolerance (72%) continued to use AVF according to prophylactic regimens (median duration of prophylaxis: 4.4 years, range: 0.9–6.8), except one who lost AVF patency just after ITI completion and received prophylaxis through the collateral veins close to the anastomosis. Similarly, all 5 children who failed ITI continued to use AVFs for treatment with by-passing agents according to licensed dosages for a median of 2.2 years (range: 1.5–4).
Discussion
Modern treatment of children with severe hemophilia is based on primary prophylaxis to prevent joint damage11 and on ITI to eradicate inhibitors.12 Both treatments are usually started at very young ages, so that venous access often represents a major barrier to treatment feasibility. CVADs have been widely used in this setting but the high rate of infectious complications, usually leading to early removal, has a negative impact on treatment feasibility and outcome, particularly in children with inhibitors who require prolonged and intensive ITI regimens.10,13,14 The use of AVF as an alternative venous access was introduced at our center in 23 hemophilic children and was associated with an acceptable rate of complications (35%) after a median follow-up period of 2.6 years.7 In this study results are provided for larger series of children who were followed-up for a median period of seven years after AVF creation. A mature AVF was ultimately obtained in all but 3 children (8%). High rates (up to 30%) of maturation failure have been reported in hemodialysis patients,15,16 however in this setting several metabolic factors, as well as female gender and radiocephalic configuration, were identified as predictors of non-maturation.17 In our cohort all but one child were males and all AVF were created proximally by using the brachial rather than the radial artery (with only one exception). A brachial artery caliber smaller than 1.2 mm was associated with failure of AVF maturation, however no correlation with age was found, suggesting that successful AVF maturation can also be achieved in young children. Moreover, an age at AVF creation younger than 2.1 years was a predictor of patency loss. In hemodialysis patients, intensive AVF use has been reported to affect patency;16 in our experience symptomatic thrombosis indeed occurred in one child who used AVF intensively for both ITI and on demand treatment with rFVIIa. Frequent injections of procoagulant agents may have favored the development of local thrombosis in this patient.
Short-term complications such as post-operative hematoma and distal ischemia syndrome were transient and did not hamper the subsequent use of AVF. Long-term complications due to modified hemodynamics of the limb (i.e. aneurysms and limb hypertrophy) were rare and never occurred before three years of use; regular Doppler ultrasound examination and echocardiography allowed early detection and proper remedial interventions. Aneurysms occurred in children with inhibitors who accessed AVF daily and for a long time, resulting in high cumulative numbers of AVF punctures. In these instances, surgical AVF dismantlement must be planned as soon as ITI is completed or when access to peripheral veins becomes available, preferably within 3–4 years from AVF creation. If ITI is still ongoing and AVF represents the only available venous access, it is advisable to remodel the anastomosis prior to dismantle it, in order to allow ITI completion without interruption.
In our cohort the need for a venous access was greater in inhibitor than in non-inhibitor children (75% and 40%, respectively). In patients without inhibitors CVAD did allow long-term prophylaxis to be maintained in a high proportion of cases, while the early occurrence of infectious complications hampered ITI completion in the majority of inhibitor patients.10 In a metanalysis on CVAD use in hemophilic children,2 it was observed that infectious complications were more frequent in the presence of inhibitors, while infections were not observed in our patients using AVF irrespective of inhibitor status. On this basis, AVF has become the first-choice venous access in children with inhibitors. Since AVF maturation was usually achieved after a median of two months, children candidate to ITI underwent AVF creation during the time period required to reach an inhibitor titer below 10 BU/mL, i.e. the value recommended as the most suitable to start ITI.12 In our previous experience with ports, ITI had to be interrupted in 50% of children because of port removal for infections occurring within the first year of treatment10 while in this series the adoption of AVF allowed ITI in 95% of children.
The surgical expertise needed for AVF creation in hemophilic children represented the main concern for its widespread use.12 A recent report of a successful experience with AVFs in a small series of hemophilic children from the US18 indicated that this limitation can be overcome by involving expert vascular surgery teams.
In conclusion, our long-term experience with AVFs in hemophilic children confirms its feasibility with an acceptable rate of complications, particularly when compared with CVAD use. Comprehensive hemophilia treatment centers with surgical expertise and assistance should consider AVF as first-choice access in children with inhibitors who usually need a long-lasting venous access for ITI, since our results highlight the advantages of AVF use in these patients. Regular follow-up and continuous surveillance on long-term outcome and complications is ongoing in our series and is recommended to centers that choose AVF as venous access in hemophiliacs.
Acknowledgments:
Acknowledgments: the authors would like to thank Prof. Pier Mannuccio Mannucci who critically revised the final version of the manuscript.
Footnotes
Authorship and Disclosures
MEM, LB and ES conceived and designed the study; MEM, CB, MR and EP contributed to the collection of clinical data; MEM, ES, CB, MR and EP contributed to the analysis and interpretation of data; MEM wrote the article; ES revised it critically. All authors approved the final version of the manuscript. The authors declare that they have no potential conflicts of interest. The authors reported no potential conflicts of interest.
