Abstract
Angiopoietin-like protein 4 (ANGPTL4) is a secreted protein that modulates the disposition of circulating triglycerides (TG) by inhibiting lipoprotein lipase (LPL). Here we examine the steps involved in the synthesis and post-translational processing of ANGPTL4, and the effects of a naturally occurring sequence variant (E40K) that is associated with lower plasma TG levels in humans. Expression of the wild-type and mutant proteins in HEK-293A cells indicated that ANGPTL4 formed dimers and tetramers in cells prior to secretion and cleavage of the protein. After cleavage at a canonical proprotein convertase cleavage site (161RRKR164), the oligomeric structure of the N-terminal domain was retained whereas the C-terminal fibrinogen-like domain dissociated into monomers. Inhibition of cleavage did not interfere with oligomerization of ANGPTL4 or with its ability to inhibit LPL, whereas mutations that prevented oligomerization severely compromised the capacity of the protein to inhibit LPL. ANGPTL4 containing the E40K substitution was synthesized and processed normally, but no monomers or oligomers of the N-terminal fragments accumulated in the medium; medium from these cells failed to inhibit LPL activity. Parallel experiments performed in mice recapitulated these results. Our findings indicate that oligomerization, but not cleavage, of ANGPTL4 is required for LPL inhibition, and that the E40K substitution destabilizes the protein after secretion, preventing the extracellular accumulation of oligomers and abolishing the ability of the protein to inhibit LPL activity.
Angiopoietin-like protein 4 (ANGPTL4)4 is a 50-kDa protein that is synthesized and secreted from several metabolically active tissues and has been implicated in the trafficking of circulating TG (1, 2). Triglycerides, either acquired from the diet or synthesized endogenously, circulate in blood as constituents of chylomicrons and very low density lipoproteins (VLDL). As these lipoproteins circulate in tissues they encounter lipoprotein lipase (LPL) at the vascular endothelial surfaces. LPL hydrolyzes the TG, producing free fatty acids that are taken up by the surrounding tissues. ANGPTL4 inhibits the activity of LPL, thereby limiting the uptake of TG-derived fatty acids by the underlying cells (3, 4). Overexpression of ANGPTL4 in mice causes severe hypertriglyceridemia, whereas mice lacking ANGPTL4 have increased LPL activity and low plasma levels of TG (5, 6). In mice, ANGPTL4 is predominantly expressed in adipose tissue and is strongly induced by fasting (2). Accordingly it has been proposed that ANGPTL4 inhibits LPL activity in adipose tissue to reroute fatty acids away from fat to muscle and other tissues when food intake is low (3, 4).
ANGPTL4 belongs to a family of seven structurally similar secreted proteins (ANGPTL1-ANGPTL7) that contain a signal sequence followed by an α-helical region predicted to form a coiled-coil, and a globular fibrinogen-like domain at the C terminus (1). Gel filtration studies of recombinant ANGPTL4 indicate that the protein assembles into oligomers that are stabilized by disulfide bonds (7). Substitution of two highly conserved cysteine residues at positions 76 and 80 in the α-helical domain prevents oligomerization of ANGPTL4 and impairs the ability of the recombinant protein to increase plasma TG levels when overexpressed in the livers of rats (7).
Upon secretion into the circulation, ANGPTL4 is cleaved into an N-terminal domain and a C-terminal fibrinogen-like domain (8). The N-terminal peptide circulates as an oligomer, and the fibrinogen-like domain circulates as a monomer (8). The N-terminal helical region of ANGPTL4 is necessary and sufficient for inhibition of LPL (9). A peptide corresponding to amino acids 1-187 of the protein binds LPL with high affinity and converts the enzyme from catalytically active dimers to inactive monomers, thereby inhibiting LPL activity (10). After disrupting the LPL dimer, ANGPTL4 is released. The LPL monomers remain folded and stable but fail to re-form active dimers. These data suggest that the N-terminal domain of ANGPTL4 interacts directly but transiently with LPL, triggering a stable conformational switch in LPL that irreversibly inactivates the enzyme.
Recently, we used a population-based resequencing strategy to examine the metabolic role of ANGPTL4 in humans (11). Resequencing the coding region of ANGPTL4 in a large (n = 3,501), multiethnic sample revealed multiple rare sequence variations that alter an amino acid in the protein and are associated with low plasma TG levels. In addition, we identified a more common variant (E40K), that was present in ∼3% of European-Americans and was associated with significantly lower plasma levels of TG and low density lipoprotein-cholesterol (LDL-C), and higher levels of high density lipoprotein (HDL)-C in two large epidemiological studies (11). These association studies confirmed that ANGPTL4 is involved in TG metabolism in humans, and also revealed additional roles in humans in the metabolism of HDL and LDL, which were not apparent from studies in genetically modified mice.
Here we examined the synthesis, secretion, and processing of ANGPTL4 and determine the mechanism by which substitution of a basic (lysine) for an acidic (glutamate) residue at residue 40 affects the function of the protein.
EXPERIMENTAL PROCEDURES
Materials—Culture medium and fetal bovine serum were obtained from Meditech, Inc. (Herndon, VA). Protease inhibitor mixture tablets were purchased from Roche Applied Science. 9,10-[3H](N)Triolein was purchased from American Radiolabeled Chemicals (St. Louis, MO). Polyclonal antibodies were raised by immunizing rabbits using N-terminal (amino acids 26-154) and C-terminal (amino acids 170-406) fragments of human ANGPTL4. Rabbit anti-human ANGPTL4 polyclonal antibody was purchased from Biovendor (Modrice, Czech Republic). Mouse anti-V5 monoclonal antibody was purchased from Invitrogen (Carlsbad, CA). Rabbit anti-human calnexin polyclonal antibody was purchase from Stressgen (Ann Arbor, MI). All other chemicals and reagents were obtained from Sigma-Aldrich unless otherwise indicated.
Animal Treatment—Male C57BL/6J mice (The Jackson Laboratory) aged 8-10 weeks were used for this study. Mice were group-housed (four mice per cage) and fed standard rodent chow. All procedures were performed according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of University of Texas Southwestern Medical Center. Animals (n = 5 per group) were injected with recombinant adenovirus (1.25 × 1011 particles/mouse) in saline solution via the tail vein. After 72 h, animals were anesthetized, blood samples were drawn, and heparin (1 unit/g body weight) was injected via the jugular vein. A second blood sample was collected 10 min after heparin administration and the animals were sacrificed by cervical dislocation. Livers were removed and snap-frozen.
