So far, prognostic evaluation of patients with myelodysplastic syndrome has mainly been based on disease-related parameters like cytopenias, karyotype, or percentage of blast cells in the bone marrow. Patients’ characteristics reflecting comorbidities like cardiovascular diseases and impaired renal or liver function were not taken into account. In this study, the authors found that the Hematopoietic Cell Transplantation Comorbidity Index (HCTCI) may be useful for patients with myelodysplastic syndrome receiving best supportive care only. See perspective article on page 602.
Keywords: myelodysplastic syndromes, comorbidity score, prognosis, Charlson Comorbidity Index, International Prognostic Scoring System, Hematopoietic Stem Cell Transplantation Comorbidity Index
Abstract
We studied the impact of comorbidities on survival and evaluated the prognostic utility of comorbidity scores in MDS patients, who received best supportive care and were assessable according to the Charlson Comorbidity Index (CCI) and the Hematopoietic Stem Cell Transplantation Comorbidity Index (HCTCI): 171 patients were identified in the Duesseldorf MDS Registry. The HCTCI captured more comorbidities. Both scoring systems had prognostic relevance, but the HCTCI more clearly distinguished between low-, intermediate- and high-risk patients. Median survival times of the different risk groups according to the HCTCI were 68, 34 and 25 months, respectively. The HCTCI showed prognostic impact in the IPSS intermediate- and high-risk group. On multivariate regression analysis, only the HCTCI remained a prognostic factor independent of IPSS. Considering their prognostic impact, comorbidities of MDS patients should receive appropriate attention in clinical trials as well as day-to-day clinical decision making.
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of bone marrow disorders that mainly occur in the elderly. Older cancer patients are more likely to have comorbidities.1 According to Feinstein, comorbidity is defined as any distinct clinical entity that has existed or may occur during the clinical course of a patient who has a condition under study.2 There are several scoring systems to evaluate comorbidity. The Charlson Comorbidity Index (CCI), which considers the one-year mortality of internal medicine inpatients and proved its relevance for patients with solid cancer,3–5 was established in 1987 by Mary Charlson.6 It is a weighted scoring system based on 19 items which in general can easily be assessed retrospectively from the patients’ charts (Online Supplementary Appendix). There is an optional extension of the CCI including the patient’s age adding one point for each decade beyond 50 years of age.7
Sorror et al. evaluated comorbidities in a group of patients with various hematologic malignant and non-malignant diseases who underwent hematopoietic stem cell transplantation (HSCT).8 Later on the Hematopoietic Cell Transplantation Comorbidity Index (HCTCI) was used to evaluate outcomes of patients specifically diagnosed with MDS or AML and given HSCT.9 These authors noted that some comorbidities included in the CCI rarely occur in transplant patients because they constitute exclusion criteria for HSCT. This is particularly true for hepatic and pulmonary disease. On the other hand, the CCI does not capture frequent comorbidities like obesity, infections, and psychiatric disorders. Therefore, Sorror et al. amended the CCI for HSCT candidates, adding the above-mentioned comorbidities and changing some definitions and weightings. The adapted scoring system was called Hematopoietic Cell Transplantation Comorbidity Index (HCTCI) (Online Supplementary Appendix).
As yet, prognostic evaluation of MDS patients has mainly been based on disease-related parameters like cytopenias, karyotype, or percentage of blast cells in the bone marrow.10 Patients’ characteristics reflecting comorbidities like cardiovascular diseases and impaired renal or liver function were not taken into account. As the widening range of treatment options for MDS requires more refined clinical decision making proper assessment of comorbidities becomes more important. However, there is currently no validated comorbidity score for patients with MDS. Nor is there any data on the prevalence and prognostic impact of comorbidities in MDS. We therefore studied the influence of comorbidities on the survival of MDS patients and evaluated the prognostic utility of the CCI and HCTCI in comparison with the International Prognostic Scoring System (IPSS).
