Abstract
This study examined whether renin expression and secretion and plasma angiotensin II (Alg II) levels were altered in adult sheep exposed to antenatal betamethasone. Pregnant sheep received injections of 0.17 mg/kg betamethasone or vehicle, at 80 and 81 days of gestation, and offspring were studied at 6 and 18 months of age. At 6 months, plasma prorenin concentrations were significantly lower in betamethasone animals (4.63 ± 0.64 vs 7.09 ± 0.83 ng angiotensin I/mL/h, P < .01). The percentage of plasma active renin was significantly higher in the betamethasone group (31.93 ± 4.09% vs 18.57 ± 2.79%, P < .01). Plasma and renocortical renin levels were similar in both groups at 18 months, but plasma renin activity was lower than at 6 months. Ang II levels were suppressed by betamethasone. The data indicate that prenatal exposure to betamethasone alters processing and secretion of renin in offspring at 6 months, but that this difference is not apparent at 18 months.
Keywords: Glucocorticoids, renin, hypertension, sodium load, renin angiotensin system, kidney
Glucorticoids play an essential role in regulating the prepartum maturation of several tissues, most notably the lungs.1 Antenatal betamethasone treatment is effective for the prevention of neonatal respiratory distress syndrome in preterm infants and substantially reduces neonatal mortality and morbidity.2 As a consequence, synthetic glucocorticoids, such as betamethasone, are routinely administered to pregnant women at risk of preterm delivery.3
High circulating maternal glucocorticoid concentrations have been associated with abnormal fetal development and altered function or disease in offspring.4 Recently, the immediate and long-term effects of perinatal steroid treatment on the fetus have been reviewed.5,6 Exposure to excess corticosteroids before birth is thought to be a key mechanism underlying the fetal origins of adult disease hypothesis.7 Indeed, an association between antenatal glucocorticoid exposure and elevations in systolic and diastolic blood pressure has been demonstrated in 14-year-old children.8
In healthy fetal sheep, late-gestation maternal glucocorticoid administration results in fetal hypertension accompanied by increased vascular resistance, mild hypoxemia, and decreased cerebral blood flow.9 Other animal studies (including ours) have also found associations between glucocorticoid exposure during pregnancy and hypertension in adult offspring.10,11 We noted that maternal betamethasone treatment at days 80 and 81 of gestation resulted in hypertension in offspring as determined at 6 months of age.10
Findings from several animal studies suggest that a potential mechanism underlying the development of adult hypertension is altered fetal kidney development.12-14 As in humans, nephrogenesis is an early developmental event in the fetal sheep.15 In mice and sheep, fetal exposure to synthetic glucocorticoids during mid- and early gestation has been demonstrated to result in decreased nephron number and hypertension in adult offspring.11,16 The mechanisms underlying these effects are not well understood.
Fetal kidney development appears to be sensitive to glucocorticoid exposure during specific gestational windows. In sheep, maternal dexamethasone treatment at 26 to 28 days leads to an approximate 40% reduction in nephron number in offspring at 7 years of age.17 Exposure to prenatal betamethasone at 80 days of gestation produces similar changes.10 Rats born to dams treated with dexamethasone (throughout gestation) exhibited a 50% reduction in nephron number and a 30% decrease in glomerular filtration rate in adulthood.12
An intact renin–angiotensin system (RAS) is essential for optimal growth of the kidney. Indeed it has been demonstrated that a local RAS is present within the ovine fetal kidney by 40 days of gestation.18 Hypertension is also programmed by protein restriction during pregnancy through suppression of the intrarenal RAS in the developing animal and consequent impairment of nephrogenesis.19 However, there is little information available regarding the effects of antenatal glucocorticoid treatment on the systemic and intrarenal RAS in adult offspring. Therefore, the primary purpose of this study was to examine the effects of antenatal betamethasone exposure on prorenin (PRC) and active (ARC) renin concentration in plasma and kidney cortex from male sheep at 6 and 18 months of age. Another objective was to compare the plasma renin response to a sodium load in adult sheep exposed to prenatal betamethasone. We also measured systemic levels of angiotensin II (Ang II). Our working hypothesis was that antenatal steroid treatment would alter the RAS in adult life.
MATERIALS AND METHODS
Animals, Treatment, and Sampling
Time-dated pregnant sheep were obtained from local suppliers and randomized to receive 2 intramuscular injections (separated by 24 hours) of 0.17 mg/kg betamethasone or vehicle (isotonic saline) on days 80 and 81 of gestation. The betamethasone dose given is analogous to that used in human pregnancy. Thereafter pregnancy was allowed to continue unimpeded and offspring were born.