Expression of ANGPTL4 in Cultured Cells—The human ANGPTL4 cDNA with a V5 epitope tag (GKPIPNPLLGLDST) at the C terminus was inserted into pcDNA3.1 under the control of the cytomegalovirus promoter-enhancer (pCMV-ANGPTL4-V5). Sequence changes were introduced into the construct using the QuikChange kit (Stratagene) according to the manufacturer's protocol. The sequences of the oligonucleotides used for mutagenesis are available on request. The presence of the desired mutation and the fidelity of each construct were confirmed by DNA sequencing.
Transfections, Cell Lysates, and Liver Lysates—Human embryonic kidney (HEK)-293A cells were seeded (1 × 105 cells/well) in 6-well plates and grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum (Cellgro). Cells were transiently transfected with expression plasmids (4 μg/well) using Lipofectamine 2000 (2.5 μl of Lipofectamine 2000/μg of DNA) according to the manufacturer's protocol (Invitrogen). After 48 h, medium was collected and centrifuged for 5 min (5,000 × g at 4 °C) to remove cell debris. Cells were washed twice with cold phosphate-buffered saline (PBS) (pH 7.4) and then lysed in 0.3 ml of 1× radioimmune precipitation assay buffer (RIPA) (50 mm Tris, pH 8.0, 150 mm NaCl, 1% (v/v) Nonidet P-40, 0.5% (v/v) sodium deoxycholate, 0.1% SDS, and complete mini EDTA-free protease inhibitor mixture (Roche)) at 4 °C. Cells were harvested by scraping with a rubber policeman and transferred to 1.5-ml tubes prior to centrifugation for 15 min (15,000 × g at 4 °C). Aliquots from medium and cells were subjected to SDS-PAGE and immunoblot analysis. Mouse livers were homogenized with a IKA T10 Ultra-Turrax hand homogenizer (Wilmington, NC), in 1× RIPA buffer and centrifuged at 15,000 × g at 4 °C for 15 min. Supernatants were collected, and aliquots (50 μg) were subjected to SDS-PAGE and immunoblot analysis.
SDS-PAGE and Immunoblot Analysis—Protein concentrations in the cell lysates were determined using the Bio-Rad bicinchoninic acid assay, according to the manufacturer's protocol. Equivalent amounts of protein from the cell lysate and medium were added to sample loading buffer (final concentration of 1×) with, or without β-mercaptoethanol (β-ME). The samples were heated to 95 °C for 5 min, loaded onto 4-15% gradient (for non-reduced samples) or 15% (for reduced samples) SDS-polyacrylamide gels, size-fractionated at 125 V, and transferred to nitrocellulose membranes. Membranes were incubated in PBST buffer (1× PBS, pH 7.4, and 0.1% Tween 20) with 5% dry milk for 60 min at room temperature before addition of the primary antibodies. Primary antibodies were diluted in PBST buffer with 5% dry milk and incubated with membranes for 60 min. Membranes were washed three times for 10 min in PBST buffer. Horseradish peroxidase-conjugated donkey anti-rabbit IgG or goat anti-mouse IgG (Pierce) was diluted (1:10,000) in PBST buffer with 5% dry milk and incubated with membranes for 60 min. Membranes were washed three times for 10 min in PBST and visualized using SuperSignal-enhanced chemiluminescence (Pierce).
In Vitro Assay of Lipoprotein Lipase—LPL activity was measured using a modification of the procedure described by Nilsson-Ehle and Schotz (12). LPL activity in adenovirus-injected mouse plasma was determined by incubating 7.5 μl of post-heparin plasma with radiolabeled substrate composed of 9,10-[3H](N)triolein (American Radiolabeled Chemicals), triolein, and phosphatidylcholine (Sigma). To determine the effect of ANGPTL4 on LPL activity, conditioned medium harvested from HEK-293A cells transfected with ANGPTL4 expression constructs was concentrated 8-fold and buffer-exchanged into 1× PBS, pH 7.4, using Amicon concentrators. The medium was then preincubated with 7.5 μl of mouse post-heparin plasma at 20 °C for 30 min before addition of radiolabeled substrate. LPL assays were performed at 37 °C and terminated after 15 min by adding heptane-chloroform-methanol (1:1.25:1.41) and then centrifuging at 3,000 × g for 15 min. A 1-ml aliquot of upper (aqueous) phase were taken into scintillation tubes and counted. The amount of 3H-fatty acid released was calculated as previously described (12, 13).
Sequence Analysis—The PSI-BLAST program (14) was used to search for homologs of human ANGPTL4 (gi 21536398) against the NCBI non-redundant data base (October 26 2008; 7,124,886 sequences; 2,457,960,432 total letters), with an inclusion E-value cutoff of 0.001. Homologs that were identified were clustered, and representative sequences containing the N-terminal domain similar to ANGPTL4 were multiple-aligned using the PROMALS3D program (15).
RESULTS
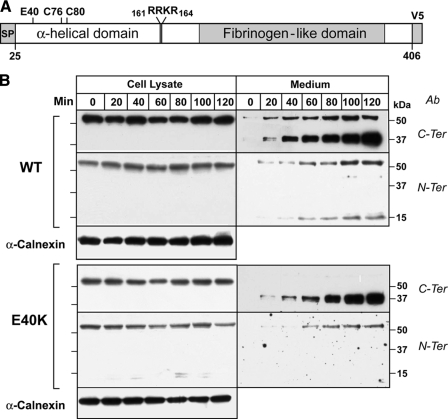
Expression and Secretion of Recombinant ANGPTL4-E40K in HEK-293A Cells—To determine the effects of the E40K substitution on expression and secretion of ANGPTL4, we performed a time course experiment in HEK-293A cells that were transfected with constructs expressing wild-type and mutant forms of the protein. Immunoblot analyses were performed using antibodies specific for the C-terminal and N-terminal fragments (Fig. 1). Wild-type ANGPTL4 was expressed as a ∼50-kDa protein, and a band of similar size was present in the medium (Fig. 1, top); this band was detected using both the C-terminal and N-terminal antibody and represents the full-length, uncleaved protein.
FIGURE 1.
Secretion time-course of ANGPTL4 wild-type (ANGPTL4-WT) and ANGPTL4-E40K mutant in vitro. A, schematic representation of ANGPTL4 showing the putative cleavage site and the cysteine residues involved in disulfide bond formation. B, HEK-293A cells were transiently transfected with ANGPTL4-WT or ANGPTL4-E40K mutant. After 48 h, the cells were washed with PBS twice and fresh Dulbecco's modified Eagle's medium was added. At the indicated time points, both cells and medium were harvested, the reduced and denatured proteins were size-fractionated on a 15% polyacrylamide gel, and immunoblot analysis was performed using anti-ANGPTL4 antibodies directed against the C terminus (C-ter) and the N terminus (N-ter). This experiment was repeated three times with similar results. Calnexin was used as loading control for the cell lyates.