Design and Methods
Study design
Our retrospective study focused on patients who received best supportive care (BSC) only, including red blood cell transfusions, platelet transfusions, and treatment with erythropoietin. Patients undergoing induction chemotherapy and/or allogeneic stem cell transplantation were excluded. Patients were retrospectively classified according to CCI and HCTCI. Both scores were calculated from the points assigned to individual comorbidity factors. The clinical data were gathered from the original patients’ charts. For each patient included in our analysis, the complete set of comorbidity factors was evaluable. Follow-up data were obtained from our outpatient clinic or by contacting the primary care physician. Local ethics approval for this study was obtained.
Patients
The Düsseldorf MDS Registry includes about 3,300 patients, diagnosed between 1975 and 2008. Among these, 1,250 are classified according to the IPSS at the time of diagnosis. The latter group includes 740 patients who received BSC only. For 171 of those patients, all comorbidity factors required for applying the CCI and HCTCI were retrievable from the original records. As this study had a retrospective character, the quality of the documentation possibly varied between different patient files. Therefore, we included all available information on the patients including imaging and functional diagnostic procedures.
This cohort included 114 male and 57 female patients. The median age at diagnosis was 69 (24–88) years. According to the IPSS, 23% of the patients under consideration were assigned to the low-risk group. The majority of patients belonged to the intermediate-1 (39%) and intermediate-2 (24%) risk groups, while the proportion of patients considered as high-risk was 14%.
Results and Discussion
Charlson Comorbidity Index
According to the CCl, 111 of 171 patients (65%) showed no comorbidities, while 37 (22%) had one, 15 (9%) had two, 2 had three, and 6 patients had four comorbidity factors. None of the patients accumulated more than four comorbidity factors.
The most frequent diagnoses were diabetes without complications (21 patients) and myocardial infarction (12 patients). There were 9 patients who suffered from a second solid tumor. Miscellaneous diagnoses included congestive heart failure, peripheral vascular disease, cerebrovascular disease, hemiplegia, chronic pulmonary disease, connective tissue disease, mild liver disease, diabetes with end organ damage, and moderate or severe renal insufficiency.
Patients included in our analysis were classified into 3 risk groups, namely low-risk (CCI of 0), intermediate-risk (CCI of 1), and high-risk (CCI of 2 or more).
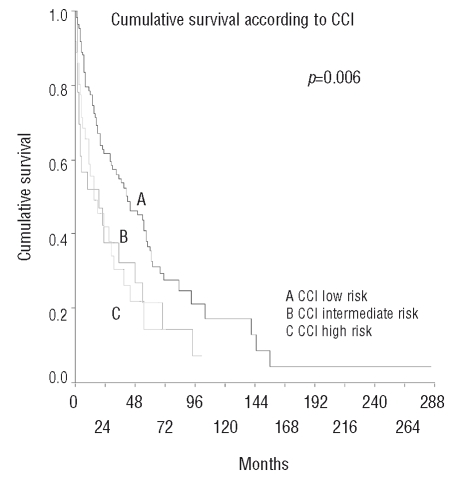
In the entire study population (n=171), median survival was 30 months. Patients with no comorbidities (CCI=0) had a median survival time of 42 months, while those with a CCI of 1 survived for only 15 months. Interestingly, patients with a CCI of 2 or more had a median survival time of 19 months as compared to 42 months in patients without comorbidities (p=0.006) (Figure 1). There was no difference in survival between the intermediate-risk and the high-risk groups according to CCI. Using the extended version of the CCI, which includes age, we identified a low-risk group of only 8 patients (5%) with a good prognosis. None remaining patients could be stratified in terms of survival.
Figure 1.
Cumulative survival according to Charlson Comorbidity Index.
We also examined the relationship between IPSS and CCI. Within the IPSS low-risk and high-risk-groups, the CCI was not able to further stratify patients according to comorbidity-related prognosis. However, prognostic information became refined in the IPSS intermediate-1 or intermediate-2 risk groups, where patients’ survival was significantly influenced by their CCI score: patients with a CCI of 0 had a median survival time of 35 months, those with a CCI of 1 had a median survival of 15 months, and patients with a CCI ≥2 survived a median of four months (p=0.03).