References
- 1.Ewenstein BM, Valentino LA, Journeycake JM, Tarantino MD, Shapiro AD, Blanchette VS, et al. Consensus recommendations for use of central venous access devices in haemophilia. Haemophilia. 2004;10:629–48. doi: 10.1111/j.1365-2516.2004.00943.x. [DOI] [PubMed] [Google Scholar]
- 2.Valentino LA, Ewenstein B, Navickis RJ, Wilkes MM. Central venous access devices in haemophilia. Haemophilia. 2004;10:134–46. doi: 10.1046/j.1365-2516.2003.00840.x. [DOI] [PubMed] [Google Scholar]
- 3.Brescia MJ, Cimino JF, Appel K, Hurwich BJ. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275:1089–92. doi: 10.1056/NEJM196611172752002. [DOI] [PubMed] [Google Scholar]
- 4.Kherlakian GM, Roedersheimer LR, Arbaugh JJ, Newmark KJ, King LR. Comparison of autogenous fistula versus expended polytetrafluoroethylene graft fistula for angioaccess in hemodialysis. Am J Surg. 1986;152:238–43. doi: 10.1016/0002-9610(86)90249-7. [DOI] [PubMed] [Google Scholar]
- 5.Bonalumi U, Civalleri D, Rovida S, Adami GF, Gianetta E, Griffanti-Bartoli F. Nine years’ experience with end-to-end arteriovenous fistula at the anatomical snuffbox for maintenance hemodialysis. Br J Surg. 1982;69:486–8. doi: 10.1002/bjs.1800690820. [DOI] [PubMed] [Google Scholar]
- 6.Lumsden AB, MacDonald J, Allen RC, Dodson TF. Hemodialysis access in the pediatric patient population. Am J Surg. 1994;168:197–201. doi: 10.1016/s0002-9610(94)80067-7. [DOI] [PubMed] [Google Scholar]
- 7.Santagostino E, Gringeri A, Berardinelli L, Beretta C, Muça-Perja M, Mannucci PM. Long-term safety and feasibility of arteriovenous fistulae as vascular accesses in children with haemophilia: a prospective study. Br J Haematol. 2003;123:502–6. doi: 10.1046/j.1365-2141.2003.04632.x. [DOI] [PubMed] [Google Scholar]
- 8.Gracz KC, Ing TS, Soung LS, Armbruster KFW, Seim SK, Merkel FK. Proximal forearm fistula for maintenance hemodialysis. Kidney Int. 1977;11:71–5. doi: 10.1038/ki.1977.9. [DOI] [PubMed] [Google Scholar]
- 9.Dixon BS, Novak L, Fangman J. Hemodialysis vascular access survival: upper-arm native arteriovenous fistula. Am J Kidney Dis. 2002;39:92–101. doi: 10.1053/ajkd.2002.29886. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso ME, Mannucci PM, Sartori A, Agliardi A, Santagostino E. Feasibility of prophylaxis and immune tolerance induction regimens in haemophilic children using fully implantable central venous catheters. Br J Haematol. 2008;141:689–95. doi: 10.1111/j.1365-2141.2008.07087.x. [DOI] [PubMed] [Google Scholar]
- 11.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 12.DiMichele DM, Hoots K, Pipe SW, Rivard GE, Santagostino E. International Workshop on Immune Tolerance Induction: Consensus Recommendations. Haemophilia. 2007;13(Suppl 1):1–22. doi: 10.1111/j.1365-2516.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 13.Valentino LA, Ewenstein B, Navickis RJ, Wilkes MM. Central venous access devices in haemophilia. Haemophilia. 2004;10:134–46. doi: 10.1046/j.1365-2516.2003.00840.x. [DOI] [PubMed] [Google Scholar]
- 14.DiMichele DM, Hay CR. The international immune tolerance study: a multicenter prospective randomized trial in progress. J Thromb Haemost. 2006;4:2271–3. doi: 10.1111/j.1538-7836.2006.02127.x. [DOI] [PubMed] [Google Scholar]
- 15.Feldman HI, Goldberg EM, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7:523–35. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 16.Erkut B, Ünlü Y, Ceviz M, Becit N, Ates A, Çolak A, et al. Primary arteriovenous fistulas in the forearm for hemodialysis: effect of miscellaneous factors in fistula patency. Ren Fail. 2006;28:275–81. doi: 10.1080/08860220600583617. [DOI] [PubMed] [Google Scholar]
- 17.Huber TS, Ozaki CK, Flynn TC, Lee WA, Berceli SA, Hirneise CM, et al. Prospective validation of an algorithm to maximize native arteriovenous fistulae for chronic hemodialysis access. J Vasc Surg. 2002;36:452–9. doi: 10.1067/mva.2002.127342. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy WJ, Valentino LA, Bonilla AS, Goncharova I, Taylor A, Pooley TA, Jacobs CE. Arteriovenous fistula for long-term venous access for boys with hemophilia. J Vasc Surg. 2007;45:986–91. doi: 10.1016/j.jvs.2006.12.060. [DOI] [PubMed] [Google Scholar]