ANGPTL4 contains a highly conserved tetrapeptide sequence (161RRKR164) that matches the preferred cleavage site [RX(K/R)R] for furin, a proprotein convertase that cleaves a broad range of proteins in the secretory pathway and on the cell surface (16). Cleavage of the protein between arginine residues 161 and 162 would be expected to generate a C-terminal fragment of 37 kDa and an N-terminal fragment of 15 kDa. Bands of the expected sizes were present in the medium and increased progressively in relative intensity over the 2-h time course of the experiment. These data are consistent with ANGPTL4 being synthesized in cells as a full-length protein, and then undergoing cleavage into a 15-kDa N-terminal and a 37-kDa C-terminal fragment. Because neither of the cleavage fragments were detected in the cells, ANGPTL4 cleavage must occur late in the secretory pathway, on the cell surface, or in the medium. Cleavage did not require addition of serum to the medium.
The E40K isoform was expressed at comparable levels to the wild-type protein in the cells, but immunoblot analysis with the C-terminal antibody revealed only the 37-kDa C-terminal fragment in the medium (Fig. 1, lower panel). None of the full-length form of the E40K isoform was detected in the medium when the proteins were immunoblotted with the C-terminal antibody. The rate of accumulation of the C-terminal fragment in the medium over the 2-h time course of the experiment was similar to that seen in cells expressing the wild-type protein. Small amounts of full-length E40K protein were visible in medium immunoblotted with the N-terminal antibody, which appears to bind with higher affinity to full-length ANGPTL4 than does the C-terminal antibody. No N-terminal fragment was detected in the medium of cells expressing ANGPTL4-E40K. From this experiment, we concluded that the E40K mutation did not interfere with synthesis, secretion, or cleavage of ANGPTL4. The reduced amount of full-length ANGPTL4 and absence of any detectable N-terminal fragment in the medium is consistent with the substitution promoting degradation of both the full-length protein and the N-terminal domain fragments.
The E40K Mutation Prevents Accumulation of ANGPTL4 Oligomers in the Medium—Residue 40 of ANGPTL4 is highly conserved through vertebrate evolution. The corresponding residue is a glutamate in all mammals for which the sequence is available and aspartic acid in fish (Table 1). A closely related family member, ANGPTL3, also has either glutamate or aspartate at this position. Glu-40 is predicted to reside in the 1st turn of an α-helix (FASWDEMNVLAHGLLQLG). This helix is followed by another helix that is predicted to be oriented at an angle to the first helix.
TABLE 1.
Sequence alignment of conserved N-terminal motif in ANGPTL
Representatives of two subfamilies (ANGPTL3 and ANGPTL4) with a conserved acidic residue at positions 40 (ANGPTL4 human number, highlighted red) are shown. Positions with semiinvariant and uncharged residues are highlighted black and yellow, respectively. NCBI gi numbers are shown on the right, consensus computed on the PROMALS3D alignment of all sequences is shown below.
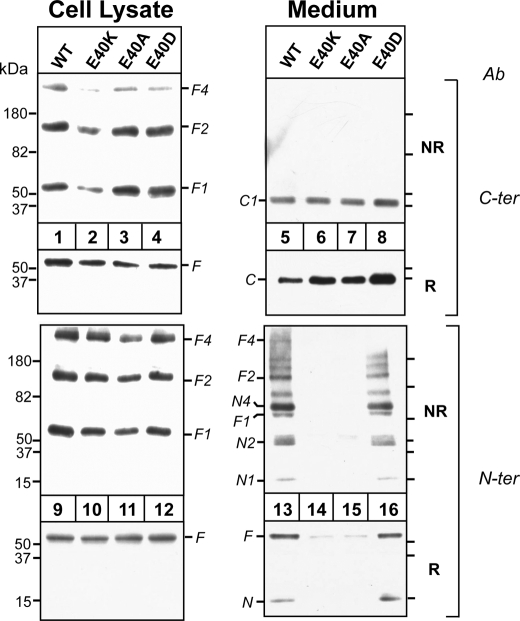
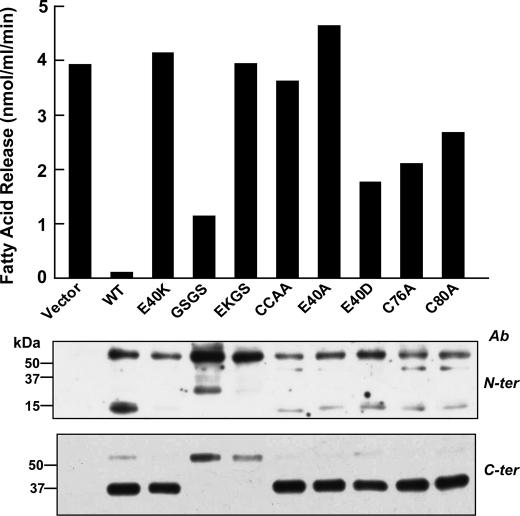
The N-terminal domain of ANGPTL4 was shown previously to form higher order oligomers that are stabilized by disulfide bonds (7). The domain contains two cysteine residues (Cys-76 and Cys-80) (Fig. 1, top) that when mutated markedly reduce the inhibitory effect of ANGPTL4 on LPL activity (7). To determine the effect of the E40K substitution on the oligomerization of ANGPTL4, we used both non-reducing (NR) and reducing (R) gels to fractionate the lysates from cells transfected with wild-type ANGPTL4 or ANGPTL4-E40K (Fig. 2). We included in the experiment cells expressing ANGPTL4 containing a neutral residue (alanine) or another acidic residue (aspartate) for glutamate to further define the role of residue 40 on the oligomerization of ANGPTL4.
FIGURE 2.
Effect of altering the Glu-40 residue on ANGPTL4 oligomerization in vitro. HEK-293A cells were transiently transfected with ANGPTL4-WT, -E40K, -E40A, and -E40D mutants. 48 h after transfection, both cells and medium were harvested. Equal amount of cell lysates (30 μl) or equivalent amounts of media were used for immunoblotting. For reduced SDS-PAGE, samples were mixed with 4× loading buffer with β-ME and loaded on 15% SDS gels. For non-reduced SDS-PAGE, samples were mixed with 4× loading buffer without β-ME and loaded on 4-15% gradient SDS gels. The gels were immunoblotted with anti-ANGPTL4 antibodies directed against the C terminus (C-ter) and the N terminus (N-ter). This experiment was repeated three times with similar results. NR, nonreducing; R, reducing.
Immunoblot analysis of non-reduced gels using antibodies to the C terminus or N terminus of ANGPTL4 revealed bands at 50, 100, and 200 kDa in lysates from cells transfected with all four constructs (lanes 1-4 and 9-12). These bands are the expected size of monomers (F1), dimers (F2), and tetramers (F4) of the full-length protein. Because ANGPTL4-E40A and -E40D oligomerized in a manner similar to the wild-type protein (lanes 3, 4, 11, and 12), the glutamic acid at residue 40 is not required for the formation of the higher order oligomers of ANGPTL4 in cells.
