Abstract
Ku is a heterodimeric protein involved in nonhomologous end-joining of the DNA double-stranded break repair pathway. It binds to the double-stranded DNA ends and then activates a series of repair enzymes that join the broken DNA. In addition to its function in DNA repair, the yeast Saccharomyces cerevisiae Ku (Yku) is also a component of telomere protein-DNA complexes that affect telomere function. The yeast telomeres are composed of duplex C1–3(A/T)G1–3 telomeric DNA repeats plus single-stranded TG1–3 telomeric DNA tails. Here we show that Yku is capable of binding to a tailed-duplex DNA formed by telomeric DNA that mimics the structure of telomeres. Addition of Cdc13p, a single-stranded telomeric DNA-binding protein, to the Yku-DNA complex enables the formation of a ternary complex with Cdc13p binding to the single-stranded tail of the DNA substrate. Because pre-loading of Cdc13p to the single-stranded telomeric tail inhibits the binding of Yku, the results suggested that loading of Yku and Cdc13p to telomeres is sequential. Through generating a double-stranded break near telomeric DNA sequences, we found that Ku protein appears to bind to the de novo synthesized telomeres earlier than that of Cdc13p in vivo. Thus, our results indicated that Yku interacts directly with telomeres and that sequential loading of Yku followed by Cdc13p to telomeres is required for both proteins to form a ternary complex on telomeres. Our results also offer a mechanism that the binding of Cdc13p to telomeres might prevent Yku from initiating DNA double-stranded break repair pathway on telomeres.
DNA damages in the form of double-stranded breaks (DSBs)4 compromise the integrity of genomes. Failure in repairing or mis-repairing double-stranded breaks can lead to chromosome instability and eventually cell death or cancer (1). Double-stranded breaks are repaired by two main pathways, the homologous recombination and nonhomologous DNA end-joining. In nonhomologous DNA end-joining, Ku is the first protein to bind to the DNA ends to initiate the repair pathway (2). Upon binding, Ku then recruits a series of repair enzymes to join the broken ends (2). Ku is a heterodimeric protein composed of 70- and ∼80-kDa subunits. In Saccharomyces cerevisiae, Ku includes Yku70 and Yku80 subunits. Because the biochemical configuration of the broken ends could be very diverse on DSBs, Ku binds to double-stranded ends in a sequence- and energy-independent manner. It is capable of binding to DNA ends with blunt 3′-overhangs or 5′-overhangs as well as double-stranded DNA with nicks, gaps, or internal loops (3–7). However, Ku does not have high affinity to single-stranded DNA. The crystal structure of human Ku heterodimer indicates that it forms a ring structure that encircles duplex DNA (7). This unique structure feature enables Ku to recognize DNA ends and achieves its high affinity binding.
In additional to the role in double-stranded break repair, Ku was shown to be a component of telomeric protein-DNA complex in yeast and mammals (8–10). Telomeres are terminal structures of chromosomes composed of short tandem repeated sequences (11, 12). Mutation of YKU70 or YKU80 causes defects in telomere structure (13–15), telomere silencing (16–19), and replication timing of telomeres (20). The function of yeast Ku (Yku) on telomeres could mediate through protein-protein interaction with Sir4p or protein-RNA interaction with Tlc1 RNA (21, 22). For example, through the interaction with Sir4p, Yku selectively affects telomeres silencing but not the silent mating type loci (17). Yku could also bind to telomerase Tlc1 RNA for telomere length maintenance (22). Judged by the DNA binding activity of Yku, it is reasonable to suggest that it may bind directly to telomeric DNA. Indeed, it was shown that human Ku is capable of binding directly to telomeric DNA in vitro (15). Moreover, because the deletion of SIR4 in budding yeast (23) or Taz1 in fission yeast (24) does not abolish the association of Ku with chromosomal ends, this suggests that Ku might bind directly to telomeric DNA in cells. However, because yeast telomeres have a short 12–14-mer single-stranded tail (25), it is uncertain whether Yku could pass the single-stranded region to reach its binding site. The direct binding of Yku to telomeric DNA has not been experimentally determined.
In contrast to double-stranded breaks, the ends of linear chromosomes are not recognized by repair enzymes as DNA damage. In S. cerevisiae, Cdc13p is the single-stranded TG1–3 DNA-binding protein that enables cells to differentiate whether the ends of a linear DNA are telomeres or broken ends (26–29). Thus, although the mechanism of how cells prevent the activation of DSB repair pathway in telomere is unclear, it is likely that binding of Cdc13p to telomeres might inhibit the initiation of DNA damage response by the Ku protein. Here, using a tailed-duplex DNA synthesized by telomeric DNA sequences to mimic telomere structure, we showed that Yku binds directly to this tailed-duplex DNA substrate and forms a ternary complex with Cdc13p. Our results also showed that Yku loaded to a de novo synthesized telomere earlier than Cdc13p in vivo. These results support the direct binding of Yku to telomeric DNA and that the spatial orientation of Cdc13p might block the activation of DSB repair pathway on telomeres.
EXPERIMENTAL PROCEDURES
Purification of Yku from Yeast—Yeast BJ2168 cells (MATa leu2 trp1 ura3-52 prb1-1122 pep4-3 prc1-407 gal2) carrying pRS425TEF-YKU70-TAP and pRS423TEF-YKU80-TAP was employed to isolate Yku heterodimer from yeast (A gift from D. E. Gottschling, Fred Hutchinson Cancer Research Center, Seattle). Purification of the Yku protein was conducted according to the procedure described by Stellwagen et al. (22).
Purification of His6-tagged Cdc13(451–693)p—The Escherichia coli expression system was used for Cdc13(451–693)p expression. Plasmids pET6H-CDC13(451–693) carrying the cdc13R635C mutation of CDC13 were constructed by ligating the 0.73-kbp BamHI-NruI cdc13R635C DNA fragment of pRS315ΔB-CDC13 with pET6H that was linearized with the same enzymes (30). E. coli BL21(DE3) pLysS was used as the host for Cdc13(451–693)p expression. The purification procedure was as described previously (31).
