Abstract
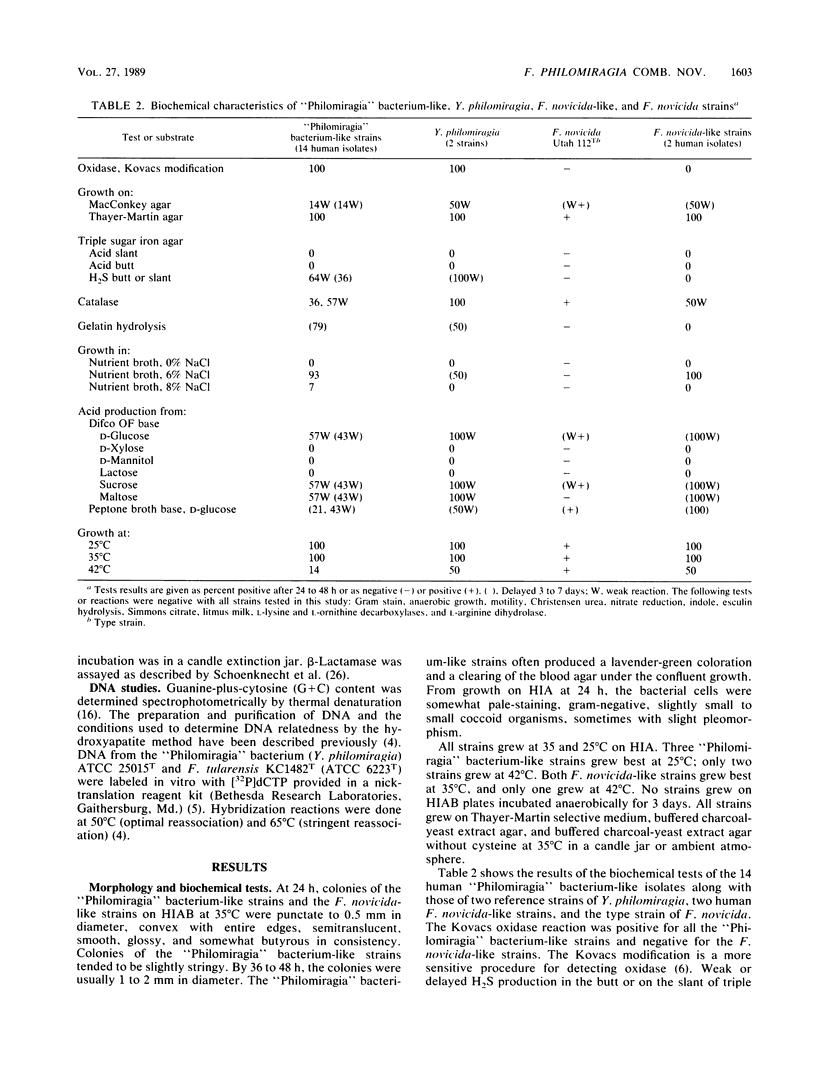
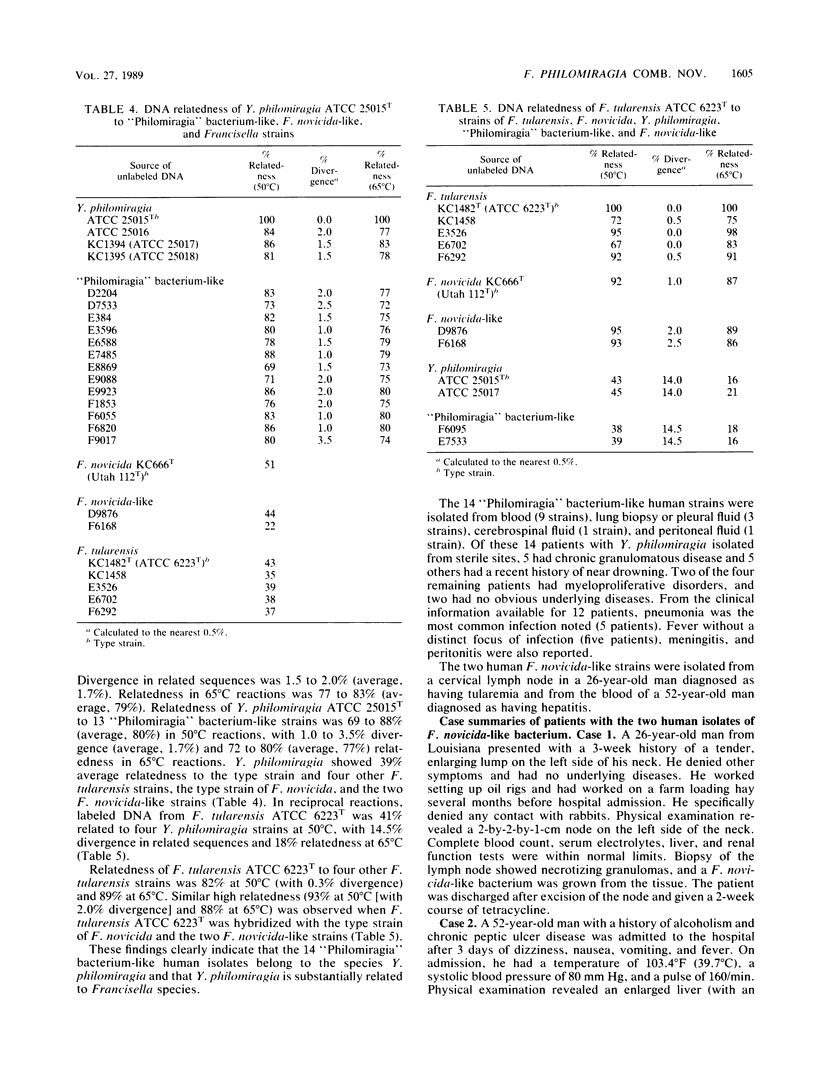
Over a 12-year period, 16 human strains of a gram-negative, catalase-positive, halophilic, aerobic, nonmotile, small coccoid bacterium were received for identification. On the bases of biochemical characteristics and cellular fatty acid profiles, 14 of these strains were similar to the "Philomiragia" bacterium (Yersinia philomiragia, species incertae sedis). Additional characteristics were growth on Thayer-Martin agar but no growth or sparse, delayed growth on MacConkey agar; oxidase positive; acid production, often weak and delayed, from D-glucose, sucrose, and maltose; urease negative; no reduction of nitrates; and H2S produced but often delayed in triple sugar iron agar. Both the human isolates and the "Philomiragia" bacterium contained C10:0, C14:0, C16:0, C18:1 omega 9c, C18:0, 3-OH C18:0, C22:0, and C24:1 as major cellular fatty acids and ubiquinone eight (Q8) as the major isoprenoid quinone. These cellular acids in these relative amounts have been found previously only in Francisella tularensis and Francisella novicida, suggesting a relationship between the "Philomiragia" bacterium and Francisella species. Of the 14 human "Philomiragia"-like isolates, 9 were from blood, 3 were from lung biopsies or pleural fluid, and one each was from peritoneal fluid and cerebrospinal fluid. DNA relatedness studies (hydroxyapatite method, 50 and 65 degrees C) showed that these 14 strains were a single group that was the same species as the "Philomiragia" bacterium. Two other human strains were oxidase negative and H2S negative. They formed a single DNA relatedness group that was indistinguishable from the type strains of both F. tularensis and F. novicida. DNA relatedness of "Philomiragia" bacterium type and other strains to strains of F. novicida and F. tularensis, including the type strains, was 35 to 46%. One of the two F. novicida- and F. tularensis-like strains was isolated from blood, and the other was isolated from a cervical lymph node. On the basis of these findings, we propose transferring Y. philomiragia from the genus Yersinia to the genus Francisella as Francisella philomiragia comb. nov. Having confirmed that F novicida and F. tularensis are the same species and having shown that F. novicida is pathogenic for humans, we further propose eliminating the species F. novicida and demoting it to a biogroup of F. tularensis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner D. J., McWhorter A. C., Knutson J. K., Steigerwalt A. G. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J Clin Microbiol. 1982 Jun;15(6):1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees S. B., Moss C. W., Hollis D. G., Weaver R. E. Chemical characterization of Flavobacterium odoratum, Flavobacterium breve, and Flavobacterium-like groups IIe, IIh, and IIf. J Clin Microbiol. 1986 Feb;23(2):267–273. doi: 10.1128/jcm.23.2.267-273.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. E., Gregory D. W., Schaffner W., McGee Z. A. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore) 1985 Jul;64(4):251–269. [PubMed] [Google Scholar]
