Abstract
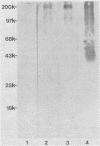
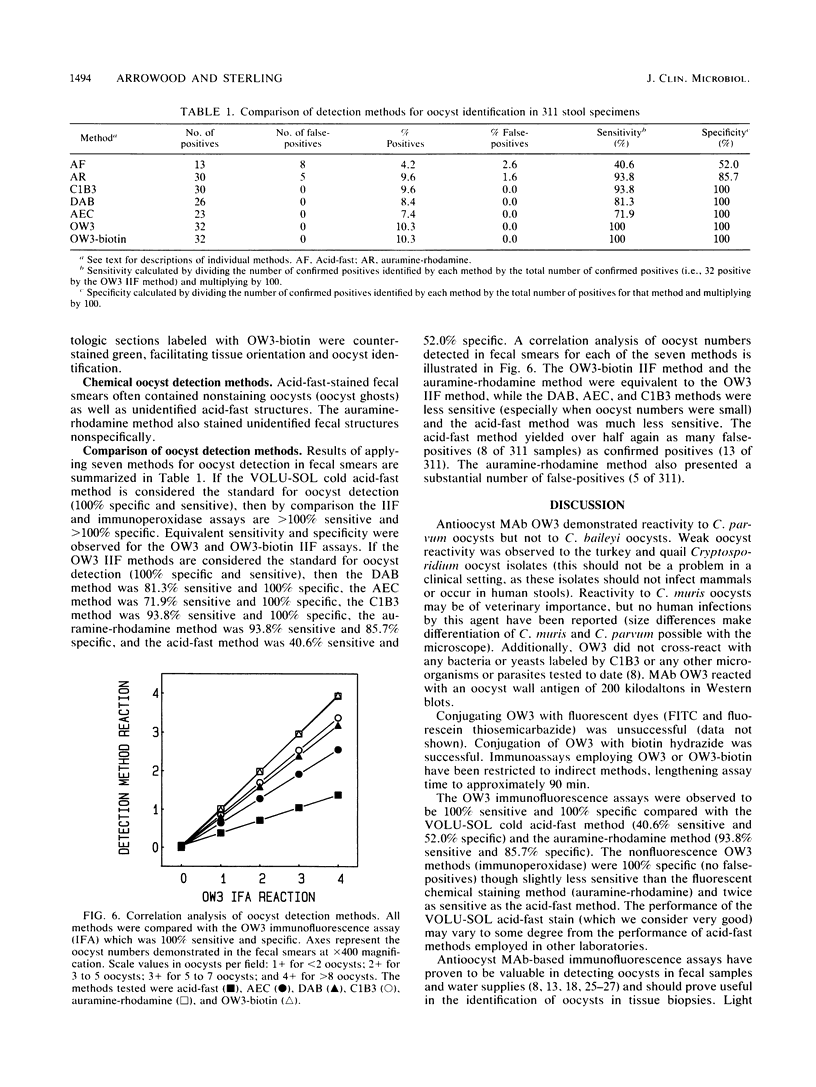
The sensitivity and specificity of seven microscopy-based Cryptosporidium oocyst detection methods were compared after application to unconcentrated fecal smears. The seven methods were as follows: (i) a commercial acid-fast (AF) stain (VOLU-SOL) method, (ii) Truant auramine-rhodamine (AR) stain method, (iii) fluorescein-conjugated C1B3 monoclonal antibody (MAb) direct fluorescence method, (iv) OW3 MAb indirect fluorescence method, (v) biotinylated OW3 indirect fluorescence method, (vi) biotinylated OW3-indirect diaminobenzidine (DAB) method, and (vii) biotinylated OW3-aminoethylcarbazole (AEC) method. A total of 281 randomly collected Formalin-fixed fecal samples (submitted to the Maricopa County Health Department, Phoenix, Ariz.) and 30 known positives (Formalin-fixed and K2Cr2O7-preserved stools from our laboratory) were examined in a blind test; 32 of 311 samples (10.3%) were confirmed positive. Of the confirmed positives, 40.6% were identified by the AF method, 93.8% were identified by the AR method, 93.8% were identified by the C1B3 method, 81.3% were identified by the OW3-DAB method, 71.9% were identified by the OW3-AEC method, 100% were identified by the OW3 indirect fluorescence method, and 100% were identified by the biotinylated OW3 indirect fluorescence method. False-positives were encountered by the AF and AR methods (52.0 and 85.7% specificity, respectively), while no false-positives encountered by the MAb-based methods. Oocysts in infected tissue sections were easily detected by the MAb-based methods.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus K. W., Campbell I., Gray E. W., Sherwood D. Staining of faecal yeasts and cryptosporidium oocysts. Vet Rec. 1981 Feb 21;108(8):173–173. doi: 10.1136/vr.108.8.173. [DOI] [PubMed] [Google Scholar]
- Arrowood M. J., Sterling C. R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987 Apr;73(2):314–319. [PubMed] [Google Scholar]
- Bronsdon M. A. Rapid dimethyl sulfoxide-modified acid-fast stain of Cryptosporidium oocysts in stool specimens. J Clin Microbiol. 1984 Jun;19(6):952–953. doi: 10.1128/jcm.19.6.952-953.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casemore D. P., Armstrong M., Sands R. L. Laboratory diagnosis of cryptosporidiosis. J Clin Pathol. 1985 Dec;38(12):1337–1341. doi: 10.1136/jcp.38.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. F., Moorhead P. D. A rapid staining technic for cryptosporidia. Mod Vet Pract. 1984 Apr;65(4):307–307. [PubMed] [Google Scholar]
- Fayer R., Ungar B. L. Cryptosporidium spp. and cryptosporidiosis. Microbiol Rev. 1986 Dec;50(4):458–483. doi: 10.1128/mr.50.4.458-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. S., Brewer T. C., Bruckner D. A. Fluorescence detection of Cryptosporidium oocysts in human fecal specimens by using monoclonal antibodies. J Clin Microbiol. 1987 Jan;25(1):119–121. doi: 10.1128/jcm.25.1.119-121.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. S., Bruckner D. A., Brewer T. C., Shimizu R. Y. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J Clin Microbiol. 1983 Jul;18(1):185–190. doi: 10.1128/jcm.18.1.185-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb R., Erlich J., Davidson G. P. Cryptosporidia detection. Med J Aust. 1985 Mar 4;142(5):329–330. doi: 10.5694/j.1326-5377.1985.tb113387.x. [DOI] [PubMed] [Google Scholar]
- Ma P., Soave R. Three-step stool examination for cryptosporidiosis in 10 homosexual men with protracted watery diarrhea. J Infect Dis. 1983 May;147(5):824–828. doi: 10.1093/infdis/147.5.824. [DOI] [PubMed] [Google Scholar]