Purification of Recombinant Yku Proteins from Sf21 Cells—Insect cell line Sf21 was used as the host for baculovirus propagation, expression, and purification of recombinant Yku70p and Yku80p. Plasmid pBac6His-YKU70 was constructed by inserting a 1.8-kbp NcoI-SalI fragment of YKU70 to NcoI- and SalI-digested pBac6His (31). Similarly, plasmid pBac6His-YKU80 was constructed by inserting a 1.9-kbp NcoI-SalI fragment of YKU80 to NcoI- and SalI-digested pBac6His. These plasmids enabled the expression of Yku70p or Yku80p with His6 tagged at the N terminus. Recombinant viruses were generated by co-transfection of plasmid pBac6His-YKU70 or pBac6His-YKU80 and Bac-N-Blue DNA to Sf21 cells (Invitrogen). To purify His6-tagged Yku70 or Yku80, ∼5 × 107 Sf21 cells were infected with 25 × 107 recombinant viruses for 4 days. Cells were washed with phosphate-buffered saline and then lysed by the addition of lysis buffer (6 m guanidine HCl, 100 mm NaH2PO4, pH 8, 10 mm Tris-HCl). The suspensions were incubated on ice for 60 min and sonicated. Total cell extracts were then collected by centrifugation. Ni-NTA-agarose (Qiagen) was utilized to purify the His6-tagged Yku70p or Yku80p. Batch purification protocol was used according to manufacturer's suggestion. The bound His6-tagged Yku70p or Yku80p protein was first washed with lysis buffer containing 20 mm imidazole and eluted by the same buffer containing 200 mm imidazole. To renature the purified Yku70p and Yku80p proteins, proteins eluted from Ni-NTA-agarose column was diluted to ∼50 μg/ml and dialyzed against renaturation buffer (100 mm Tris, pH 8.0, 2 mm EDTA, 2 mm dithiothreitol, 0.4 m l-arginine, 20% glycerol) at 4 °C for 12 h. Purified protein was aliquoted and frozen by dry ice/ethanol bath.
Electrophoretic Mobility Shift Assay (EMSA)—Oligonucleotide (Table 1) was first 5′-end-labeled with [γ-32P]ATP (3000 mCi/mm; PerkinElmer Life Sciences) using T4 polynucleotide kinase (New England Biolabs) and subsequently purified from a 10% sequencing gel after electrophoresis. To prepare the duplex or tailed-duplex DNA substrates, the 5′-end-labeled DNA was mixed with an excess amount of unlabeled complementary DNA and incubated at 100 °C for 5 min. The DNA mixtures were then cooled under room temperature and subsequently separated by a native 8% polyacrylamide gel to isolate the duplex or tailed-duplex DNA substrates. To perform the assays, purified proteins were mixed with 2.0 ng of 32P-labeled DNA with a total volume of 15 μl containing 50 mm Tris-HCl, pH 7.5, 1 mm EDTA, 50 mm NaCl, and 1 mm dithiothreitol. The mixtures were allowed to incubate at room temperature for 10 min. 3 μl of 80% glycerol was then added, and the mixtures were loaded on a 6% nondenaturing polyacrylamide gel, which were pre-run at 125 V for 10 min. Electrophoresis was carried out in TBE (89 mm Tris borate, 2 mm EDTA) at 125 V for 105 min. The gels were dried and autoradiographed. Binding activity was quantified using a PhosphorImager (GE Healthcare).
TABLE 1.
Oligonucleotides used in this study
DNase I Footprint Analysis—Telomeric DNA RT1 were 5′-labeled, annealed to RT2, and purified as described above. The DNA was mixed with Yku protein in 50 μl of reaction buffer (10 mm Tris-HCl, pH 8.0, 2.5 mm MgCl2, 0.5 mm CaCl2) and incubated at room temperature for 10 min. DNase I (0.2 unit) was added and incubated at 37 °C for another 10 min. The reaction was stopped with 10 μl of 250 mm EGTA. The DNA was then precipitated at –70 °C by adding 1 μl of 10 mg/ml oyster glycogen and 150 μl of ethanol. The precipitant was collected by centrifugation, dried, and analyzed by electrophoresis using 10% polyacrylamide sequencing gel.
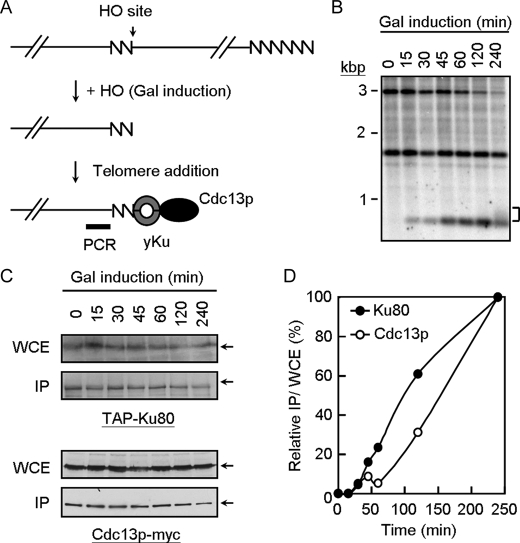
Chromatin Immunoprecipitation Analysis on a de Novo Synthesized Telomere—Yeast strain YJL0801 was generated by introducing a CDC13-myc9::TRP1 into UCC5706 (MATa-inc ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1::GAL1-HO-LEU-2 VII-L::ADE2-TG1–3-HO site-LYS2 rad52::hisG, a gift of D. E. Gottschling (32)) and then transformed with plasmid pRS423TEF-KU80-TAP (22). The strain was used in chromatin immunoprecipitation experiments on de novo synthesized telomeres (32). Briefly, yeast cells were grown in medium (2.5% raffinose) lacking histidine, tryptophan, and lysine until the cells reach the concentration of 7 × 106 cells/ml. The cells were then arrested at M phase using 10 μg/ml nocodazole at 30 °C until ∼90% of cells with the morphology of large buds. To induce HO endonuclease expression, the nocodazole-treated cells were centrifuged and resuspended in prewarmed medium containing 3% galactose and 10 μg/ml nocodazole. HO cutting efficiency was determined by Southern blotting analysis using probe near the HO site at different time points (see Fig. 7). Chromatin immunoprecipitation experiments were performed as described (33), except that the TAP-tagged Ku80 was precipitated directly by IgG-Sepharose (Amersham Biosciences), and anti-Myc antibody followed by protein G-Sepharose (Sigma) was used for the Cdc13p immunoprecipitations. Quantification of the immunoprecipitated DNAs was achieved using real time PCR on a StepOne real time system (Applied Biosystems). Enrichment of DNA from the sequences inserted near the HO cut site (XIP) over an internal control DNA (XIPi) located about 47 kb from the left arm of chromosome VII was determined after normalization. Primer pairs used in amplifying XIP and XIPi were as described (34). Values determined from immunoprecipitates were then normalized against values obtained from total cell extracts (input).
