Abstract
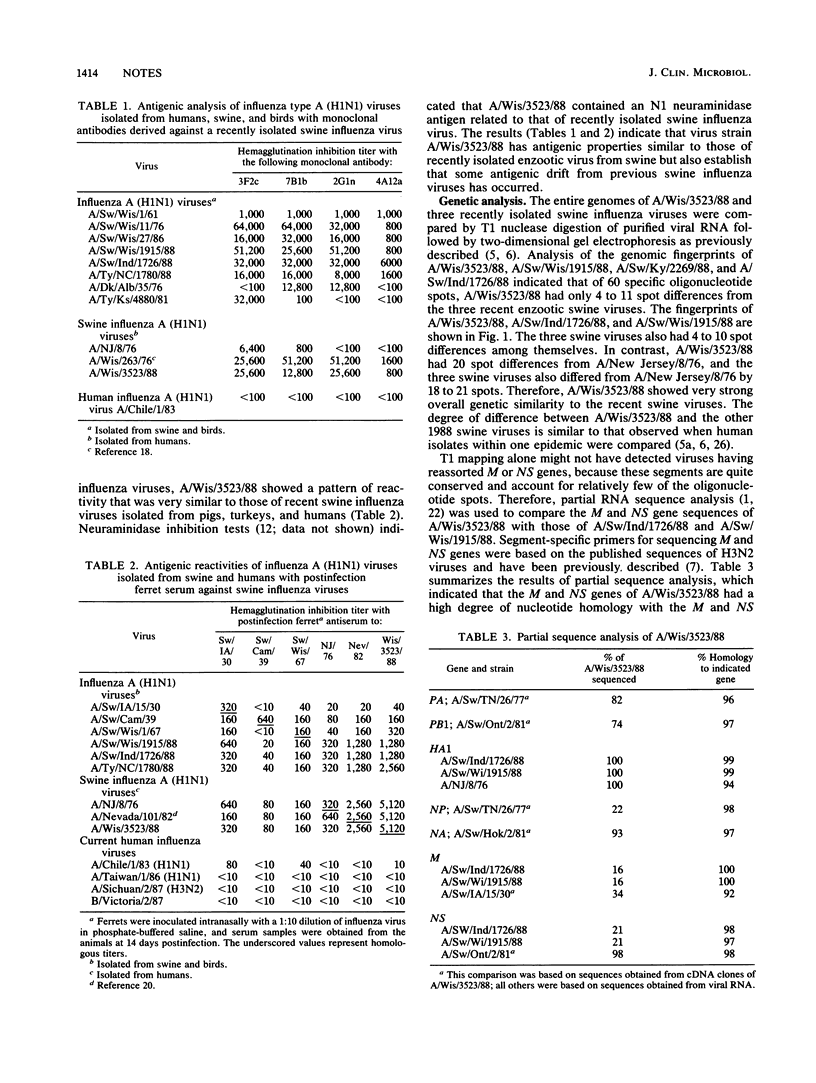
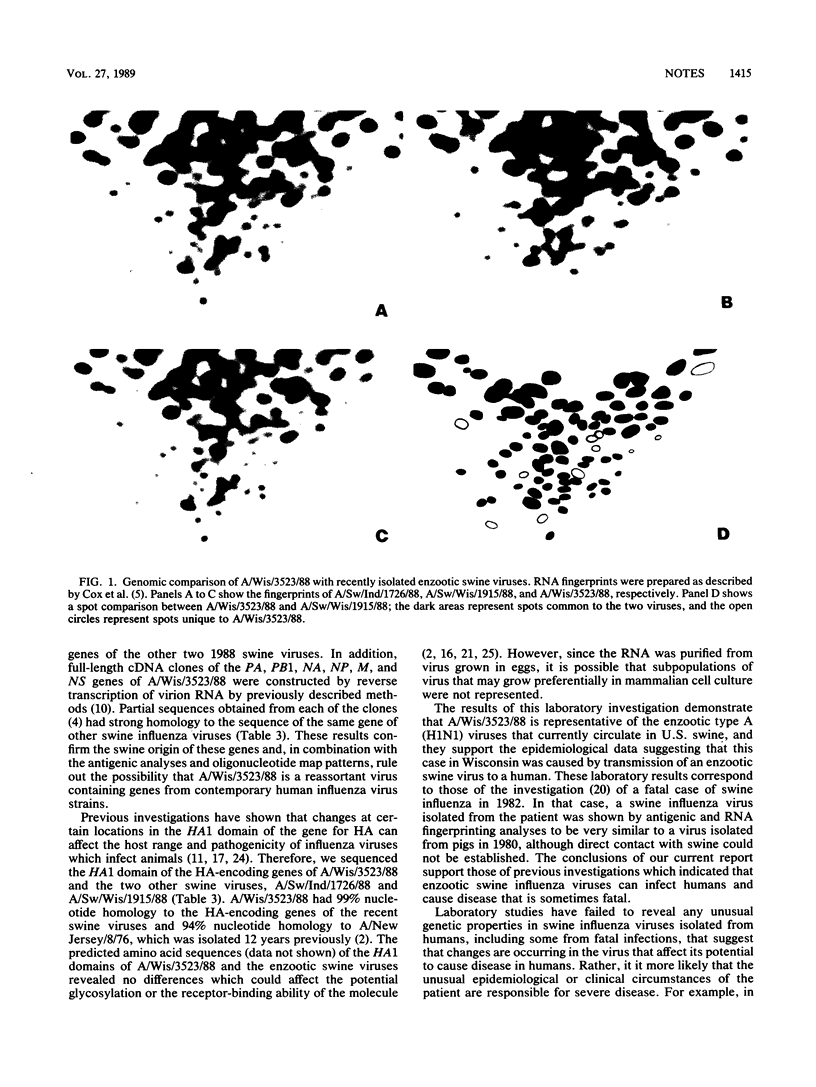
A swine influenza virus-like type A (H1N1) virus, designated A/Wisconsin/3523/88, was isolated in September 1988 from a Wisconsin woman who had died with primary viral pneumonia. Antigenic analyses with hemagglutinin-specific monoclonal antibodies and postinfection ferret serum indicated that the hemagglutinin of A/Wisconsin/3523/88 was antigenically closely related to viruses currently circulating in swine. Genetic analysis of the A/Wisconsin/3523/88 virus by RNA fingerprinting and partial RNA sequence analysis of seven of the eight segments indicated that the genome of the human isolate was similar to that of enzootic swine viruses. These laboratory data supported the epidemiologic findings that this human infection occurred by transmission of an enzootic swine influenza virus and that the virus showed no major genetic changes potentially related to increased pathogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M. Nucleotide sequence coding for the "signal peptide" and N terminus of the hemagglutinin from an asian (H2N2) strain of influenza virus. Virology. 1979 Sep;97(2):468–472. doi: 10.1016/0042-6822(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Both G. W., Shi C. H., Kilbourne E. D. Hemagglutinin of swine influenza virus: a single amino acid change pleiotropically affects viral antigenicity and replication. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6996–7000. doi: 10.1073/pnas.80.22.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Bai Z. S., Kendal A. P. Laboratory-based surveillance of influenza A(H1N1) and A(H3N2) viruses in 1980-81: antigenic and genomic analyses. Bull World Health Organ. 1983;61(1):143–152. [PMC free article] [PubMed] [Google Scholar]
- Cox N. J., Black R. A., Kendal A. P. Pathways of evolution of influenza A (H1N1) viruses from 1977 to 1986 as determined by oligonucleotide mapping and sequencing studies. J Gen Virol. 1989 Feb;70(Pt 2):299–313. doi: 10.1099/0022-1317-70-2-299. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Kendal A. P. Genetic stability of A/Ann Arbor/6/60 cold-mutant (temperature-sensitive) live influenza virus genes: analysis by oligonucleotide mapping of recombinant vaccine strains before and after replication in volunteers. J Infect Dis. 1984 Feb;149(2):194–200. doi: 10.1093/infdis/149.2.194. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Kitame F., Kendal A. P., Maassab H. F., Naeve C. Identification of sequence changes in the cold-adapted, live attenuated influenza vaccine strain, A/Ann Arbor/6/60 (H2N2). Virology. 1988 Dec;167(2):554–567. [PubMed] [Google Scholar]