Immunoblot analysis of ANGPTL4 in the medium of the cells under both nonreduced and reduced conditions is shown in the right panel of Fig. 2. When the C-terminal antibody was used for immunoblotting, only the 37-kDa fragment, corresponding to the cleaved C terminus (C1), was present in the medium (a band corresponding to the full-length protein was only apparent on very long exposures (data not shown)). Similar amounts of the C-terminal fragment were present in medium from cells expressing wild-type (lane 5) and mutant forms of ANGPTL4 (lanes 6-8). Thus, the absence of a glutamic acid residue at position 40 in ANGPTL4 did not interfere with the synthesis, oligomerization, secretion, or cleavage of ANGPTL4.
When duplicate aliquots of the medium were subjected to immunoblotting with the N-terminal antibody under nonreduced conditions, significant differences were apparent between the wild-type and mutant forms of the protein. A 15-kDa band and a series of bands of higher molecular weight that were multiples of 15 kDa were present in the medium from cells expressing wild-type ANGPTL4 (lane 13). These bands correspond to monomers (N1), dimers (N2) and tetramers (N4) of the 15-kDa N-terminal fragment. Bands of the expected size of the full-length protein and of oligomers of the intact protein were also detected with the N-terminal antibody (F1, F2, and F4). These full-length oligomers were visible when the C-terminal antibody was used for immunoblotting only after a prolonged exposure of the film to the immunoblot (data not shown). In addition to oligomers of the N-terminal and full-length protein, fragments with an estimated molecular mass of 65 and 134 kDa were also present in the medium of cells expressing the wild-type protein. These bands presumably represent additional oligomers of the N terminus or of the N terminus bound to the full-length protein (see below). These data indicate that the N-terminal domains of wild-type ANGPTL4 remain oligomerized after cleavage, whereas the C-terminal domains dissociate into monomers.
In contrast to these results, no immunodetectable N-terminal fragment or higher molecular weight oligomers were present in the medium of cells expressing ANGPTL4-E40K (lane 14) or ANGPTL4-E40A (lane 15). Substitution of aspartate for glutamic acid at residue 40 produced a banding pattern that was similar to the wild-type protein (lane 16). Thus, substitution of a positively charged or neutral residue for a negatively charged amino acid at position 40 does not interfere with the oligomerization, secretion, or catalytic cleavage of ANGPTL4, but rather with stabilization of oligomers of the full-length protein, and of the N-terminal fragments. These data suggest that a negatively charged amino acid at residue 40 is required to stabilize higher order oligomers of ANGPTL4 after cleavage.
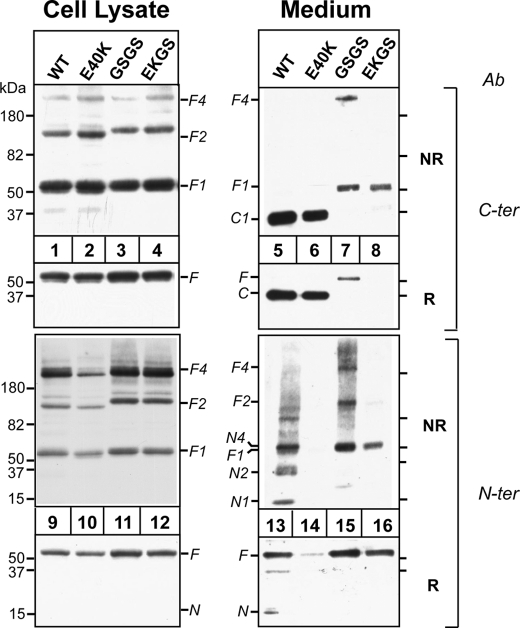
The E40K Substitution Affects ANGPTL4 Oligomers Independently of Cleavage—Next we examined the effect of protein cleavage on the stability of the N-terminal domain. Substitution of the residues at the highly conserved furin cleavage site (161RRKR164) with the sequence GSGS abolished cleavage of the protein (Fig. 3, lane 7). Because cleavage proceeded normally in furin-deficient cells, a protein other than furin must also be capable of cleaving ANGPTL4 (supplemental Fig. S1). To determine the effect of the E40K substitution in a protein that could not be cleaved, we introduced the substitution into the ANGPTL4-GSGS construct (EKGS). In cells, oligomer formation by ANGPTL4 containing the GSGS sequence (±E40K) (lanes 3, 4, 11, and 12) was indistinguishable from that of wild-type ANGPTL4, and the mutant proteins were secreted into medium (lanes 7, 8, 15, and 16). As expected, full-length ANGPTL4 (F1) but no C-terminal fragment (C1), was present in the medium of cells expressing ANGPTL4-GSGS (lane 7) and ANGPTL4-EKGS (lane 8). A band with an estimated molecular mass of 200 kDa, the predicted size of the full-length tetramer (F4), was present in the medium of the GSGS cells (lane 7), and a band at the expected size of the dimer (F2) was seen when the N-terminal antibody was used (lane 15). No full-length dimers or tetramers were present in medium from cells expressing ANGPL4-EKGS. Only the full-length monomer of ANGPTL4-EKGS was present in the medium (lanes 8 and 16). These results are consistent with the RRKR being the site of cleavage of ANGPTL4 and demonstrate that cleavage is not required for formation or secretion of ANGPTL4 oligomers. Moreover, the absence of any oligomers in the medium from the cells expressing ANGPTL4-EKGS demonstrates that substitution of lysine for glutamate at position 40 interferes with maintenance of stable oligomers of ANGPTL4, irrespective of whether or not the protein undergoes catalytic cleavage.
FIGURE 3.
Effect of E40K substitution on oligomerization of cleavage-deficient ANGPTL4 mutants in vitro. HEK-293A cells were transiently transfected with ANGPTL4-WT, -E40K, -GSGS, and -EKGS constructs 48 h after transfection, both cells and medium were harvested. Equal amount of cell lysates (30 μl) or equivalent amounts of medium were used for immunoblotting. For reduced SDS-PAGE, samples were mixed with 4× loading buffer with β-ME and loaded on 15% SDS gels. For non-reduced SDS-PAGE, samples were mixed with 4× loading buffer without β-ME and loaded on 4-15% gradient SDS gels. The gels were immunoblotted with anti-ANGPTL4 antibodies directed against the C terminus (C-ter) and the N terminus (N-ter). This experiment was repeated three times with similar results.
