Abstract
Bruton’s tyrosine kinase (Btk) is a critical transducer of signals originating from the B cell antigen receptor (BCR). Dosage, sequential phosphorylation, and protein interactions are interdependent mechanisms influencing Btk function. Phosphopeptide-specific mAbs recognizing two distinct phosphotyrosine modifications were used to quantify Btk activation by immunofluorescent techniques during B cell stimulation. In a population of cultured B cells stimulated by BCR crosslinking and analyzed by flow cytometry, transient phosphorylation of the regulatory Btk tyrosine residues (551Y and 223Y) was detected. The kinetics of phosphorylation of the residues were temporally distinct. Tyrosine 551, a transactivating substrate site for Src-family kinases, was maximally phosphorylated within ≈30 seconds of stimulation as monitored by flow cytometry. Tyrosine 223, an autophosphorylation site within the SH3 domain, was maximally phosphorylated at ≈5 minutes. Btk returned to a low tyrosine phosphorylation level within 30 minutes, despite persistent elevation of global tyrosine phosphorylation. Colocalization of activated Btk molecules with the crosslinked BCR signaling complex was observed to coincide with the period of maximal Btk tyrosine phosphorylation when stimulated B cells were analyzed with confocal microscopy. The results of these in situ temporal and spatial analyses imply that Btk signaling occurs in the region of the Ig receptor signaling complex, suggesting a similar location for downstream targets of its activity.
Bruton’s tyrosine kinase (Btk) is an essential component of B cell signaling pathways linking receptor activation to important downstream processes such as the control of intracellular free calcium (1–4). The biological importance of its signaling function was shown by naturally occurring Btk loss of function mutations in human X linked agammaglobulinemia (XLA) and murine X linked immunodeficiency (xid) syndromes (5, 6). XLA manifests as a severe humoral immunodeficiency with an absence of functional B cells in the periphery. xid results in an alteration of normal B cell development that reduces the total peripheral B cell population by ≈50% and impairs functional responses to certain T cell-independent antigens, activation of the BCR, interleukin-5 receptor, interleukin-10 receptor, CD38, and CD40 on B cells; the Fc ε receptor on mast cells; and non-integrin collagen receptors on platelets (7–15). Transgenic model systems demonstrate a Btk dose-dependent phenotype of immunodeficiency and B cell dysfunction in vivo (16). When Btk expression is absent, specific BCR responses to type II T cell-independent antigens are undetectable. A low level of Btk expression confers partial recovery of these responses. Strikingly, transgenic overexpression of the wild-type allele diminishes antigen responses compared with normal B cell function. When Btk activity is strongly enhanced by expression of a constitutively activated allele (E41K, Btk*), B cell marrow development is disrupted, yielding an accentuated phenotype of murine immunodeficiency (17). Detailed analysis of the role of Btk in such signal transduction pathways would be aided by the development of techniques and reagents capable of quantifying the intermediate stages in the activation and inactivation of this kinase.
One mechanism controlling Btk kinase activity occurs through the sequential phosphorylation of specific regulatory tyrosine residues (18–21). Src-family kinases (such as Lyn in B cells) associated with immunoreceptor tyrosine activation motif-containing subunits link stimulated cell surface receptors to Btk function through phosphorylation of Btk 551Y in the kinase domain “activation loop.” This phosphorylation dramatically increases Btk enzymatic and biological activities. Btk is further activated by autophosphorylation of the 223Y residue within the Src homology type 3 domain ligand-binding pocket (22). Targeted deletion of Lyn in murine B cells alters Btk-dependent antigen responses, consistent with Lyn’s role as a modifier of Btk function (23, 24). A role for a Src-family kinase in the activation of a Btk homolog during Drosophila egg development has been shown by using genetic analysis, revealing a striking evolutionary preservation of this interaction in a nonimmunological context (25, 26). Additional phosphorylated regulatory tyrosine, serine, and possibly threonine residues are present (19, 27). Protein kinase C directly binds to the Btk pleckstrin homology domain and phosphorylates Btk in vitro (28). The in vivo biological importance of this modification is suggested by the protein kinase C βI(−/−) mouse, which manifests an xid-like B cell immunodeficiency. (29).