Plasma samples from male offspring (9 control, 9 betamethasone) were obtained at 6 and 18 months of age for the assessment of plasma active and prorenin concentrations. Ang II concentrations were measured in a subset of animals at 6 months, and in all at 18 months. A total of 16 animals (8 control, 8 betamethasone) were sacrificed at 18 months of age and the kidneys collected for determination of active and prorenin content.
All experimental procedures were approved by the Institutional Animal Care and Use Committee.
Plasma Active Renin Concentrations
Plasma active renin concentrations were assessed by measuring active renin activity as a function of the amount of angiotensin I (Al) generated from angiotensinogen using a commercial kit (CA-1553, Diasorin, Inc, Stillwater, Minnesota). To measure renin concentrations independent of endogenous angiotensinogen, the method was slightly modified from that described for renin activity. Excess renin substrate (0.5 mL of adult nephrectomized sheep plasma) was added to each aliquot (0.3 mL) of plasma along with the enzyme inhibitor phenylmethylsulfonyl fluoride and maleate buffer (to assure an optimal pH of 6.0). Half of this cocktail was then incubated at 37°C whereas the remainder was stored at 4°C for 1.75 hours. The AI generated was measured with the RIA kit. All samples from an animal were analyzed simultaneously in duplicate, and all assays included samples from control and betamethasone-treated animals. Results are expressed as ng AI (37°C ng/mL minus 4°C ng/mL) per milliliter plasma per hour of incubation.
Prorenin Concentrations
Prorenin concentrations were determined by measuring active renin before and after treatment of plasma or kidney cortex homogenate with bovine pancreatic trypsin (TRL3, Worthington Biochemical Corporation, Lakewood, New Jersey) at a concentration determined to yield maximum renin activation. Each lot of trypsin was tested with pooled plasma or kidney homogenate by constructing a trypsin dose–response curve. Once the optimal dose of trypsin was established for a lot, this dose was used for subsequent assays. Trypsin activation was at 4°C and pH 7.3 for 30 minutes. The activation was stopped by addition of trypsin inhibitor (Type I-S: from soybean T9003, Sigma, St Louis, Missouri) at room temperature for 15 minutes. Total trypsin-activated renin minus active renin gave the PRC for each sample.
Tissue Renin Concentrations
Approximately 50 mg of renal cortex was homogenized on ice for 45 seconds in 2 mL of saline. The homogenate was then centrifuged at 2100 × g for 10 minutes, and the supernatant collected. Samples were diluted with saline containing 5.2 mM BAL (2,3 dimercapto-1-propanol), 0.59 mM 8-hydroxyquinoline, and 10 mM disodium EDTA. ARC and PRC were determined as for plasma and are expressed as ng AI per mg of protein per hour of incubation.
Plasma Angiotensin II Concentrations
Blood was collected in prechilled EDTA-containing tubes, centrifuged, and the plasma flash frozen and stored at −80°C until extracted. The peptide was extracted from the plasma onto solid-phase extraction cartridges (Sep-Pak Vac300, 200 mg, Waters, Milford, Massachusetts) and eluted with 86% ethanol/4% glacial acetic acid, and then dried in a Speedvac Concentrator and stored at −20°C. Based on the recovery of I125 labeled angiotensin II added to each sample, 64 ± 1.9% (mean ± SEM) of the peptide was extracted.
To determine Ang II concentration, samples were reconstituted with the assay buffer supplied with the Angiotensin II RIA Kit (Alpco Diagnostics, Salem, New Hampshire) and assayed according to the manufacturer's directions.
Sodium Infusion Studies
A total of 77 betamethasone-exposed animals and 5 control animals were studied at 18 months of age. Surgery was performed to insert vascular and bladder catheters and sheep were then placed in study carts. Five days after surgery, a sodium load of hypertonic NaCl (0.0275 mEq/kg/min at 0.55 mL/min) was infused over 60 minutes. Blood samples were obtained before, at the end of the infusion, and 90 minutes after the infusion was stopped (recovery) for renin and sodium measurement (Medica EasyLyte, Bedford, Massachusetts).
Kidneys were collected from these animals for the previously described analysis at least 3 days after sodium infusion.
Statistical Analysis
Data are expressed as mean ± SEM. Comparisons were made using Student t test or analysis of variance where appropriate. Tukey test was used for post hoc analysis. Differences were considered significant when P < .05.