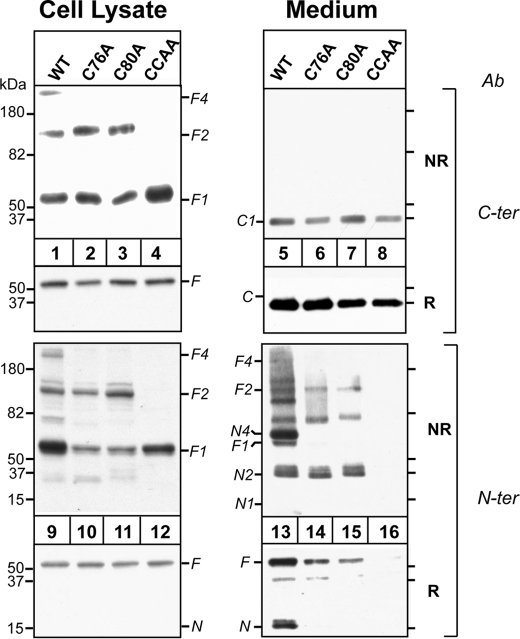
The Cysteine Residues in the N Terminus Are Required for N-terminal Oligomerization of ANGPTL4—The N-terminal domain of ANGPTL4 contains two cysteine residues (Cys-76 and Cys-80) that when mutated markedly reduce the inhibitory effect of ANGPTL4 on LPL activity (7). We mutated each of the cysteines to alanine individually (C76A, C80A) and together (CCAA) to determine the effects on oligomerization (Fig. 4). Full-length monomer (F1) and dimer (F2) were both present in the cells expressing ANGPTL4 that contained a single cysteine substitution (lanes 2, 3, 10, and 11). No band at the expected size of a tetramer (F4) was detected in these cells, perhaps because tetramer formation requires both cysteines to form a disulfide bond with different molecules of ANGPTL4. In cells in which alanines were substituted for both cysteine residues, only the monomer was present in the cells (lanes 4 and 12). Thus, as reported by Ge et al. (7), cysteines 76 and 80 in ANGPTL4 are required for oligomerization of the protein.
FIGURE 4.
Effect of Cys residures (Cys-76 and Cys-80) on ANGPTL4 oligomerization. HEK-293A cells were transiently transfected with ANGPTL4-WT, -C76A, -C80A, and -CC/AA mutants. 48 h after transfection, both cells and medium were harvested. Equal amounts of cell lysates (30 μl) and medium were used for immunoblotting. For reduced SDS-PAGE, samples were mixed with 4× loading buffer with β-ME and loaded on 15% SDS gels. For nonreduced SDS-PAGE, samples were mixed with 4× loading buffer without β-ME and loaded on 4-15% gradient SDS gels. The gels were immunoblotted with anti-ANGPTL4 antibodies directed against the C terminus (C-ter) and the N terminus (N-ter). This experiment was repeated three times with similar results.
Substitution of alanines for the cysteines in the N terminus did not interfere with the secretion of ANGPTL4 or with the cleavage of the protein. Levels of the C-terminal fragment in the three cysteine mutants were similar to that seen in the medium of cells expressing the wild-type protein (lanes 5-8). In contrast, the cysteine mutations significantly altered the pattern of bands observed with the N-terminal antibody. In the medium from cells expressing the single cysteine mutants, no monomers of the N-terminal fragment were seen (Fig. 4, lanes 14 and 15); only fragments of the expected size of the dimer of the N terminus (N2) and of the full-length protein (F2) were present. In addition, a band of ∼65 kDa was seen in the medium, which is the expected size of a full-length monomer linked to an N-terminal fragment. Mutating both cysteines resulted in no full-length oligomers forming in the cells or being detected in the medium (lanes 8 and 16). The C-terminal domain accumulated at normal levels in the medium, indicating that the monomer undergoes secretion and cleavage. However, no N-terminal fragments or full-length protein were detected in the medium, suggesting that they are rapidly degraded. These findings suggest that oligomerization stabilizes ANGPTL4 in the medium.
The E40K Substitution Abolishes ANGPTL4-mediated Inhibition of LPL Activity—ANGPTL4 inhibits the TG hydrolase activity of LPL (3, 10). To examine the effect of the E40K substitution on ANGPTL4-mediated suppression of LPL activity, we added conditioned medium from cells expressing wild-type or mutant forms of ANGPTL4 to an in vitro LPL assay system comprising mouse post-heparin plasma and an emulsion of [3H]triolein (17). Medium from cells transfected with an empty vector had no effect on LPL-mediated hydrolysis of triolein, whereas medium from cells transfected with wild-type ANGPTL4 inhibited LPL activity by more than 95% (Fig. 5). Medium from cells transfected with ANGPTL4-E40K failed to inhibit LPL activity, even at concentrations severalfold higher than wild-type (supplemental Fig. S2).
FIGURE 5.
Effect of E40K substitution and other mutants on LPL activity. Wild-type or mutant forms of ANGPTL4 were expressed in HEK-293A cells and conditioned medium was collected and concentrated. The medium was added to post-heparin mouse plasma (7.5 μl) and incubated for 30 min at 20 °C. An emulsion containing 1.13 μmol of [3H]triolein was added, and the release of fatty acid was determined as described under “Experimental Procedures.” Equal amounts of proteins were used in the assay as determined by immunoblotting (bottom panel) using antibodies against the C terminus (C-ter) and the N terminus (N-ter). This experiment was repeated three times with similar results.
Medium containing the cleavage-defective mutant form of ANGPTL4 (GSGS) inhibited LPL, though not as effectively as did medium from cells expressing the wild-type protein. Thus, cleavage of ANGPTL4 is not required for inhibition of LPL. Introduction of the E40K mutation into the cleavage defective mutant (EKGS) resulted in a complete lack of LPL inhibition. Moreover, substitution of the two cysteine residues involved in oligomerization of the N-terminal domain inhibited the activity of ANGPTL4 (CCAA), whereas residual inhibitory activity was seen if only a single cysteine was mutated. Substitution of alanine (E40A), but not aspartic acid (E40D) for glutamate at amino acid 40 completely destroyed the ability of ANGPTL4 to inhibit LPL activity. Thus, the presence of cysteine residues at positions 76 and 80 and an acidic residue at residue 40 (glutamate or aspartate) are required for ANGPTL4 to inhibit LPL activity at levels similar to the wild-type protein. These findings indicate that oligomerization of ANGPTL4 is required for extracellular accumulation of the N-terminal domain of the protein and inhibition of LPL activity.