Hematopoietic Cell Transplantation Comorbidity Index
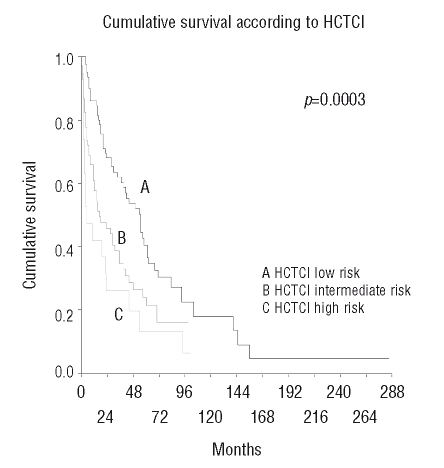
According to the HCTCI, 82 patients (48%) had no comorbidities. The distribution of patients among the HCTCI scores of 1, 2, 3, 4, and 5 was 7, 22, 14, 4, and 1, respectively. The HCTCI defines 3 risk-groups: a score of 0 indicates low risk, a score of 1 or 2 defines intermediate risk, and a score of 3 or more is equivalent to high risk. These risk groups included 82, 69, and 20 of our patients, respectively. The most frequent comorbidities were infection (n=39) and diabetes (n=25). There was no correlation between cardiac comorbidities and degree of anemia. Also there was no correlation between absolute neutrophil count and infection.
Patients with an HCTCI of 0 had a median survival time of 68 months, those with an HCTCI of 1 or 2 lived for 34 months, and those with an HCTCI of ≥3 survived for 25 months (p<0.001) (Figure 2).
Figure 2.
Cumulative survival according to Hematopoietic Cell Transplantation Comorbidity Index.
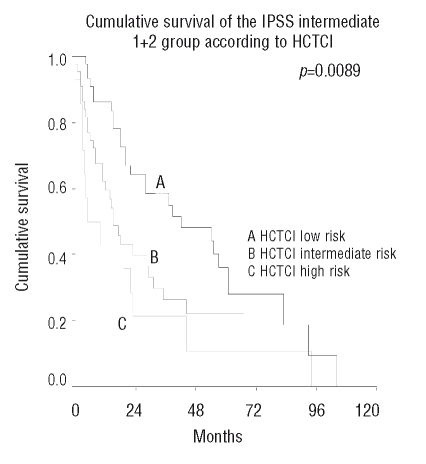
The HCTCI was able to further subdivide the IPSS intermediate-2 (p=0.016) and high-risk groups (p=0.0026). For patients in the IPSS intermediate-1 and low-risk groups, the HCTCI provided no additional prognostic information. The results for the IPSS intermediate groups are demonstrated in Figure 3.
Figure 3.
Cumulative survival of the IPSS intermediate 1 + 2 group according to Hematopoietic Cell Transplantation Comorbidity Index.
On univariate analysis, the presence of pulmonary disease, GI tract ulcers, cardiac disorders, and infection were independent prognostic factors for survival.
Multivariate analysis
Multivariate analysis using the Cox Regression Model indicated that the HCTCI yielded prognostic information independent of the IPSS (p=0.006), whereas the CCI did not enter the regression model (p=0.27). Age was not included into the regression analyses.
Non-leukemic death
Comparing the 3 different risk groups of the HCTCI we found that the percentage of non-leukemic deaths differed significantly, 53% of the low-risk patients, 61% of the intermediate patients and 85% of the high-risk patients succumbed to non-leukemic death (p=0.0093).
To the best of our knowledge, this is the first study concentrating on the prognostic impact of comorbidities in patients with MDS. Based on 171 patients treated with BSC only, we found that about 50% of MDS patients have one or more comorbidities (according to the HCTCI). The presence of comorbidities was associated with a worse prognosis. This was reliably captured by the HCTCI, which added prognostic information to the IPSS. The CCI was less well suited as a prognostic tool in this patient population.