RESULTS
Plasma Active, Prorenin, and Angiotensin II Concentrations
Plasma ARCs were significanty higher at 6 months compared with at 18 months in both groups (P = .0002, F = 9.01; Figure 1). There were no between group differences at either age.
Figure 1.
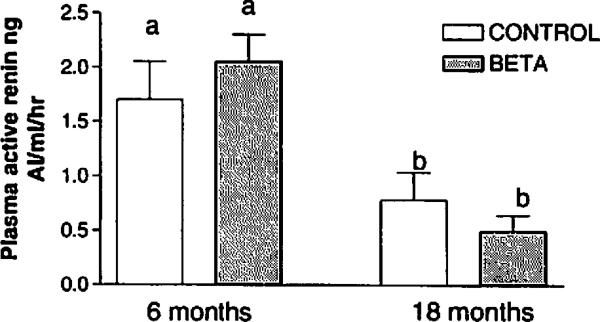
Plasma active renin concentration in control and betamethasone-treated sheep; n = 9 for both groups at 6 months of age and n = 8 for both at 18 months. Different letters indicate significant between-group differences (F = 9.01, P = .0002)
Prorenin concentrations were significantly lower in the betamethasone compared with the control group at 6 months (P = .03, F = 3.40; Figure 2). In contrast, there was no between group difference apparent at 18 months. PRCs decreased significantly in the control group between 6 and 18 months (P = .002).
Figure 2.

Plasma prorenin concentration in control and betamethasone-treated sheep; n = 9 for both groups at 6 months of age and n = 8 for both at 18 months. Different letters indicate significant between-group differences (F = 3.40, P = .03)
As shown in Figure 3, active renin comprised a significantly greater percentage of the total plasma renin concentration in the betamethasone compared with the control group at 6 months of age (P = .016). The ARC percentage was similar in both groups at 18 months, at which time a significant decrease from the 6 month percentage was apparent for the betamethasone group (P = .016).
Figure 3.

Percentage of active renin in plasma from control and betamethasone-treated sheep; n = 9 for both groups at 6 months of age and n = 8 for both at 18 months. Different letters indicate significant between-group differences (P < .015).
There was no between group difference apparent in Ang II concentration at 6 months (Figure 4). At 18 months, however, plasma Ang II levels were significantly lower in betamethasone compared with similarly aged control and 6-month betamethasone animals (P = .011, F = 4.168).
Figure 4.

Plasma angiotensin II concentrations in control and betamethasone-treated sheep; n = 5 and n = 6 for the control and betamethasone groups, respectively, at 6 months of age and n = 8 for both at 18 months. Different letters indicate significant between-group differences (F = 4.618, P = .034).
Renocortical Active and Prorenin Concentrations
Neither renocortical active or prorenin concentrations, nor the percentage active renin were different between the 2 groups at 18 months (Table 1).
Table 1.
Active Renin Concentration (ARC), Prorenin Concentration (PRC), and Percentage of Active Renin Concentration (%ARC) in Kidney Tissues From Control (n = 7) and Betamethasone-Treated (n = 8) Sheep at 18 Months of Age
| Treatment |
|||||
|---|---|---|---|---|---|
| Control |
Betamethasone |
||||
| Variable | Mean | SEM | Mean | SEM | P Value |
| ARC (ng AI/mL/h) | 756.7 | 301.7 | 600.5 | 140.8 | 0.6 |
| PRC (ng AI/mL/h) | 269.3 | 108.3 | 258.7 | 152.7 | 0.9 |
| %ARC | 72.8 | 8.0 | 78.6 | 12.2 | 0.9 |
Effect of Sodium Infusion on Plasma Active Renin, Prorenin, and Sodium Concentrations
Sodium infusion did not significantly alter ARC during or after the infusion (Figure 5). Similarly, percentage ARC values did not change in either group (Figure 6). Plasma PRCs were similar in control and betamethasone sheep (5.84 ± 0.49 vs 6.31 ± 0.70 ng AI/mL/h) and did not change following sodium infusion (data not shown).
Figure 5.

Plasma active renin concentration in control and betamethasone-treated sheep at baseline, after 60 minutes of sodium infusion, and 90 minutes after the infusion was stopped; n = 5 and n = 4 for the control and betamethasone groups, respectively. There were no significant between-group or within-group differences (F = 3.83, P > .05).
Figure 6.

Percentage of active renin in plasma from control and betamethasone-treated sheep at baseline, after sodium infusion, and at the end of the recovery period; n = 5 and n = 7 in the control and betamethasone groups, respectively. There were no significant between-group or within-group differences (F = 5.20, P > .05).