FIGURE 7.
Loading of Yku to telomeres is earlier than that of Cdc13p. A, schematic representation of the de novo telomere addition assay (32). The 81 bp of TG1–3 sequence (zigzag line) and the recognition site for the HO endonuclease was placed near the telomere of chromosome VII-L. HO is induced by the addition of galactose to the media. The cells then add TG1–3 to the TG1–3/HO end to repair the end. The probe used to monitor the TG1–3/HO end is shown as a thick bar. Yeast cells (YJL0801) were treated with nocodazole, transferred into medium containing galactose to induce HO expression, and then samples were taken at the time indicated. B, Southern blot analysis of SpeI cut genomic DNA is shown. The band labeled at ∼3 kbp is the SpeI fragment from the construct on chromosome VII-L. After cleavage with HO, this fragment is converted into a new band with the size of ∼0.7 kbp. The 1.6-kbp SpeI fragment represents the endogenous ade2-101 locus is also indicated. This band serves as a DNA loading control. Bracket indicates the newly synthesized telomeres from the 0.7-kbp fragment. C, immunoblotting analysis of the total yeast extracts and immunoprecipitates (IP) using antibodies against TAP or Cdc13p. WCE, whole cell extract. D, real time PCR analysis of the immunoprecipitated DNA. The results from real time PCR experiments are expressed as the relative value of immunoprecipitates/input using time = 0 as 0% and time = 240 min as 100%. The values presented are the average of 3–4 independent experiments.
RESULTS
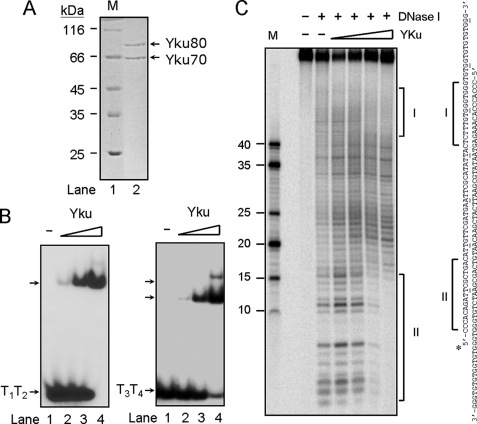
Yku Bound to Duplex and Tailed-duplex TG1–3 DNA in Vitro— Human Ku binds to double-stranded ends in a sequence-independent manner. It was shown that the exact structure of the DNA ends does not appear to be crucial for its binding. Human Ku binds to DNA ends with 5′- or 3′-overhangs, or with hairpin loops (5, 35), and it was also shown to bind to tailed-duplex DNA structure formed by telomeric DNA (15). Thus, it is interesting to test if Yku also bind to telomeric DNA in vitro. Using TAP-tagged Yku80, Yku was purified to near-homogeneity after two rounds of affinity purifications (Fig. 1A). The DNA binding activity of the purified Yku was accessed by electrophoretic mobility shift assays (EMSA). Similar to the property of mammalian Ku, the purified Yku bound to double-stranded DNA formed by two oligonucleotides with random sequences, did not bind to single-stranded DNA with the same sequences, and required a DNA end for binding (data not shown). To determine whether Yku can bind to duplex telomeric DNA, two complementary oligonucleotides with telomeric DNA sequences were synthesized and annealed (T1T2). The resulting duplex DNA has 9 bp of telomeric DNA on both ends. Yku appeared to bind efficiently to T1T2 telomeric DNA duplex (Fig. 1B). We next designed a tailed-duplex DNA substrate by annealing T3 and T4 oligonucleotides. The resulting DNA mimics the structure of telomere that has a 9-bp telomeric DNA duplex and a 15 mer-single-stranded G-tail on both ends. As shown in Fig. 1B, Yku formed complexes to the tailed-duplex DNA, and it also appeared that two complexes were formed with the DNA substrate indicating that Yku might bind to both ends of the DNA substrate. To further investigate the binding of Yku on tailed-duplex telomeric DNA, DNase I footprint experiments were conducted. The length of the duplex DNA substrate was extended to 65 bases (RT1RT2) to increase the resolution of the experiment. Results shown in Fig. 1C showed the autoradiograms of DNase I footprint analysis and the summary of the footprint results. Our results clearly indicated that Yku protein made two distinct footprints on the tailed-duplex DNA. Analysis of the locations of these two DNase I-protected regions indicated that Yku bound to both ends of the duplex DNA with protected regions of 13 and 15 bp on both ends (Fig. 1C). Because DNase I preferentially digests duplex over single-stranded DNA, the binding of Yku on single-stranded telomeric DNA cannot be determined here. Nevertheless, our results clearly indicated that Yku bound to the duplex and single-stranded junctions of the tailed duplex telomeric DNA.
FIGURE 1.
Yku binds to duplex and tail-duplex DNA substrates formed by telomeric DNA. A, purification of Ku from yeast. A TAP-tagged Yku80 was employed to purify Yku from yeast BJ2168. A Coomassie Blue-stained 10% SDS-polyacrylamide gel is given. Lane 1 (M) shows the molecular weight marker; lane 2 shows 1 μg of purified Yku. B, Yku binds to tailed-duplex DNA formed by telomeric DNA. ∼2 ng of 32P-radiolabeled DNA formed by T1T2 (left panel) or T3T4 (right panel) was incubated with 50 nm (lanes 2), 150 nm (lanes 3), or 450 nm (lanes 4) of purified Yku at room temperature for 10 min and then analyzed by a 6% polyacrylamide gel. An autoradiogram is shown here. C, DNase I footprints of Yku to tailed-duplex DNA. The footprint was done using a tailed-duplex DNA substrate formed by RT1 and RT2. Five nm each of the DNA substrates were used in the reactions. The Yku concentration was 7, 66, 133, and 200 nm, respectively. The autoradiograms of DNase I footprints (left) and the DNA sequences of tailed-duplex DNA (right) are shown. The regions protected from DNase I digestion are bracketed.
