Abstract
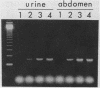
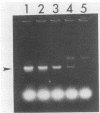
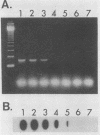
The polymerase chain reaction was used to amplify a 188-base pair (bp) segment of the repetitive 195-bp nuclear DNA sequence of Trypanosoma cruzi that is the most abundant sequence in this organism. The reaction amplified this repetitive element in four T. cruzi isolates from widely separated geographic regions. No amplification of the 188-bp fragment occurred when DNAs extracted from Leishmania spp., African trypanosomes, or blood samples from mice and humans were used. Amplification of one-half of the DNA from a single T. cruzi parasite produced an amount of the 188-bp element that was readily visible in a gel stained with ethidium bromide. Hybridization of a radiolabeled probe to membrane-bound amplification products increased the sensitivity to a level at which 1/200 of the DNA in a single parasite could be detected. T. cruzi DNA was readily detected in DNA extracted from the abdominal contents of infected insect vectors reared in the laboratory. No parasite DNA was detected in the blood samples of two individuals known to be infected with T. cruzi, possibly because in such patients the number of circulating parasites are extremely low or because parasitemias are intermittent. These results represent a considerable increase in sensitivity over previously reported methods for the detection of T. cruzi infections. Polymerase chain reaction amplification can be used to evaluate large numbers of samples in a single day and thus should be useful in large-scale studies of the prevalence of T. cruzi in both insect vectors and mammalian hosts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashall F., Yip-Chuck D. A., Luquetti A. A., Miles M. A. Radiolabeled total parasite DNA probe specifically detects Trypanosoma cruzi in mammalian blood. J Clin Microbiol. 1988 Mar;26(3):576–578. doi: 10.1128/jcm.26.3.576-578.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Donelson J. E. Mini-exon gene repeats of Trypanosoma (Nannomonas) congolense have internal repeats of 190 base pairs. Mol Biochem Parasitol. 1987 Aug;25(1):113–122. doi: 10.1016/0166-6851(87)90024-7. [DOI] [PubMed] [Google Scholar]
- Degrave W., Fragoso S. P., Britto C., van Heuverswyn H., Kidane G. Z., Cardoso M. A., Mueller R. U., Simpson L., Morel C. M. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol Biochem Parasitol. 1988 Jan 1;27(1):63–70. doi: 10.1016/0166-6851(88)90025-4. [DOI] [PubMed] [Google Scholar]
- Embury S. H., Scharf S. J., Saiki R. K., Gholson M. A., Golbus M., Arnheim N., Erlich H. A. Rapid prenatal diagnosis of sickle cell anemia by a new method of DNA analysis. N Engl J Med. 1987 Mar 12;316(11):656–661. doi: 10.1056/NEJM198703123161103. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Prediger E., Huecas M. E., Nogueira N., Lizardi P. M. Minichromosomal repetitive DNA in Trypanosoma cruzi: its use in a high-sensitivity parasite detection assay. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3356–3360. doi: 10.1073/pnas.81.11.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D. J., Bwayo J. J. Isopycnic isolation of African trypanosomes on Percoll gradients formed in situ. Acta Trop. 1982 Dec;39(4):363–366. [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Gam A. A., Gilliam F. C. American trypanosomiasis (Chagas' disease) in Central American immigrants. Am J Med. 1987 May;82(5):915–920. doi: 10.1016/0002-9343(87)90152-5. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Hieny S., Shiver G. M., Snary D., Sher A. Cryptic epitope explains the failure of a monoclonal antibody to bind to certain isolates of Trypanosoma cruzi. J Immunol. 1984 Nov;133(5):2731–2735. [PubMed] [Google Scholar]
- Kirchhoff L. V., Kim K. S., Engman D. M., Donelson J. E. Ubiquitin genes in trypanosomatidae. J Biol Chem. 1988 Sep 5;263(25):12698–12704. [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A., de la Cruz V. F., Ferreira P. C., Morel C., Simpson L. Evolution of parasitism: kinetoplastid protozoan history reconstructed from mitochondrial rRNA gene sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4779–4783. doi: 10.1073/pnas.85.13.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanar D. E., Levy L. S., Manning J. E. Complexity and content of the DNA and RNA in Trypanosoma cruzi. Mol Biochem Parasitol. 1981 Sep;3(5):327–341. doi: 10.1016/0166-6851(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Rice-Ficht A. C., Kelly G., Esser K. M., Donelson J. E. Characterization of the genes specifying two metacyclic variable antigen types in Trypanosoma brucei rhodesiense. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6642–6646. doi: 10.1073/pnas.81.21.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden P. D., Barreto A. C., Cuba C. C., Gama M. B., Ackers J. Improvements in routine xenodiagnosis with first instar Dipetalogaster maximus (Uhler 1894) (Triatominae). Am J Trop Med Hyg. 1979 Jul;28(4):649–652. [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Brentano S. T., Rice-Ficht A. C., Dorfman D. M., Donelson J. E. DNA rearrangements of the variable surface antigen genes of the trypanosomes. J Protozool. 1984 Feb;31(1):65–73. doi: 10.1111/j.1550-7408.1984.tb04291.x. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Piesman J., Sherlock I. A., Mota E., Todd C. W., Hoff R., Weller T. H. Association between household triatomine density and incidence of Trypanosoma cruzi infection during a nine-year study in Castro Alves, Bahia, Brazil. Am J Trop Med Hyg. 1985 Sep;34(5):866–869. doi: 10.4269/ajtmh.1985.34.866. [DOI] [PubMed] [Google Scholar]
- Rogers W. O., Wirth D. F. Kinetoplast DNA minicircles: regions of extensive sequence divergence. Proc Natl Acad Sci U S A. 1987 Jan;84(2):565–569. doi: 10.1073/pnas.84.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield C. J. Control of Chagas' disease vectors. Br Med Bull. 1985 Apr;41(2):187–194. doi: 10.1093/oxfordjournals.bmb.a072048. [DOI] [PubMed] [Google Scholar]
- Sloof P., Bos J. L., Konings A. F., Menke H. H., Borst P., Gutteridge W. E., Leon W. Characterization of satellite DNA in Trypanosoma brucei and Trypanosoma cruzi. J Mol Biol. 1983 Jun 15;167(1):1–21. doi: 10.1016/s0022-2836(83)80031-x. [DOI] [PubMed] [Google Scholar]
- WOODY N. C., WOODY H. B. American trypanosomiasis (Chagas' disease); first indigenous case in the United States. J Am Med Assoc. 1955 Oct 15;159(7):676–677. doi: 10.1001/jama.1955.02960240042010a. [DOI] [PubMed] [Google Scholar]