Baseline plasma sodium concentrations were not different in control and betamethasone animals so the two were combined to examine the effect of the sodium infusion. Plasma sodium concentrations increased significantly from baseline after sodium infusion (145.5 ± 0.9 to 147.0 ± 1.4 mEq/L, P < .03).
DISCUSSION
We and others have observed that fetal sheep exposed to glucocorticoids in the prenatal period have elevated blood pressure in adulthood.20,21 It is possible that this is a consequence of glucocorticoid-induced disregulation of the mechanisms controlling renin expression and/or secretion. The main aim of this study was to determine if treatment of pregnant ewes with clinically relevant doses of betamethasone would affect intrarenal renin expression and renin secretion in adult male offspring. We found that plasma PRCs were lower in betamethasone-exposed sheep compared with controls at 6 months of age. Such a difference was not apparent at 18 months. In addition, the percentage of ARC in plasma was higher in the betamethasone group at 6 months. Again, no between-group differences were evident at 18 months. These data suggest that prenatal exposure to betamethasone causes a transient alteration in the processing and secretion of renin in offspring at 6 months of age.
A key element of the fetal origins of adult disease hypothesis is that the insult occurs during a critical period of development (which may differ for each organ system), thus permanently altering the structure and function of an organ system.22 The kidney, and in particular the renin–angiotensin system (RAS), may be critically affected in models of fetal programming and may contribute to the subsequent development of disease.23 In some models of fetal programming, it has been demonstrated that offspring exposed to glucocorticoids in utero develop high blood pressure in adulthood.20,21,24 Further to this, Woods et al25 have reported that pharmacologic suppression of the RAS during development leads to a reduced number of glomeruli and hypertension in adulthood. These findings support the idea that there is a strong relationship between the intrarenal RAS and blood pressure. Gender-specific effects have also been noted with males being more susceptible to programming effects.26
It is known that renin can be secreted in 2 forms, one of which is inactive and is the renin biosynthetic precursor, prorenin. We have previously shown in sheep that acute stimulation causes selective release of active renin but has little effect on prorenin secretion.27 However, with more prolonged stimulation, prorenin secretion is also increased in both sheep and humans.28,29 In the present study, although plasma prorenin levels were significantly decreased in betamethasone-exposed animals at 6 months, plasma ARCs remained the same with a resultant increase in the percentage of active renin. These data suggest that prenatal betamethasone may interfere with the processing of renin in young adult male offspring.
During fetal life, the activity of the RAS changes markedly.30 The persistent increase in plasma renin activity, despite increased Ang II levels, suggests RAS upregulation before and after birth, resulting in an heightened set point and attenuated feedback.31 There is also evidence that the RAS is essential for normal kidney growth and development.25,32-34
Plasma renin activity decreases with increasing postnatal age.31 Velaphi et al31 demonstrated that plasma renin levels in lambs were 3 times higher than in adult sheep over the first month of life, suggesting enhancement of RAS activity and Ang II production. In the present study, we found that there was a decrease in plasma renin concentrations between 6 and 18 months of age. This was accompanied by a significant decline in plasma Ang II levels in the steroid-treated animals. Carbone et al30 have also reported that basal levels of plasma renin activity are significantly lower in adult sheep than in newborn lambs. Taken together, the data suggest that there is a continuing decline of renin secretion and Ang II concentrations during the first year of postnatal life. Basal mean arterial pressure increases during the first few months of life in several species.35-37 It is likely that the gradual elevation in blood pressure that occurs in the first year of life contributes to the decline in plasma renin levels via baroreceptor mediated inhibition of renin secretion.38
Renin secretion is inversely related to plasma sodium concentration.39,40 Increases or decreases in tubular fluid NaCl concentrations at the level of the macula densa are followed by inverse changes in the amount of renin released from juxtaglomerular cell stores, and subsequent changes in plasma renin concentrations and the rate of Ang II formation. Prostaglandins, specifically PGE2, and PGI2, are known regulators of renin release and have been implicated in macula densa–mediated renin release.41,42 There is considerable support for the notion that PGE2, is generated in macula densa cells through the action of cyclooxygenase-2 and that it is released when macula densa NaCl concentrations acutely reduced.43
In the present study we detected a small, but significant increase in plasma sodium concentrations from baseline after sodium infusion. Although there was a tendency for plasma active renin levels to decline in control animals, the decrease was not statistically significant and no such trend was apparent in betamethasone-treated animals. The fact that there was no significant change in the PRC following sodium infusion may be because the relatively large variation in the plasma renin levels impeded our ability to detect a significant difference. Also we used a short-term infusion (1 hour) that increased plasma Na levels by approximately 2 mEq/L only. Thus the intensity of the stimulus or its duration may not have been sufficient to promote a significant decrease in plasma renin concentration. The sodium infusion did not alter the percentage of active renin in the plasma. This suggests that acute changes in NaCl delivery to the macula densa do not alter the processing of renin or the relative proportions of ARC and PRC secreted.