Using EMSA as an assay, we next determined the binding affinity of Yku to duplex DNA or tailed-duplex DNA (Table 2). For a better comparison, we have designed a set of DNA substrates with the same length (Table 1). Under our assay conditions, Yku bound to telomeric DNA duplex with an affinity similar to that of random-sequence DNA duplex (Table 2, compare T11T12 with R5R6). The addition of a 15-mer single-stranded tail with random sequence decreased slightly the binding affinity (compare R7R8 with R5R6). Interestingly, the single-stranded telomeric tail reduced the binding affinity by ∼3-fold (compare T13T14 with R5R6). Thus, our results indicated that Yku is capable of binding to tailed-duplex telomeric DNA, although with an affinity moderately lower than that of other double-stranded ends.
TABLE 2.
Binding affinity of Yku to DNA substrates
The apparent binding constant for each substrate was determined from an average of three experiments.
| DNA substrate | Description | Length (duplex + single strand tail) | Kd,app |
|---|---|---|---|
| nm | |||
| R5R6 | Duplex | 18 + 0 | 53 ± 13 |
| T11T12 | Duplex | 18 + 0 | 88 ± 28 |
| R7R8 | Tailed-duplex | 18 + 15 | 77 ± 30 |
| T13T14 | Tailed-duplex | 18 + 15 | 186 ± 43 |
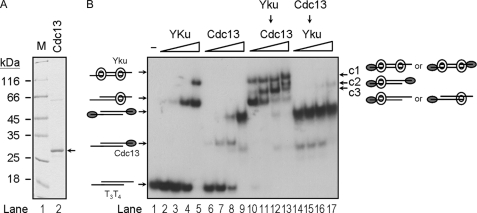
Sequential Formation of Ternary Complex on Telomeres by Yku and Cdc13p—Except for late S phase, a single-stranded tail with 12–14-mer TG1–3 sequence was detected in most parts of the cell cycle (25). We were next interested in testing whether such a single-stranded tail is sufficient for binding by both Yku and Cdc13p. The T3T4 tailed-duplex DNA was used as the substrate in our studies. We have also expressed a His6-tagged Cdc13(451–693)p in E. coli and purified this tagged protein using Ni-NTA-agarose (Fig. 2A). The His6-tagged Cdc13(451–693)p contains the DNA binding domain and has been shown previously that it has the single-stranded telomeric DNA binding activity similar to that of full-length Cdc13p (31). The result shown in Fig. 2B demonstrated that this His6-tagged Cdc13(451–693)p is capable of binding to the tailed-duplex DNA substrate (lanes 6–9). Interestingly, after initial loading of Yku, the substrate could then be bound by Cdc13(451–693)p to form several ternary complexes (Fig. 2B, lanes 11–13). Judging by the relative migrations and supershift analyses, the c1 complex may have two molecules of Yku and one molecule of Cdc13 on one DNA, whereas c2 and c3 each has one molecule of Yku and one or two molecules of Cdc13p, respectively. Thus, our DNA substrate that mimics the structure of telomere enables the formation of ternary complex by both Yku and Cdc13p. Moreover, because pre-loading of Cdc13p appeared to inhibit the binding of Yku to DNA, the result also indicated that the formation of these ternary complexes was sequential (Fig. 2B, lanes 14–17).
FIGURE 2.
Sequential formation of a ternary complex on tailed-duplex DNA by Yku and Cdc13(451–693)p. A, purification of His6-tagged Cdc13(451–693)p. The DNA binding domain of Cdc13p, Cdc13(451–693)p, was purified from E. coli. Two μg of the purified protein were analyzed by 12% SDS-PAGE and stained with Coomassie Blue. B, binding of tailed-duplex DNA by Yku and Cdc13p. Around 2 ng of duplex DNA formed by T3 and T4 were incubated with Yku or Cdc13(451–693)p and then analyzed by a 6% polyacrylamide gel. Lane 1 does not have any protein. Lanes 2–5 contain 17, 50, 150, and 450 nm Yku, respectively. Lanes 6–9 contain 1.1, 3.3, 10, and 33 nm Cdc13(451–693)p, respectively. In lanes 10–13, the DNA substrate was incubated with 450 nm of Yku at room temperature for 10 min, followed by the addition of 1.1, 3.3, 10, and 33 nm Cdc13(451–693)p, respectively. In lanes 14–17, the DNA substrate was incubated with 33 nm of Cdc13(451–693)p at room temperature for 10 min, followed by the addition of 17, 50, 150, and 450 nm Yku, respectively. The positions and graphic representations of the Yku-Cdc13(451–693)p-DNA complexes are indicated.