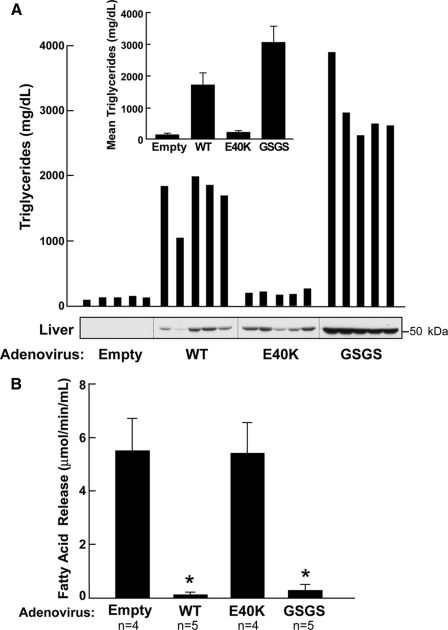
The E40K Substitution Abolishes ANGPTL4-induced Hypertriglyceridemia in Mice—Overexpression of ANGPTL4 in mice leads to marked hypertriglyceridemia (3). To assess the effect of the E40K substitution on ANGPTL4-induced hypertriglyceridemia, we used recombinant adenoviruses to express the wild-type and mutant forms of the protein in mice. Mice infected with adenovirus expressing wild-type ANGPTL4 had a 13-fold increase in plasma TG levels 3 days after infection (Fig. 6A). In contrast, mice infected with adenoviruses expressing ANGPTL4-E40K had little or no increase in plasma TG concentrations despite hepatic levels of ANGPTL4 that were comparable to those observed in mice expressing the wild-type protein (Fig. 6A). Consistent with these findings, post-heparin plasma from mice infected with virus expressing wild-type ANGPTL4 completely suppressed LPL activity (Fig. 6B) whereas no change in LPL activity was seen when post-heparin plasma from the mice expressing ANGPTL4-E40K was used in the assay. Mice overexpressing the cleavage-defective form of ANGPTL4 (GSGS) consistently developed even higher plasma TG levels than did the mice expressing the wild-type protein (Fig. 6A). The cleavage defective form of ANGPTL4 also potently inhibited LPL activity in vitro. These data are consistent with our in vitro data showing that post-translational cleavage of ANGPTL4 is not required for the protein to be active against LPL (Fig. 6B).
FIGURE 6.
Effect of E40K substitution on mouse plasma triglyceride level and LPL activity by adenovirus overexpression. A, adenoviruses expressing no protein, ANGPTL4-WT, -E40K, or -GSGS were injected into wild-type C57/B6 mice (n = 5 in each group) via tail vein. 72 h after injection, post-heparin plasma, and livers were collected. Plasma triglyceride levels were measured in each individual mouse, and mean triglyceride levels from each group are shown on top. Livers were homogenized, and equal amounts of protein (50 μg) were loaded on 12% SDS-PAGE. ANGPTL4 protein in liver was detected using antibody against the N terminus (N-ter). B, LPL activity in post-heparin mouse plasma (7.5 μl) from each group was measured as described under “Experimental Procedures.”
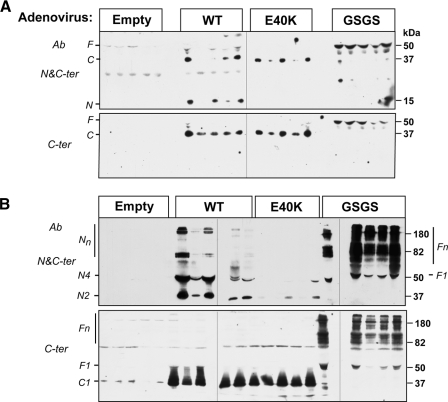
The E40K Substitution Prevents Accumulation of the N-terminal Fragment of ANGPTL4 in the Circulation—Analysis of cultured cells indicated that the E40K substitution destabilized the N-terminal oligomers of ANPTL4 after secretion (Fig. 1). To assess the in vivo relevance of this observation, we performed immunoblot analysis of plasma samples from mice infected with adenoviruses overexpressing wild-type and mutant forms of ANGPTL4 (Fig. 7). In mice expressing wild-type ANGPTL4, almost all circulating ANGPTL4 was present in the cleaved form (Fig. 7A). Immunoblot analysis of plasma fractionated on nonreducing gels indicated that the N-terminal domains were present as oligomers (N2, N4, Nn), whereas the C-terminal domain circulated as a monomer (Fig. 7B). In mice overexpressing the E40K isoform, circulating levels of the C-terminal domain were comparable to those observed in mice overexpressing the wild-type protein. However the N-terminal fragment was either not detected or present at very low levels in the mice infected with the ANGPTL4-E40K virus. Mice infected with the cleavage-defective mutant of ANGPTL4 accumulated high levels of full-length protein, almost all of which circulated as oligomers.
FIGURE 7.
Oligomerization of ANGPTL4-WT, -E40K, and -GSGS in mice. Empty control, ANGPTL4-WT, -E40K, or -RRKR/GSGS adenovirus was injected into wild-type C57/B6 mice (n = 5 in each group) via the tail vein. 72 h after injection, plasma was collected, and 2 μl of plasma from each mouse were used for immunoblotting. For reduced SDS-PAGE (A), samples were mixed with 4× loading buffer with β-ME and loaded on 12% SDS gels. For non-reduced SDS-PAGE (B), samples were mixed with 4× loading buffer without β-ME and loaded on 8% SDS gels. The gels were immunoblotted with anti-ANGPTL4 antibodies directed against the C terminus (C-ter) and the N terminus (N-ter).
DISCUSSION
The major finding of this study is that oligomerization and cleavage of ANGPTL4 have distinct roles in the function of the protein. Our data indicate that ANGPTL4 assembles into dimers and tetramers in the cell, and this oligomerization is required for the inhibitory effect of ANGPTL4 on LPL activity. Mutations that prevent oligomerization of ANGPTL4 do not block secretion of the protein but limit extracellular accumulation of the full-length and N-terminal domains that are required for inhibition of LPL. The E40K variant of ANGPTL4, an allele found in ∼3% of Caucasians (11), does not inhibit synthesis, oligomerization, cleavage, or secretion of the protein yet it virtually abolishes the inhibitory action of ANGPTL4 on LPL activity by preventing the extracellular accumulation of the full-length protein and of the N-terminal fragments. Cleavage of ANGPTL4 is not required for inhibition of LPL activity in vitro or in vivo. Mutations that disrupt the cleavage site had little effect on the inhibitory action of ANGPTL4 on LPL activity. The cleavage defective mutant accumulated to much higher levels in the circulation than did the wild-type protein when expressed in mice. Thus, cleavage of ANGPTL4 may promote clearance of the protein.