- Huddleston J. A., Brownlee G. G. The sequence of the nucleoprotein gene of human influenza A virus, strain A/NT/60/68. Nucleic Acids Res. 1982 Feb 11;10(3):1029–1038. doi: 10.1093/nar/10.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y., Webster R. G. Evolution of the A/Chicken/Pennsylvania/83 (H5N2) influenza virus. Virology. 1985 Oct 15;146(1):130–137. doi: 10.1016/0042-6822(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Kida H., Brown L. E., Webster R. G. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology. 1982 Oct 15;122(1):38–47. doi: 10.1016/0042-6822(82)90375-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Naeve C. W., Hinshaw V. S., Webster R. G. Mutations in the hemagglutinin receptor-binding site can change the biological properties of an influenza virus. J Virol. 1984 Aug;51(2):567–569. doi: 10.1128/jvi.51.2.567-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve C. W., Webster R. G. Sequence of the hemagglutinin gene from influenza virus A/Seal/Mass/1/80. Virology. 1983 Sep;129(2):298–308. doi: 10.1016/0042-6822(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Patriarca P. A., Kendal A. P., Zakowski P. C., Cox N. J., Trautman M. S., Cherry J. D., Auerbach D. M., McCusker J., Belliveau R. R., Kappus K. D. Lack of significant person-to-person spread of swine influenza-like virus following fatal infection in an immunocompromised child. Am J Epidemiol. 1984 Feb;119(2):152–158. doi: 10.1093/oxfordjournals.aje.a113733. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983 Jun;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F., Burgert E. O., Jr, Dowdle W. R., Noble G. R., Campbell R. J., Van Scoy R. E. Isolation of swine influenza virus from autopsy lung tissue of man. N Engl J Med. 1976 Mar 25;294(13):708–710. doi: 10.1056/NEJM197603252941308. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Bean W. J., Van Wyke K. L., Geraci J. R., St Aubin D. J., Petursson G. Characterization of an influenza A virus from seals. Virology. 1981 Sep;113(2):712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Young J. F., Desselberger U., Palese P. Evolution of human influenza A viruses in nature: sequential mutations in the genomes of new H1N1. Cell. 1979 Sep;18(1):73–83. doi: 10.1016/0092-8674(79)90355-6. [DOI] [PubMed] [Google Scholar]