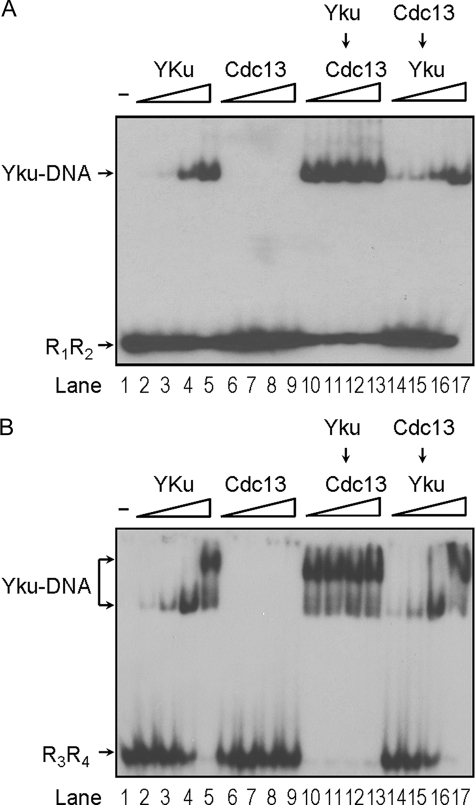
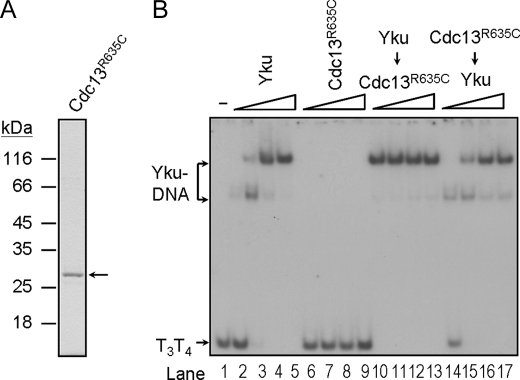
To further demonstrate that specific binding of Cdc13p to single-stranded telomeric tail is required for the formation of ternary complex, similar experiments were conducted using DNA substrates that were either without the single-stranded tail (R1R2) or with a random-sequence single-stranded tail (R3R4). Results shown in Fig. 3 clearly indicated that a single-stranded telomeric tail is required for Cdc13p to bind and to form ternary complexes with Yku. We have previously isolated several cdc13 mutants that are defective in binding to telomeric DNA (30). One of the mutants with an alternation from Arg-635 to Cys (R635C) failed to interact with single-stranded telomeric DNA. The Arg-635 was shown to be located at the DNA-binding surface of Cdc13p (36). We next analyzed if the telomere binding activity of Cdc13p is required for ternary complex formation. Using the procedures similar to that in purifying wild-type protein, we have purified Cdc13(451–693)p with the R635C mutation to homogeneity (Fig. 4A). Formation of the ternary complex by Cdc13R635C mutant was then tested on T3T4 tailed-duplex DNA. The purified Cdc13R635C mutant failed to bind to the DNA substrate (Fig. 4B, lanes 5–8). It also failed to form a ternary complex with Yku and did not affect the binding of Yku to DNA. Thus, our results indicated that Cdc13p binds to the single-stranded telomeric DNA portion of the tailed-duplex DNA, and its binding activity is required for the formation of ternary complex with Yku.
FIGURE 3.
A single-stranded telomeric tail is required for ternary complex formation. Around 2 ng of 32P-labeled DNA substrates that do not have a single-stranded tail (A) or have a single-stranded tail with random sequences (B) were incubated with Yku or Cdc13(451–693)p and analyzed by EMSA. The amounts of Yku and Cdc13(451–693)p used were the same as those for Fig. 2B. Autoradiograms are shown here.
FIGURE 4.
Telomeric DNA binding activity of Cdc13(451–693)p is required for ternary complex formation. A, purification of His6-tagged Cdc13(451–693)R635Cp. Two μg of the purified protein were analyzed by 10% SDS-PAGE and stained with Coomassie Blue. B, Cdc13(451–693)R635Cp fails to form complex with Yku-DNA. Around 2 ng of 32P-labeled T3T4 DNA substrate were incubated with Yku and/or Cdc13(451–693) R635Cp and analyzed by EMSA. The amounts of Yku used in lanes 2–5 and 14–17 were 50, 150, 450, and 1350 nm, respectively. Lanes 10–13 each has 1350 nm Yku. The amounts of Cdc13(451–693)R635Cp were the same as those for Fig. 2B. An autoradiogram is shown here.
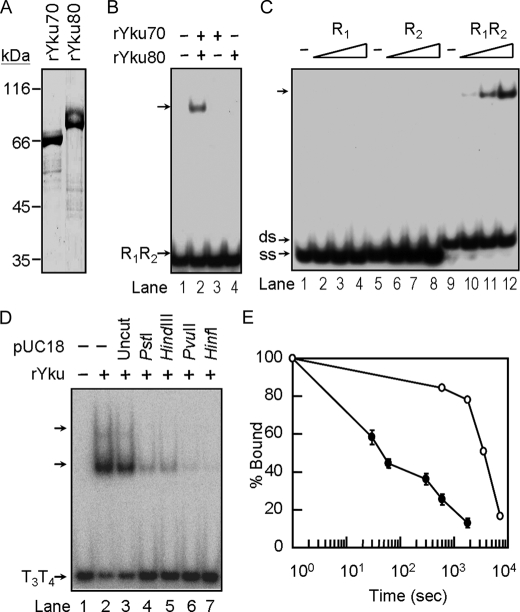
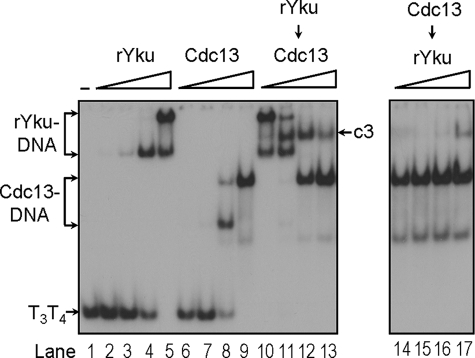
Stably Formed Yku Ring Is Required for Formation of Yku-Cdc13p-DNA Ternary Complex—Because human Ku heterodimer forms a ring structure to encircle DNA and it binds DNA through the open ends (7), it is reasonable to postulate that Yku interacts with telomeres in the form of a ring structure. Thus, a simple interpretation on the inhibition of Yku binding by pre-loaded Cdc13p would be Cdc13p blocking the passage of Yku ring to its target site. However, it is still possible that the pre-loaded Cdc13p occupies the DNA-binding site of Yku to prevent the binding of Yku. To rule out this possibility, we prepared recombinant Yku70 and Yku80 (rYku) separately from insect Sf21 cells using baculovirus expression systems (Fig. 5A). Although neither of these two recombinant proteins bound to blunt-end duplex DNA, the Yku activity could be readily reconstituted by mixing these two proteins (Fig. 5B). The reconstituted Yku did not bind to single-stranded DNA (Fig. 5C). The binding activity of the reconstituted Yku to the DNA ends was further confirmed by competition analysis using close circle and restriction enzyme-digested plasmid DNA. The rYku-DNA complex cannot be competed away by close circular plasmid, whereas it was readily competed away by DNAs that were digested with restriction enzymes to expose DNA ends (Fig. 5D). The degree of competition did not appear to be dependent on the type of free DNA ends generated as 3′-overhang generated by PstI competed as well as 5′-overhang generated by HindIII or blunt ends generated by PvuII. Instead, competition was increased as the number of free DNA ends increased (Fig. 5D, lane 7). Thus, the binding activity of reconstituted Yku to telomere tail-duplex DNA was specific for DNA ends, and its binding activity is similar to that of endogenous Yku protein. However, in contrast to the Yku isolated from yeast that has a half-life on DNA for >60 min, the reconstituted protein did not stably bind to DNA. It had an estimated half-life on DNA for ∼1 min (Fig. 5E). The reconstituted Yku heterodimer did not appear to form a stable ring-like structure on DNA. Although the mechanism of how reconstituted Yku is less stable on DNA is not clear to us, it is possible that the ring-like structure was not stably formed during reconstitution, or additional modification is required in yeast to enable the formation of stable Yku heterodimer. Nevertheless, this property enabled us to test if the binding site