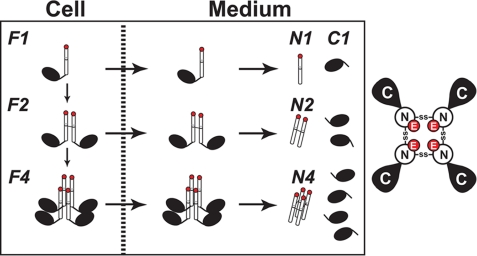
The post-translational processing of ANGPTL4 is shown schematically in Fig. 8. Here we have shown that oligomerization of ANGPTL4 occurs prior to secretion of the protein from cells: immunoblot analysis of cell lysates size-fractionated on nonreducing gels revealed bands corresponding to dimers and tetramers of the full-length protein (Fig. 2). No protein at the expected size of a trimer of ANGPTL4 was detected in cells. The pattern of oligomerization of both the full-length protein and of the N terminus in the medium was similar to that seen in the cell: only dimers and tetramers of both intact ANGPTL4 and of the N-terminal fragment were present in medium expressing the wild-type protein. These findings are consistent with the oligomers of the N terminus found in the medium representing complexes that formed within the cell. Mutation of two cysteine residues in the N-terminal domain (Cys-76 and Cys-80) that stabilize the oligomeric structure of ANGPTL4 (7) prevented formation of oligomers in the cell (Fig. 4, lane 4). Shan et al. (18) reported that mutation of the cysteines at residues 76 and 80 to serine did not prevent oligomerization or LPL-inhibition by the N-terminal fragment of ANGPTL4 expressed in bacteria. These data suggest that disulfide bonds formed by Cys-76 and Cys-80 are not essential for ANGPTL4 oligomer formation in vitro. However our data, and those of Ge et al. (7), indicate that these cysteines form intermolecular disulfide bonds that are essential for the stability of ANGPTL4 oligomers in vivo.
FIGURE 8.
A schematic model of the post-translational processing of ANGPTL4. Left, ANGPTL4 undergoes oligomerization to dimers and tetramers by forming disulfide bonds between cysteine residues in the N-terminal domain of the protein (Cys-76 and Cys-80). The orientation of the multimers has not been defined. Oligomers are shown here in a parallel orientation. The monomers, dimers, and tetramers of ANGPTL4 are secreted, and the protein is cleaved between Arg-161 and Arg-164 either late in the secretory pathway, on the cell surface, or in the medium. The N-terminal disulfide linkages are retained, and the C termini are released as monomers. Glutamate at residue 40 is shown in red. Right, a schematic of the ANGPTL4 tetramer as viewed from the top of the complex. The glutamic acid residues may form a cluster of negative charges at the N terminus of the oligomeric complex.
Failure to oligomerize did not prevent secretion or cleavage of the mutant protein, as indicated by the accumulation of the C-terminal domain in the medium of cells expressing ANGPTL4-CCAA (Fig. 4). However medium from cells expressing this mutant form of ANGPTL4 had no immunodetectable full-length or N-terminal oligomers, and failed to inhibit LPL activity. Thus, oligomerization of ANGPTL4 is not required for secretion or catalytic cleavage of the protein (Fig. 4), but is essential for inhibition of LPL activity.
When only one of the two cysteines in the N-terminal fragment was changed to an alanine, dimers but no tetramers of the full-length protein were present both in the cells and in the medium (Fig. 4). Thus, the tetramers likely form when the two cysteine residues form disulfide bonds with different ANGPTL molecules. Our experiments do not allow us to define the orientation of the ANGPTL4s in either the dimer or the tetramer, i.e. are they oriented in parallel or are they antiparallel? Our data are consistent with the notion that cleavage of the protein results in the C terminus being released from the ANGPTL4 complex as a monomer, while the N-terminal regions remains linked either to one or to two molecules of ANGPTL4, forming dimers or tetramers of the N-terminal domain (Fig. 8).
The role of cleavage in the function of ANGPTL4 had not been examined previously. The N-terminal fragment of ANGPTL4 is sufficient for activity (10), but it is not known if cleavage is required for activation of the protein. Although ANGPTL4 was efficiently cleaved in cells lacking furin (supplemental Fig. S1), disruption of a canonical furin recognition motif, 161RRKR164, prevented cleavage of the protein as noted previously by Chomel et al. (19). These data confirm the R161RKR16 motif as the cleavage site (Fig. 3), although the protease(s) involved remain to be identified. Previously, it was shown that cleavage of rat ANGPTL4 required addition of serum to the cultured cells and could be inhibited by addition of protease inhibitors (8). Serum was not required for cleavage of human ANGPTL4, and addition of protease inhibitors failed to prevent cleavage of the protein (data not shown). It is possible that there are significant differences in the processing of human and rat ANGPTL4.
Cells expressing the cleavage-defective form of ANGPTL4 secreted oligomers of the full-length protein (dimers and tetramers) into the medium (Fig. 3, lanes 7 and 15). Conditioned medium from these cells inhibited LPL activity in postheparin plasma (Fig. 5). Moreover, mice overexpressing the cleavage-defective mutant form of the protein developed severe hypertriglyceridemia and LPL activity in their postheparin plasma was very low (Fig. 6). These data indicate that cleavage is not required to activate the LPL inhibitory effect of ANGPTL4. Plasma levels of oligomeric ANGPTL4 were substantially higher in mice expressing the cleavage defective form of the protein than in animals expressing the wild-type protein, which may contribute to their higher plasma levels of TG (Fig. 7). The accumulation of ANGPTL4 oligomers in mice expressing ANGPTL4-GSGS suggests that cleavage normally facilitates inactivation of the protein by promoting clearance of the active portion (the N terminus) from the circulation.
Shan et al. (18) reported that the substitution of lysine for glutamate at residue 40 of ANGPTL4 greatly decreased the affinity of purified recombinant N-terminal fragments of the protein for LPL. Our results indicate that the primary effect of the E40K substitution in vivo is to prevent extracellular accumulation of the N-terminal domain, thereby severely limiting the amount of functional ANGPTL4 available to interact with LPL. The mechanism by which the E40K substitution prevents the extracellular accumulation of ANGPTL4 oligomers is not known. Although the presence of a lysine at residue 40 did not prevent oligomer formation in cells or secretion of the C-terminal domain of the protein (Fig. 2), it might specifically prevent secretion of the N-terminal oligomer. This model would require that ANGPTL4 cleavage occurs prior to secretion into the medium. Although we cannot formally rule out the possibility that the protein undergoes cleavage just prior to secretion, we found no evidence that cleavage occurs intracellularly. We did not see any of the C-terminal or N-terminal fragment in our cell lysates (Fig. 1), which is consistent with prior studies of rat ANGPTL4, where the cleaved protein fragments could only be detected in the medium and not in cell lysates (8).