Btk is also regulated through binding interactions with signaling proteins and second-messenger molecules. G protein subunits bind to the pleckstrin homology domain and increase the catalytic domain enzymatic activity (30–32). Two alleles, Btk* and R28C (xid), illustrate the importance of phosphatidylinositol 3-kinase (PI 3-kinase) as a coregulator of Btk activation in receptor signaling pathways. Cell fractionation studies localize the wild-type allele in a cytosolic compartent, whereas a small percentage of the constitutively activated Btk* associates with the membrane fraction (33). Modulation of Btk signaling, presumably by enhanced membrane localization, is strongly modulated by the membrane lipid products of PI 3-kinase (1, 2, 27, 34). The xid allele does not up-regulate its signaling function in the presence of enhanced PI 3-kinase activity because it binds poorly to phosphatidylinositol 3,4,5-trisphosphate, whereas alleles constitutively targeted to the membrane have increased activity (2, 27, 35–38).
The activated BCR assembles a membrane-signaling complex (“signalosome”) containing the Ig chains, the α and β subunits, associated signal-transducing enzymes, and docking proteins (7). The intracellular location of Btk relative to the BCR signalosome structure may be dynamically regulated during the course of antigen receptor stimulation by crosslinking. The correlation of Btk phospholipid binding and signaling function suggests that activated Btk molecules can localize within the signalosome structure. This hypothesis can be directly tested by analysis of the subcellular location of Btk protein in individual cells. Such analysis would complement prior genetic and kinetic studies by providing spatial data linking Btk activation to BCR signaling. In this paper, we use phosphopeptide-specific antibodies recognizing the phosphorylated 551Y and 223Y residues to define Btk activation at the single-cell level. Btk localizes to the BCR signaling complex coincident with phosphorylation of the regulatory residues 551Y and 223Y. The essential signals transduced by Btk likely occur in the region of the receptor-signaling complex.
MATERIALS AND METHODS
Materials.
Peptides and phosphopeptides were prepared by Chris Turck (Howard Hughes Medical Institute, University of California, San Francisco) using a Model 350 Multiple Peptide Synthesizer (Advanced ChemTech). BALB/c and severe combined immunodeficient mice were used following university animal care guidelines. Keyhole limpet hemocyanin carrier protein and maleimide-activated plates for antibody screening were obtained from Pierce. Glutaldehyde for coupling of peptides to the carrier was obtained from Sigma. Anti-human Igβ was obtained from PharMingen. Phalloidin Texas Red-X was obtained from Molecular Probes. Antibodies coupled to alkaline phosphatase, fluorochromes, and horseradish peroxidase were obtained from Southern Biotechnology Associates, Jackson ImmunoResearch, PharMingen, and Bio-Rad. The antibody for Ramos cell stimulation [Goat F(ab′)2 anti-human IgM] was obtained from Southern Biotechnology Associates. ELISA plates were analyzed with a Molecular Devices Vmax plate reader. Wortmannin was obtained from Calbiochem. The general phosphotyrosine antibody 4G10 was from Upstate Biotechnology (Lake Placid, NY). Ramos B cells were obtained from American Type Culture Collection.
Generation of mAbs.
Immunogens were prepared by coupling Btk phosphopeptides [223PY, KVVALY(PO4)DYMPMNAND; 551PY, KVLDDEY(PO4)TSSVGS] to keyhole limpet hemocyanin (the lysine was added to the 551PY peptide to aid coupling) (21). BALB/c mice were immunized with carrier-coupled phosphopeptides in Freund’s complete (GIBCO; first dose only) or incomplete adjuvant. Immune sera were tested in ELISA and immunoblot assays. Splenocyte fusion with murine myeloma cells and hybridoma subcloning were performed by Susan Ou (California Institute of Technology) using standard techniques. Hybridoma culture supernatants were screened by ELISA and immunoblot assay for recognition of Btk phosphorylation sites. Antibody-containing ascites were generated in immunodeficient C.B-17 scid/scid mice and purified by protein A or protein G Sepharose. The mAb 223PYmAb (IgG1 κ) corresponds to a hybridoma clone designated 4F10/1A1 and 551PYmAb (IgG2b λ) corresponds to 8D11/1B4 .