- Madore M. S., Rose J. B., Gerba C. P., Arrowood M. J., Sterling C. R. Occurrence of Cryptosporidium oocysts in sewage effluents and selected surface waters. J Parasitol. 1987 Aug;73(4):702–705. [PubMed] [Google Scholar]
- McLauchlin J., Casemore D. P., Harrison T. G., Gerson P. J., Samuel D., Taylor A. G. Identification of cryptosporidium oocysts by monoclonal antibody. Lancet. 1987 Jan 3;1(8523):51–51. doi: 10.1016/s0140-6736(87)90753-7. [DOI] [PubMed] [Google Scholar]
- McNabb S. J., Hensel D. M., Welch D. F., Heijbel H., McKee G. L., Istre G. R. Comparison of sedimentation and flotation techniques for identification of Cryptosporidium sp. oocysts in a large outbreak of human diarrhea. J Clin Microbiol. 1985 Oct;22(4):587–589. doi: 10.1128/jcm.22.4.587-589.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead J. R., Arrowood M. J., Current W. L., Sterling C. R. Field inversion gel electrophoretic separation of Cryptosporidium spp. chromosome-sized DNA. J Parasitol. 1988 Jun;74(3):366–369. [PubMed] [Google Scholar]
- Mead J. R., Arrowood M. J., Sterling C. R. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J Parasitol. 1988 Feb;74(1):135–143. [PubMed] [Google Scholar]
- Musial C. E., Arrowood M. J., Sterling C. R., Gerba C. P. Detection of Cryptosporidium in water by using polypropylene cartridge filters. Appl Environ Microbiol. 1987 Apr;53(4):687–692. doi: 10.1128/aem.53.4.687-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoh S. H., Gordon C., Potter A., Zola H. The purification of mouse monoclonal antibodies from ascitic fluid. J Immunol Methods. 1986 Jul 24;91(2):231–235. doi: 10.1016/0022-1759(86)90483-7. [DOI] [PubMed] [Google Scholar]
- O'Shannessy D. J., Dobersen M. J., Quarles R. H. A novel procedure for labeling immunoglobulins by conjugation to oligosaccharide moieties. Immunol Lett. 1984;8(5):273–277. doi: 10.1016/0165-2478(84)90008-7. [DOI] [PubMed] [Google Scholar]
- Pohjola S., Jokipii L., Jokipii A. M. Dimethylsulphoxide-Ziehl-Neelsen staining technique for detection of cryptosporidial oocysts. Vet Rec. 1985 Apr 20;116(16):442–443. doi: 10.1136/vr.116.16.442. [DOI] [PubMed] [Google Scholar]
- Sterling C. R., Arrowood M. J. Detection of Cryptosporidium sp. infections using a direct immunofluorescent assay. Pediatr Infect Dis. 1986 Jan-Feb;5(1 Suppl):S139–S142. doi: 10.1097/00006454-198601001-00022. [DOI] [PubMed] [Google Scholar]
- Sterling C. R., Seegar K., Sinclair N. A. Cryptosporidium as a causative agent of traveler's diarrhea. J Infect Dis. 1986 Feb;153(2):380–381. doi: 10.1093/infdis/153.2.380. [DOI] [PubMed] [Google Scholar]
- Stibbs H. H., Ongerth J. E. Immunofluorescence detection of Cryptosporidium oocysts in fecal smears. J Clin Microbiol. 1986 Oct;24(4):517–521. doi: 10.1128/jcm.24.4.517-521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotish R. L., Wang C. C., Meyenhofer M. Structure and composition of the oocyst wall of Eimeria tenella. J Parasitol. 1978 Dec;64(6):1074–1081. [PubMed] [Google Scholar]
- Upton S. J., Current W. L. The species of Cryptosporidium (Apicomplexa: Cryptosporidiidae) infecting mammals. J Parasitol. 1985 Oct;71(5):625–629. [PubMed] [Google Scholar]
- Willson P. J., Acres S. D. A comparison of dichromate solution floatation and fecal smears for diagnosis of cryptosporidiosis in calves. Can Vet J. 1982 Aug;23(8):240–246. [PMC free article] [PubMed] [Google Scholar]
- Zierdt W. S. Concentration and identification of Cryptosporidium sp. by use of a parasite concentrator. J Clin Microbiol. 1984 Nov;20(5):860–861. doi: 10.1128/jcm.20.5.860-861.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]