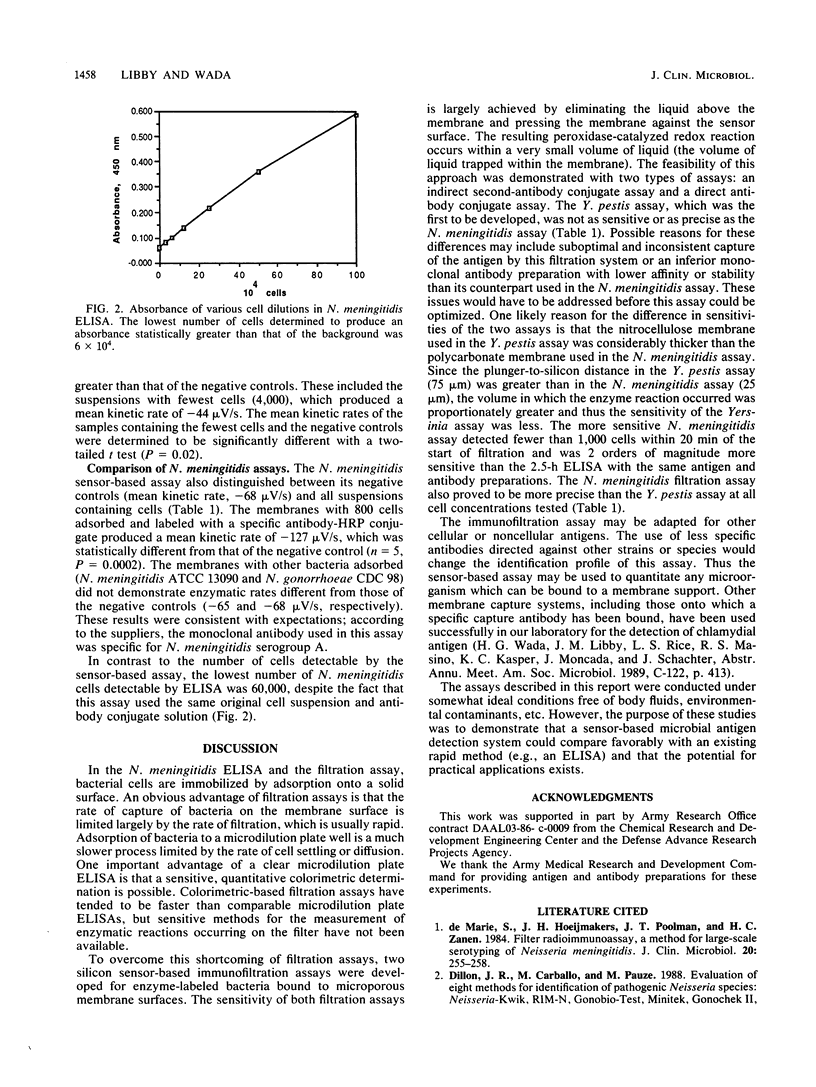
Abstract
A light-addressable potentiometric (silicon) sensor was used in an immunofiltration procedure for the detection of pathogenic bacteria. Yersinia pestis was detected by filtering the cells onto nitrocellulose membranes and then filtering anti-Y. pestis mouse monoclonal antibody and anti-mouse immunoglobulin G-horseradish peroxidase conjugate. For Neisseria meningitidis detection, mouse monoclonal antibody to the major outer membrane protein of this bacterium was coupled directly to horseradish peroxidase. N. meningitidis cell suspensions were filtered onto polycarbonate membranes, and the enzyme conjugate was allowed to react with the filtered bacteria. The presence of both enzyme conjugates was determined potentiometrically with the silicon sensor. The sensitivity of this technique relative to that of an enzyme-linked immunosorbent assay for N. meningitidis was determined. Fewer than 1,000 bacterial cells could be detected with the silicon sensor in a 20-min assay, whereas a 2.5-h enzyme-linked immunosorbent assay with the same antigen and antibody preparations was significantly less sensitive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dillon J. R., Carballo M., Pauzé M. Evaluation of eight methods for identification of pathogenic Neisseria species: Neisseria-Kwik, RIM-N, Gonobio-Test, Minitek, Gonochek II, GonoGen, Phadebact Monoclonal GC OMNI Test, and Syva MicroTrak Test. J Clin Microbiol. 1988 Mar;26(3):493–497. doi: 10.1128/jcm.26.3.493-497.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D. G., Parce J. W., McConnell H. M. Light-addressable potentiometric sensor for biochemical systems. Science. 1988 May 27;240(4856):1182–1185. doi: 10.1126/science.3375810. [DOI] [PubMed] [Google Scholar]
- Hosmer M. E., Cohenford M. A., Ellner P. D. Preliminary evaluation of a rapid colorimetric method for identification of pathogenic Neisseria. J Clin Microbiol. 1986 Jul;24(1):141–142. doi: 10.1128/jcm.24.1.141-142.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa E., Imagawa M., Hashida S., Yoshitake S., Hamaguchi Y., Ueno T. Enzyme-labeling of antibodies and their fragments for enzyme immunoassay and immunohistochemical staining. J Immunoassay. 1983;4(3):209–327. doi: 10.1080/15321818308057011. [DOI] [PubMed] [Google Scholar]
- Janda W. M., Zigler K. L., Bradna J. J. API QuadFERM+ with rapid DNase for identification of Neisseria spp. and Branhamella catarrhalis. J Clin Microbiol. 1987 Feb;25(2):203–206. doi: 10.1128/jcm.25.2.203-206.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S., Schell R. F., Pennell D. R. Rapid method for the differentiation of gram-positive and gram-negative bacteria on membrane filters. J Clin Microbiol. 1988 Jul;26(7):1378–1382. doi: 10.1128/jcm.26.7.1378-1382.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soergel M. E., Schaffer F. L., Blank H. F. Solid-phase radioimmunoassay for detection of plague antigen in animal tissue. J Clin Microbiol. 1982 Nov;16(5):953–956. doi: 10.1128/jcm.16.5.953-956.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistnes A. I., Rosenqvist E., Frøholm L. O. Spin membrane immunoassay for use in meningococcal serology. J Clin Microbiol. 1983 Oct;18(4):905–911. doi: 10.1128/jcm.18.4.905-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. E., Arntzen L., Tyndal G. L., Isaäcson M. Application of enzyme immunoassays for the confirmation of clinically suspect plague in Namibia, 1982. Bull World Health Organ. 1986;64(5):745–752. [PMC free article] [PubMed] [Google Scholar]
- Williams J. E., Gentry M. K., Braden C. A., Leister F., Yolken R. H. Use of an enzyme-linked immunosorbent assay to measure antigenaemia during acute plague. Bull World Health Organ. 1984;62(3):463–466. [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Moran E. E., Connelly H., Mandrell R. E., Brandt B. Monoclonal antibodies to serotype 2 and serotype 15 outer membrane proteins of Neisseria meningitidis and their use in serotyping. Infect Immun. 1984 Oct;46(1):260–266. doi: 10.1128/iai.46.1.260-266.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marie S., Hoeijmakers J. H., Poolman J. T., Zanen H. C. Filter radioimmunoassay, a method for large-scale serotyping of Neisseria meningitidis. J Clin Microbiol. 1984 Aug;20(2):255–258. doi: 10.1128/jcm.20.2.255-258.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