of Yku is occupied by pre-loaded Cdc13p. We reasoned that if we preloaded Yku to prevent Cdc13p from occupying the Yku-binding site, when the reconstituted Yku falls off from DNA, the ability of Yku to rebind to Cdc13p-loaded DNA could then be used as a criterion to determine whether Yku binds DNA in a pre-formed ring. A pre-formed Yku ring should not rebind to DNA in the presence of pre-loaded Cdc13p. Using the reconstituted Yku, we conducted similar experiments described in Fig. 2B. The reconstituted Yku readily bound to the tailed-duplex DNA with an affinity similar to that of Yku isolated from yeast (Fig. 6, lanes 2–5). However, when Cdc13p was added to the rYku-DNA complexes, the binding patterns were shifted to rYku-Cdc13p-DNA complex and finally to Cdc13p-DNA complex (Fig. 6, lanes 10–13). Because the pre-loaded Cdc13p inhibited the binding of reconstituted rYku (Fig. 6, lanes 14–17), our results indicated that the reconstituted rYku did not re-bind to DNA when it fell off the DNA. Thus, the binding of Yku to tailed-duplex telomere DNA is in a pre-formed ring structure.
FIGURE 5.
Stably formed Yku is required for ternary complex formation. A, purification of Yku70 and Yku80 from insect cells. Yku70 and Yku80 with His6 tag were purified from Sf21 using a Ni-NTA-agarose column, respectively (see “Experimental Procedures”). Five μg each of purified Yku70 and Yku80 were analyzed on a 10% SDS-polyacrylamide gel and stained with Coomassie Blue, respectively. B, Yku heterodimer is essential for DNA binding activity. Yku70 and/or Yku80 of 100 nm was incubated with 2 ng of radiolabeled R1R2 DNA duplex and analyzed by EMSA. An autoradiogram is presented. C, reconstituted Yku binds to duplex DNA. 10, 33, or 100 nm of reconstituted Yku was incubated with 2 ng of radiolabeled oligonucleotide R1, R2, or R1R2 duplex DNA and then analyzed by EMSA. An autoradiogram is presented. D, Yu binds to the ends of the tailed-duplex DNA. Around 2 ng each of the tailed-duplex DNA formed by T3 and T4 were first mixed without (lanes 1 and 2) or with 200 ng of pUC18 plasmid DNA that was undigested (lanes 3) or digested by PstI (1 cut, lanes 4), HindIII (1 cut, lane 5), PvuII (2 cuts, lane 6), or HinfI (6 cuts, lanes 7). The DNA mixtures were then incubated with 150 nm of Yku and then analyzed by electrophoresis using a 6% polyacrylamide gel. An autoradiogram is shown here. E, DNA binding stability of Yku. Yku of 100 nm isolated from yeast (open circle) or reconstituted Yku (close circle) were incubated with 0.2 ng of radiolabeled R1R2 at room temperature for 10 min. Twenty ng of unlabeled R1R2 were added to the reaction mixtures. At the time indicated, an aliquot of the reaction mixtures was withdrawn and analyzed by EMSA. The amounts of DNA remained bound by Yku were quantified by a PhosphorImager. The value obtained at time = 0 was taken as 100%. The data show the average of three experiments.
FIGURE 6.
Stably bound Yku is required to form ternary complex. Around 2 ng of 32P-labeled T3T4 DNA substrate were incubated with reconstituted Yku and/or Cdc13p and analyzed by EMSA. The amounts of rYku used in lanes 2–5 were 50, 150, 450, and 1350 nm, respectively. Lanes 10–13 each has 1350 nm Yku. The amounts of Cdc13p were the same as those for Fig. 2B. An autoradiogram is shown here. The position of the Yku-Cdc13p-DNA complex is marked c3.
Loading of Yku to de Novo Synthesized Telomeres Is Earlier than Cdc13p—To confirm the observation of sequential loading of Yku and Cdc13p to telomeres in vitro, we have adopted a de novo telomere addition system into our analysis in vivo (32). Here we tagged Yku80 with TAP and Cdc13p with the Myc epitope in yeast cells that carrying the 81 bp of TG1–3 sequence and the recognition site for the HO endonuclease near the telomere of chromosome VII-L (YJL0801, Fig. 7A). Upon cleavage by HO endonuclease, the TG1–3 sequences are added to the TG1–3/HO end to repair the end that could be detected by Southern blotting analysis using the probe near the TG1–3/HO end. Yeast cells (YJL0801) were treated with nocodazole, transferred into medium containing galactose to induce HO endonuclease expression, and then analyzed. As shown in Fig. 7B, Southern blotting analysis of SpeI-digested genomic DNA indicated that the ∼3-kbp SpeI fragment from the construct on chromosome VII-L was converted into a new band with the size of ∼0.7 kbp. New TG1–3 sequences were apparent at ∼240 min after HO cutting. Parallel experiments were conducted to precipitate Yku and Cdc13p, respectively (Fig. 7C). The precipitates were then analyzed for the DNA near the TG1–3/HO end and DNA ∼47 kbp away from the left end of chromosome VII. Taking the level of Yku80 loading to telomeres at 240 min after HO endonuclease induction as 100%, our results indicated that loading of Yku to telomeres was apparent at ∼45–60 min, whereas for Cdc13p it was 60–120 min (Fig. 7D). We estimated that the time required for half-loading of Yku to telomeres was ∼100 min. As a comparison, the time required for loading Cdc13p was ∼150 min. Thus, our results clearly showed that loading of Yku to newly formed telomeres is earlier than that of Cdc13p in vivo.