The kidney appears to have a dual role with regard to the RAS, both as an effector and a target organ. Increasing evidence indicates that the intrarenal RAS serves as an important regulator of renal function.44 For instance, renin within the kidney, independent of the systemic RAS, may induce Na retention.45 In addition there is now evidence that renin and prorenin receptors are present in the kidney.46,47 Thus, renin could participate directly in the genesis of hypertension in offspring prenatally exposed to glucocorticoids possibly by acting on this newly discovered receptor. Our data suggest that the elevated blood pressure caused by antenatal steroid exposure does not result from elevated intrarenal renin concentrations.
In summary, our data suggest that prenatal exposure to betamethasone causes a transient alteration in the processing and secretion of renin in 6-month-old offspring. Further studies are needed to determine if this change alters the regulation of renal function and blood pressure.
Acknowledgments
This research was supported by NIH grants HD17644 and HD47584.
REFERENCES
- 1.Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev. 1994;6:141–150. doi: 10.1071/rd9940141. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Statement Online The effect of antenatal steroids for fetal maturation on perinatal outcomes. 1994 Feb 28 2;Mar 28 2;12(2):1–24. [PubMed] [Google Scholar]
- 3.NIH Consensus Development Conference 1995 Effects of corticosteroid for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 4.Moritz KM, Boon WM, Witour EM. Glucocorticoid programming of adult disease. Cell Tissue Res. 2005;322:81–88. doi: 10.1007/s00441-005-1096-6. [DOI] [PubMed] [Google Scholar]
- 5.Fowden A, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 6.Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. Am J Obstet Gynecol. 2004;190:878–881. doi: 10.1016/j.ajog.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Doyle L, Ford G, Davis N, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci. 2000;98:137–142. [PubMed] [Google Scholar]
- 9.Miller S, Chai M, Loose J, et al. The effects of maternal betamethasone administration on the intrauterine growth-restricted fetus. Endocrinology. 2007;148:1288–1295. doi: 10.1210/en.2006-1058. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res. 2005;58:510–515. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 11.Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002;40:729–734. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- 12.Celsi G, Kistner A, Aizman R, et al. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 14.Nwagwu MO, Cook A, Langley-Evans SC. Evidence of progressive deterioration of renal function in rats exposed to a maternal low-protein diet in utero. Br J Nutr. 2000;83:79–85. [PubMed] [Google Scholar]
- 15.Gimonet V, Bussieres L, Medjebeur AA, Gasser B, Lelongt B, Laborde K. Nephrogenesis and angiotensin II receptor subtypes gene expression in the fetal lamb. Am J Physiol. 1998;274:F1062–F1069. doi: 10.1152/ajprenal.1998.274.6.F1062. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol. 2007;292:R453–R461. doi: 10.1152/ajpregu.00481.2006. [DOI] [PubMed] [Google Scholar]
- 17.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549:929–935. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wintour EM, Alcorn D, Butkus A, et al. Ontogeny of hormonal and excretory function of the meso- and metanephros in the ovine fetus. Kidney Int. 1996;50:1624–1633. doi: 10.1038/ki.1996.478. [DOI] [PubMed] [Google Scholar]
- 19.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci. 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- 21.Figueroa JP, Acuna G, Rose JC, Massmann GA. Maternal antenatal steroid administration at 0.55 gestation increases arterial blood pressure in young adult sheep offspring. J Soc Gynecol Invest. 2004;11:358A. [Google Scholar]
- 22.Vehaskari VM, Woods LL. Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol. 2005;16:2545–2556. doi: 10.1681/ASN.2005030300. [DOI] [PubMed] [Google Scholar]