Up to now, prognostic assessment of patients with MDS has mainly been based on parameters reflecting disease biology, like medullary blast count, cell counts, karyotype and LDH.10,11 Parameters reflecting the patient’s biology have not been harnessed for prognostication. In the realms of internal medicine, however, outcome in terms of survival has been shown to be influenced by comorbidities. Charlson and co-workers6,7 proposed a scoring system (CCI) that is based on 19 variables of interest and defines 3 risk groups. When applied to our patient cohort, the CCI had little prognostic impact and did not add valuable information to the IPSS. The CCI detects comorbidities in only 35% of patients. This may partly be due to the fact that many CCI comorbidity parameters, like lymphoma, myeloma, or leukemia, are hardly applicable to an MDS patient population. Furthermore, common comorbidities like myocardial infarction or congestive heart failure, which are very relevant for anemic MDS patients, have a low weighting in the CCI.
The HCTCI was originally developed to predict the outcome of allogeneic stem cell transplantation in patients with MDS or AML. Sorror et al.9 reported that patients with an HCTCI of 0–2 had a 2-year overall survival of 70% and 57%, depending on low or high-risk disease, after non-myeloablative conditioning, and of 78% and 50%, respectively, after myeloablative conditioning. For patients with an HCTCI ≥3 the overall survival after two years was significantly worse: 41% and 29% for non-myeloablative, and 45% and 24% after myeloablative conditioning, respectively. Even though patients undergoing HSCT are generally younger and therefore not representative of the majority of MDS patients, we found that the HCTCI successfully identified MDS patients with relevant comorbidities. The HCTCI includes parameters that are very relevant for intensive hematologic treatment approaches like induction chemotherapy or allogeneic stem cell transplantation. In particular, cardiovascular disorders and infections are more thoroughly considered, thus identifying one or more comorbidities in about 52% of patients. The infection parameter not only applied to 34 of our 171 patients (20%) but also strongly influenced overall survival (p=0.015). Patients without infection had a median survival of 35 months, whereas patients suffering from an infection had a median survival of only 11 months. Another important feature of the HCTCI is its consideration of coronary artery disease, which is relevant for anemic patients. We conclude that the HCTCI yields prognostic information independent of the IPSS because it takes into account relevant patient characteristics that are independent of the biology of the underlying bone marrow disease. The HCTCI was able to stratify patients within the IPSS intermediate-2 and high-risk groups, but not within the lower-risk groups, where only a few fatal events had occurred.
The HCTCI was developed for patients receiving aggressive treatment, namely allogeneic stem cell transplantation. Interestingly, we found that this scoring system is also useful for MDS patients receiving best supportive care only. Still, an even better prognostic tool may be devised for the majority of MDS patients who receive low-intensity treatment including blood transfusions, iron chelation, hematopoietic growth factors, epigenetic treatment, or immunomodulatory drugs or induction chemotherapy. In our opinion, an optimized comorbidity scoring system for MDS patients should focus on cardiac disorders, liver dysfunction (especially due to iron overload), renal impairment, infections, and previous malignancies.
Supplementary Material
Footnotes
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
All authors contributed equally to the writing and conception of the manuscript. Paper presented in part during the 9th International Symposium on Myelodysplastic Syndromes, Florence, 2007.
The authors reported no potential conflicts of interest.
References
- 1.Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35:181–200. doi: 10.1016/s1040-8428(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 2.Feinstein AR. The pretherapeutic classification of comorbidity in chronic disease. J Chron Dis. 1970;23:455–68. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 3.Sabin SL, Rosenfeld RM, Sundaram K, Har-el G, Lucente FE. The impact of comorbidity and age on survival with laryngeal cancer. Ear Nose Throat J. 1999;78:578. [PubMed] [Google Scholar]
- 4.Singh B, Bhaya M, Stern J, Roland JT, Zimbler M, Rosenfeld RM, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107:1469–75. doi: 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Lubke T, Monig SP, Schneider PM, Holscher AH, Bollschweiler E. Does Charlson-comorbidity index correlate with short-term outcome in patients with gastric cancer? Zentralbl Chir. 2003;128:970–6. doi: 10.1055/s-2003-44805. [DOI] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 8.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–54. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 11.Aul C, Gattermann N, Heyll A, Germing U, Derigs G, Schneider W. Primary myelodysplastic syndromes: analysis of prognostic factors in 235 patients and proposals for an improved scoring system. Leukemia. 1992;6:52–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.