DISCUSSION
A main question in the telomere field is how telomeres escape from being recognized as DNA damages. Our results provide a simple answer to this question. It is well documented that the unique DNA structure of telomeres provides a platform for the formation of a specialized protein-DNA complex to protect telomeres (37). Direct binding of telomeric DNA by telomere-binding proteins then recruits other telomere-associated factors to telomeres. Our in vitro data showed that Cdc13p did not compete for the same binding sites of Yku, and a ternary complex was formed upon binding of these two proteins to a tailed-duplex telomeric DNA. Moreover, sequential binding of Yku protein and Cdc13p to telomeres was also demonstrated both in vitro and in vivo, and loading of Cdc13p prevents further binding of Ku to telomeres. The sequential loading of Yku protein and Cdc13p specified a spatial orientation of these two proteins that might contribute to their capping function on telomeres. Through masking the activity of Yku protein by proximal binding of Cdc13p, the subsequent activation of DSB repair enzymes to telomere ends might be prevented. This model, although simple, explains the capping activity of Cdc13p and Yku well. However, it does not fully explain several functions of Yku proteins on telomeres. For example, the involvement of Yku protein in processing single-stranded telomeric DNA cannot be explained by this simple model (9).
Here we have adopted a de novo telomere addition system to determine the sequential loading of Yku protein and Cdc13p onto telomeres. In this system, the binding of telomere proteins is initiated on telomeric DNA sequences near a double-stranded break (32). The Yku protein first recognizes the double-stranded break and recruits other proteins to process the break, probably mediated by the exonuclease activity of Mre11p-Rad50p-Xrs2p (MRX) complex (34). A single-stranded telomeric tail is then generated that enables the binding by Cdc13p. The sequential binding of Yku and Cdc13p onto the de novo synthesized telomeres could reflect, at least partially, on normal telomere replication. During telomere replication, the leading strand synthesis generates a blunted end similar to double strand break. Because the DNA substrate formed after leading strand telomere synthesis favors Yku binding, the loading of Yku and Cdc13p should be similar to that in de novo telomere formation. However, the lagging strand telomere synthesis generates a 3′-tail after removal of the RNA primer. Thus, formation of telomeric protein-DNA complex after lagging strand telomere synthesis might present a different scenario. Judged by the high binding affinity of Cdc13p to single-stranded telomeric DNA (31, 38) and that pre-loading of Cdc13p to telomeres inhibits further binding of Yku protein, it is possible that Yku protein might not bind to lagging strand synthesized telomeres. Alternatively, because Yku protein is one of the most abundant proteins in the nucleus, the number of Yku molecules might compensate for its affinity to interact with telomeres. It is less certain whether Yku protein and Cdc13p also load onto lagging strand synthesized telomeres in a sequential manner. Nevertheless, our results provide a strong indication for the sequential formation of telomeric DNA-protein complex by Yku protein and Cdc13p in normal telomere replication.
Yku protein appears to have diverse roles on telomeres. However, the question of how it renders its function on telomeres is less clear. Here we show that Yku heterodimer is capable of binding directly to DNA substrates that mimic the structure of telomeres. Thus, in addition to two previous characterized modes of interactions involving Sir4p (21, 39) and telomerase Tlc1 RNA (22, 40) to recruit Yhu to telomeres, our result adds a new interaction mode for Yku protein to bind to telomeres. These three different modes of interactions might cooperate to tether Yku protein to telomeres. Failures in any of these interactions might not dramatically affect the localization of Yku protein on telomeres. For example, a yku80 mutant that failed to interact with Sir4p still interacted with telomeres (39). Moreover, it was shown by chromatin immunoprecipitation experiments that Yku80 still interacted with telomeres in a sir4 mutant or a tlc1Δ48 mutant that failed to interact with Yku (23, 41). Because the DN-binding Sir4p interaction and Tlc1 RNA binding were mapped to separate regions of the Yku80 protein (22, 39), it is tempting to speculate that these three different modes of interactions might affect different function of Yku on telomeres and that these interactions might occur simultaneously on the same Yku molecule. However, because direct binding of Yku has an off rate of >60 min, efficient redistribution of Yku from telomeres to double-stranded breaks upon DNA damages might not be easily achieved by the telomeric DNA-bound Yku protein (23). Stable binding of Yku protein to DNA does not appear to favor its dynamic function on telomeres. Here we favor the model of specific interactions mediated by different subsets of Yku molecules. The Yku proteins on telomeres could be divided into at least three functional subsets according to their specific interactions with telomeres. In addition to its direct binding of telomeres, a different subset of Yku molecules interact with Sir4p or Tlc1 to mediate its dynamic regulation of telomere functions. Several lines of evidence also support this model. It was shown that only a portion of telomere-associated Yku was relocalized upon DNA damages (23). Moreover, analysis of a yku80 mutant that failed to interact with Sir4p indicated that although the mutant protein still co-immunoprecipitated with telomeric DNA, it failed to co-immunoprecipitate the Y′-subtelomeric DNA (39). Thus, selective binding of Yku protein to different partners on telomeres might contribute to its multiple functions on telomeres.
Acknowledgments
We thank Drs. J.-Q. Zhou and C.-L. Hsu for reading the manuscript and helpful suggestions. We are grateful to Dr. D. E. Gottschling for providing Yku reagents and yeast strain for de novo telomere addition experiments.
This work was supported by National Science Council, Taiwan, Grants 97-2311-B-010-005-MY3 and 97-3112-B-010-013 and National Health Research Institute Grant NHRI-EX97-9625SI.
Footnotes
The abbreviations used are: DSB, double-stranded break; EMSA, electrophoretic mobility shift assay; Ni-NTA, nickel-nitrilotriacetic acid; r, recombinant.