- 23.Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology. 2002;143:4455–4463. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- 24.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol. 2005;289:R955–R962. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- 25.Woods LL, Rasch R. Perinatal ANG II programs adult blood pressure, glomerular number, and renal function in rats. Am J Physiol. 1998;275:R1593–R1599. doi: 10.1152/ajpregu.1998.275.5.R1593. [DOI] [PubMed] [Google Scholar]
- 26.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 27.Chen K, Carey LC, Valego NK, Liu J, Rose JC. Thyroid hormone modulates renin and ANG II receptor expression in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1006–R1014. doi: 10.1152/ajpregu.00046.2005. [DOI] [PubMed] [Google Scholar]
- 28.Rosnes JS, Valego N, Wang J, Zehnder T, Rose JC. Active renin, prorenin, and renin gene expression after reduced renal perfusion pressure in term ovine fetuses. Am J Physiol. 1998;275:R141–R147. doi: 10.1152/ajpregu.1998.275.1.R141. [DOI] [PubMed] [Google Scholar]
- 29.Toffelmire EB, Slater K, Corvol P, Menard J, Schambelan M. Response of plasma prorenin and active renin to chronic and acute alterations of renin secretion in normal humans. J Clin Invest. 1989;83:679–687. doi: 10.1172/JCI113932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbone GM, Sheikh AU, Rogers S, Brewer G, Rose JC. Developmental changes in renin gene expression in ovine kidney cortex. Am J Physiol. 1993;264:R591–R596. doi: 10.1152/ajpregu.1993.264.3.R591. [DOI] [PubMed] [Google Scholar]
- 31.Velaphi SC. The renin-angiotensin system in conscious newborn sheep: metabolic clearance rate and activity. Pediatr Res. 2007;61:681–686. doi: 10.1203/pdr.0b013e3180534252. [DOI] [PubMed] [Google Scholar]
- 32.Fogo A, Yoshida Y, Yared A, Ichikawa I. Importance of angiogenic action of angiotensin II in the glomerular growth of maturing kidneys. Kidney Int. 1990;38:1068–1074. doi: 10.1038/ki.1990.314. [DOI] [PubMed] [Google Scholar]
- 33.Tufro-McReddie A, Johns DW, Geary KM, et al. Angiotensin II type 1 receptor: role in renal growth and gene expression during normal development. Am J Physiol. 1994;266(6 Pt 2):F911–F918. doi: 10.1152/ajprenal.1994.266.6.F911. [DOI] [PubMed] [Google Scholar]
- 34.Gomez RA. Role of angiotensin in renal vascular development. Kidney Int. 1998;67(Suppl):S12–S16. doi: 10.1046/j.1523-1755.1998.06703.x. [DOI] [PubMed] [Google Scholar]
- 35.Davidson D. Circulating vasoactive substances and hemodynamic adjustments at birth in lambs. J Appl Physiol. 1987;63:676–684. doi: 10.1152/jappl.1987.63.2.676. [DOI] [PubMed] [Google Scholar]
- 36.Assali NS. Some aspects of fetal life in utero and the changes at birth. Am J Obstet Gynecol. 1967;97:324–331. doi: 10.1016/0002-9378(67)90493-0. [DOI] [PubMed] [Google Scholar]
- 37.Wilson TA, Kaiser DL, Wright EM, Jr, Peach MJ, Carey RM. Ontogeny of blood pressure and the renin-angiotensin-aldosterone system. Sequential studies in the newborn lamb. Circ Res. 1981;49:416–423. doi: 10.1161/01.res.49.2.416. [DOI] [PubMed] [Google Scholar]
- 38.Robillard JE, Nakamura KT. Neurohormonal regulation of renal function during development. Am J Physiol. 1988;254:F771–F779. doi: 10.1152/ajprenal.1988.254.6.F771. [DOI] [PubMed] [Google Scholar]
- 39.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 40.Ganong WF. Review of Medical Physiology. McGraw Hill Medical; New York, NY: 2005. [Google Scholar]
- 41.Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol. 2007;292:F415–F422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
- 42.Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274(2 Pt 2):R263–R279. doi: 10.1152/ajpregu.1998.274.2.R263. [DOI] [PubMed] [Google Scholar]
- 43.Peti-Peterdi J, Komlosi P, Fuson AL, et al. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris RC, Cheng HF. The intrarenal renin-angiotensin system: a paracrine system for the local control of renal function separate from the systemic axis. Exp Nephrol. 1996;4:2–7. [PubMed] [Google Scholar]
- 45.Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin-angiotensin system. Contrib Nephrol. 2004;143:117–130. doi: 10.1159/000078716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catanzaro DF. Physiological relevance of renin/prorenin binding and uptake. Hypertens Res. 2005;28:97–105. doi: 10.1291/hypres.28.97. [DOI] [PubMed] [Google Scholar]