- Jantzen E., Berdal B. P., Omland T. Cellular fatty acid composition of Francisella tularensis. J Clin Microbiol. 1979 Dec;10(6):928–930. doi: 10.1128/jcm.10.6.928-930.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen W. I., Owen C. R., Jellison W. L. Yersinia philomiragia sp. n., a new member of the Pasteurella group of bacteria, naturally pathogenic for the muskrat (Ondatra zibethica). J Bacteriol. 1969 Dec;100(3):1237–1241. doi: 10.1128/jb.100.3.1237-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSON C. L., WICHT W., JELLISON W. L. A new organism resembling P. tularensis isolated from water. Public Health Rep. 1955 Mar;70(3):253–258. [PMC free article] [PubMed] [Google Scholar]
- Lambert M. A., Patton C. M., Barrett T. J., Moss C. W. Differentiation of Campylobacter and Campylobacter-like organisms by cellular fatty acid composition. J Clin Microbiol. 1987 Apr;25(4):706–713. doi: 10.1128/jcm.25.4.706-713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Kai A., Lambert M. A., Patton C. Isoprenoid quinone content and cellular fatty acid composition of Campylobacter species. J Clin Microbiol. 1984 Jun;19(6):772–776. doi: 10.1128/jcm.19.6.772-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN C. R., BUKER E. O., JELLISON W. L., LACKMAN D. B., BELL J. F. COMPARATIVE STUDIES OF FRANCISELLA TULARENSIS AND FRANCISELLA NOVICIDA. J Bacteriol. 1964 Mar;87:676–683. doi: 10.1128/jb.87.3.676-683.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenza J. M., Klotz S. A., Penn R. L. Isolation of Francisella tularensis from blood. J Clin Microbiol. 1986 Sep;24(3):453–455. doi: 10.1128/jcm.24.3.453-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter D. B., Gerloff R. K. Deoxyribonucleic acid hybridization among some species of the genus Pasteurella. J Bacteriol. 1966 Dec;92(6):1838–1839. doi: 10.1128/jb.92.6.1838-1839.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R. A., Hollis D. G., Weaver R. E., Hitzig W. H. Chronic granulomatous disease: fatal septicemia caused by an unnamed gram-negative bacterium. J Clin Microbiol. 1982 Nov;16(5):821–825. doi: 10.1128/jcm.16.5.821-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Lawton W. D. Transformation of Pasteurella novicida. J Bacteriol. 1969 Nov;100(2):1112–1113. doi: 10.1128/jb.100.2.1112-1113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