References
- 1.O'Driscoll, M., and Jeggo, P. A. (2006) Nat. Rev. Genet. 7 45–54 [DOI] [PubMed] [Google Scholar]
- 2.Lieber, M. R., Ma, Y., Pannicke, U., and Schwarz, K. (2003) Nat. Rev. Mol. Cell Biol. 4 712–720 [DOI] [PubMed] [Google Scholar]
- 3.Mimori, T., and Hardin, J. A. (1986) J. Biol. Chem. 261 10375–10379 [PubMed] [Google Scholar]
- 4.Paillard, S., and Strauss, F. (1991) Nucleic Acids Res. 19 5619–5624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzon, M., Fewell, J. W., and Kuff, E. L. (1993) J. Biol. Chem. 268 10546–10552 [PubMed] [Google Scholar]
- 6.Ono, M., Tucker, P. W., and Capra, J. D. (1994) Nucleic Acids Res. 22 3918–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker, J. R., Corpina, R. A., and Goldberg, J. (2001) Nature 412 607–614 [DOI] [PubMed] [Google Scholar]
- 8.Hsu, H.-L., Gilley, D., Blackburn, E. H., and Chen, D. J. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 12454–12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravel, S., Larrivee, M., Labrecque, P., and Wellinger, R. J. (1998) Science 280 741–744 [DOI] [PubMed] [Google Scholar]
- 10.d'Adda di Fagagna, F., Hande, M. P., Tong, W. M., Roth, D., Lansdorp, P. M., Wang, Z. Q., and Jackson, S. P. (2001) Curr. Biol. 11 1192–1196 [DOI] [PubMed] [Google Scholar]
- 11.Blackburn, E. H., Greider, C. W., and Szostak, J. W. (2006) Nat. Med. 12 1133–1138 [DOI] [PubMed] [Google Scholar]
- 12.Blackburn, E. H. (1990) J. Biol. Chem. 265 5919–5921 [PubMed] [Google Scholar]
- 13.Boulton, S. J., and Jackson, S. P. (1996) Nucleic Acids Res. 24 4639–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter, S. E., Greenwell, P. W., Ritchie, K. B., and Petes, T. D. (1996) Nucleic Acids Res. 24 582–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi, A., and de Lange, T. (1999) J. Biol. Chem. 274 21223–21227 [DOI] [PubMed] [Google Scholar]
- 16.Nugent, C. I., Bosco, G., Ross, L. O., Evans, S. K., Salinger, A. P., Moore, J. K., Haber, J. E., and Lundblad, V. (1998) Curr. Biol. 8 657–660 [DOI] [PubMed] [Google Scholar]
- 17.Laroche, T., Martin, S. G., Gotta, M., Gorham, H. C., Pryde, F. E., Louis, E. J., and Gasser, S. M. (1998) Curr. Biol. 8 653–656 [DOI] [PubMed] [Google Scholar]
- 18.Boulton, S. J., and Jackson, S. P. (1998) EMBO J. 17 1819–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra, K., and Shore, D. (1999) Curr. Biol. 9 1123–1126 [DOI] [PubMed] [Google Scholar]
- 20.Cosgrove, A. J., Nieduszynski, C. A., and Donaldson, A. D. (2002) Genes Dev. 16 2485–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukamoto, Y., Kato, J., and Ikeda, H. (1997) Nature 388 900–903 [DOI] [PubMed] [Google Scholar]
- 22.Stellwagen, A. E., Haimberger, Z. W., Veatch, J. R., and Gottschling, D. E. (2003) Genes Dev. 17 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, S. G., Laroche, T., Suka, N., Grunstein, M., and Gasser, S. M. (1999) Cell 97 621–633 [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi, T., Sadaie, M., Kanoh, J., and Ishikawa, F. (2003) J. Biol. Chem. 278 1924–1931 [DOI] [PubMed] [Google Scholar]
- 25.Larrivee, M., LeBel, C., and Wellinger, R. J. (2004) Genes Dev. 18 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, J.-J., and Zakian, V. A. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 13760–13765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nugent, C. I., Hughes, T. R., Lue, N. F., and Lundblad, V. (1996) Science 274 249–252 [DOI] [PubMed] [Google Scholar]
- 28.Bourns, B. D., Alexander, M. K., Smith, A. M., and Zakian, V. A. (1998) Mol. Cell. Biol. 18 5600–5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang, T.-L., Wang, C. Y., Hsu, C.-L., Chen, M. Y., and Lin, J.-J. (2003) J. Biol. Chem. 278 9318–9321 [DOI] [PubMed] [Google Scholar]
- 30.Lin, Y.-C., Lee, Y.-H. W., and Lin, J.-J. (2007) Biochem. J. 403 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, Y.-C., Hsu, C.-L., Shih, J.-W., and Lin, J.-J. (2001) J. Biol. Chem. 276 24588–24593 [DOI] [PubMed] [Google Scholar]
- 32.Diede, S. J., and Gottschling, D. E. (1999) Cell 99 723–733 [DOI] [PubMed] [Google Scholar]
- 33.Taggart, A. K. P., Teng, S.-C., and Zakian, V. A. (2002) Science 297 1023–1026 [DOI] [PubMed] [Google Scholar]
- 34.Diede, S. J., and Gottschling, D. E. (2001) Curr. Biol. 11 1336–1340 [DOI] [PubMed] [Google Scholar]
- 35.Rathmell, W. K., and Chu, G. (1994) Mol. Cell. Biol. 14 4741–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitton-Fry, R. M., Anderson, E. M., Hughes, T. R., Lundblad, V., and Wuttke, D. S. (2002) Science 296 145–147 [DOI] [PubMed] [Google Scholar]
- 37.Ferreira, M. G., Miller, K. M., and Cooper, J. P. (2004) Mol. Cell 13 7–16 [DOI] [PubMed] [Google Scholar]
- 38.Anderson, E. M., Halsey, W. A., and Wuttke, D. S. (2002) Nucleic Acids Res. 30 4305–4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy, R., Meier, B., McAinsh, A. D., Feldmann, H. M., and Jackson, S. P. (2004) J. Biol. Chem. 279 86–94 [DOI] [PubMed] [Google Scholar]
- 40.Peterson, S. E., Stellwagen, A. E., Diede, S. J., Singer, M. S., Haimberger, Z. W., Johnson, C. O., Tzoneva, M., and Gottschling, D. E. (2001) Nat. Genet. 27 64–67 [DOI] [PubMed] [Google Scholar]
- 41.Fisher, T., Taggart, A. K. P., and Zakian, V. A. (2004) Nat. Struct. Mol. Biol. 11 1198–1205 [DOI] [PubMed] [Google Scholar]