Mutations that prevent oligomerization of ANGPTL4 do not inhibit secretion of the C-terminal domain but prevent the accumulation of the N-terminal domain in the medium. Therefore an alternative possibility is that the E40K substitution promotes dissociation of the oligomers after secretion. This possibility seems unlikely, however, as the available data are consistent with a model in which the N-terminal oligomer is stabilized by disulfide bonds, and it is not clear how the substitution of lysine for glutamic acid at residue 40 could promote dissociation of a complex stabilized by covalent interactions.
A third possibility is that the E40K mutation results in rapid degradation of the N-terminal oligomers after secretion. The substitution may cause structural changes in the protein that render it more susceptible to proteolysis, or it may prevent it from interacting with components in the extracellular milieu (such as the extracellular matrix) that protect the protein from proteolytic cleavage, as suggested by Chomel et al. (19). If the monomers of ANGPTL4 are all oriented in parallel in the oligomeric complex, there would be a cluster of negatively charged residues, including glutamic acid at residue 40, at the N terminus of the complex. Adjacent to the glutamic acid is an aspartic acid (Asp-39), which is completely conserved (Table 1). These negatively charged residues may interact with a positively charged protein (or even LPL) that shields ANGPTL4 from degradation. This mechanism is consistent with the finding that no immunodetectable N-terminal fragments of ANGPTL-E40K were present in the medium despite normal expression of the protein in cells and normal levels of the C-terminal fragment in the medium (Fig. 2).
Whereas our study focused primarily on the role of these post-translational processes in TG metabolism, several lines of evidence indicate that ANGPTL4 may also play a role in maintaining the integrity of the vascular endothelium. Chomel et al. (19) reported that the N-terminal domain of ANGPTL4 binds to the extracellular matrix, and speculated that cleavage of the protein releases the C-terminal fibrinogen-like domain to transduce antiangiogenic activities (20). The proteolytic cleavage, and the highly conserved C-terminal domain of ANGPTL4, which appear to play little role in TG metabolism, may be essential for its role in the vasculature.
Supplementary Material
Acknowledgments
We thank Liangcai Nie, Stephenie Blankenship, and Daniel Smith for excellent technical assistance, and Dr. Charles Dann III and Dr. Sander Kersten for helpful discussions.
This work was supported by National Institutes of Health Grant HL092550, the Donald W. Reynolds Foundation, the American Heart Association, and a Welch Foundation grant I-1505 (to N. V. G.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Author's Choice—Final version full access.
Footnotes
The abbreviations used are: ANGPTL4, angiopoietin-like protein 4; TG, triglyceride; PBS, phosphate-buffered saline; β-ME, β mercaptoethanol; LPL, lipoprotein-lipase; WT, wild type.
References
- 1.Yoon, J. C., Chickering, T. W., Rosen, E. D., Dussault, B., Qin, Y., Soukas, A., Friedman, J. M., Holmes, W. E., and Spiegelman, B. M. (2000) Mol. Cell Biol. 20 5343-5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kersten, S., Mandard, S., Tan, N. S., Escher, P., Metzger, D., Chambon, P., Gonzalez, F. J., Desvergne, B., and Wahli, W. (2000) J. Biol. Chem. 275 28488-28493 [DOI] [PubMed] [Google Scholar]
- 3.Yoshida, K., Shimizugawa, T., Ono, M., and Furukawa, H. (2002) J. Lipid Res. 43 1770-1772 [DOI] [PubMed] [Google Scholar]
- 4.Oike, Y., Akao, M., Kubota, Y., and Suda, T. (2005) Trends Mol. Med. 11 473-479 [DOI] [PubMed] [Google Scholar]
- 5.Koster, A., Chao, Y. B., Mosior, M., Ford, A., Gonzalez-DeWhitt, P. A., Hale, J. E., Li, D., Qiu, Y., Fraser, C. C., Yang, D. D., Heuer, J. G., Jaskunas, S. R., and Eacho, P. (2005) Endocrinology 146 4943-4950 [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein, L., Berbee, J. F., van Dijk, S. J., van Dijk, K. W., Bensadoun, A., Kema, I. P., Voshol, P. J., Muller, M., Rensen, P. C., and Kersten, S. (2007) Arterioscler. Thromb. Vasc. Biol. 27 2420-2427 [DOI] [PubMed] [Google Scholar]
- 7.Ge, H., Yang, G., Yu, X., Pourbahrami, T., and Li, C. (2004) J. Lipid Res. 45 2071-2079 [DOI] [PubMed] [Google Scholar]
- 8.Ge, H., Yang, G., Huang, L., Motola, D. L., Pourbahrami, T., and Li, C. (2004) J. Biol. Chem. 279 2038-2045 [DOI] [PubMed] [Google Scholar]
- 9.Ge, H., Cha, J. Y., Gopal, H., Harp, C., Yu, X., Repa, J. J., and Li, C. (2005) J. Lipid Res. 46 1484-1490 [DOI] [PubMed] [Google Scholar]
- 10.Sukonina, V., Lookene, A., Olivecrona, T., and Olivecrona, G. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 17450-17455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romeo, S., Pennacchio, L. A., Fu, Y., Boerwinkle, E., Tybjaerg-Hansen, A., Hobbs, H. H., and Cohen, J. C. (2007) Nat. Genet. 39 513-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson-Ehle, P., and Schotz, M. C. (1976) J. Lipid Res. 17 536-541 [PubMed] [Google Scholar]
- 13.Berbee, J. F., van der Hoogt, C. C., Sundararaman, D., Havekes, L. M., and Rensen, P. C. (2005) J. Lipid Res. 46 297-306 [DOI] [PubMed] [Google Scholar]
- 14.Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D. J. (1997) Nucleic Acids Res. 25 3389-3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei, J., Kim, B. H., and Grishin, N. V. (2008) Nucleic Acids Res. 36 2295-2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas, G. (2002) Nat. Rev. Mol. Cell Biol. 3 753-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romeo, S., Yin, W., Kozlitina, J., Pennacchio, L. A., Boerwinkle, E., Hobbs, H. H., and Cohen, J. C. (2009) J. Clin. Investig. 119 70-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan, L., Yu, X. C., Liu, Z., Hu, Y., Sturgis, L. T., Miranda, M. L., and Liu, Q. (2009) J. Biol. Chem. 284 1419-1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chomel, C., Cazes, A., Faye, C., Bignon, M., Gomez, E., Ardidie-Robouant, C., Barret, A., Ricard-Blum, S., Muller, L., Germain, S., and Monnot, C. (2009) Faseb J. 23 940-949 [DOI] [PubMed] [Google Scholar]
- 20.Yang, Y. H., Wang, Y., Lam, K. S., Yau, M. H., Cheng, K. K., Zhang, J., Zhu, W., Wu, D., and Xu, A. (2008) Arterioscler. Thromb. Vasc. Biol. 28 835-840 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.