Immunostaining of Cells.
Aliquots of cells (0.1 ml) were transferred into 1 ml of ice-cold fixative solution (PBS with 4% paraformaldehyde and 1 mM sodium vanadate). After fixation for 10 minutes, cells were collected by centrifugation (≈1,000 × g for 0.5 min) and washed twice with 1 ml of Tris (0.1 M, pH 8.0). Cells were permeabilized and stained by incubation for 4–6 hr at 25°C in 0.1 ml of permeabilization buffer (PBS with 2% BSA, 2% fetal bovine serum, 1 mM sodium vanadate, 1 mM EDTA, 0.2% Tween 20, and 0.01% SDS) supplemented with the indicated antibodies. Cells were washed once with 1 ml of permeabilization buffer and incubated for 1 hr at 25°C with the indicated fluorochrome-labeled secondary reagents diluted in permeabilization buffer. The stained cells were washed with 1 ml PBS and analyzed by flow cytometry. For confocal microscopy, stained cells were air dried on glass slides and mounted in VectaShield (Vector Laboratories) anti-quenching solution. Fluorescent secondary reagents were chosen based on the combination of primary antibodies and technique, either confocal microscopic analysis (MRC 1024, Bio-Rad) or flow cytometry (FACScan, Becton Dickinson).
Flow Cytometry.
Single-cell suspensions were analyzed by FACScan after exclusion of small debris by defining a lymphocyte gate based on forward- and side-scatter profiles. Four-quadrant, two-dimensional plots were generated by logarithmic amplification of fluorescence emitted by single cells. Control (double negative) quadrants were determined by evaluation of cells in which species or isotype Ig controls were substituted for specific antibodies.
RESULTS AND DISCUSSION
Specificity of mAbs for Intracellular Staining.
Hybridomas secreting mAbs that specifically recognize the phosphorylated Btk 551Y or 223Y residues were generated by fusion of immunized murine splenocytes with myeloma cells. To identify potential clones with desired specificity, culture supernatants from ≈300 (551PYmAb) or ≈700 (223PYmAb) hybridoma fusion wells were screened for reactivity against the immunizing phosphopeptide and cognate peptides in an ELISA. Hybridomas producing antibodies highly reactive to the phosphopeptide were retested by using immunoblot analysis. From each fusion, ≈5 clones producing IgG were subcloned. A single clone recognizing each phosphorylated site was chosen for this study based on the relative reactivity in ELISA, immunoblot, and immunoprecipitation assays,
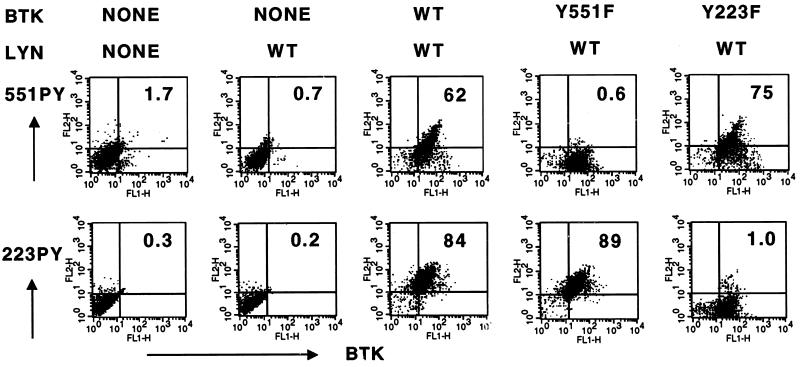
Purified mAbs were then tested for the capacity to recognize specifically phosphorylated Btk residues in situ in a lymphocyte immunofluorescence staining assay. Alternative Btk alleles (wild-type, Y551F, and Y223F) were heterologously expressed by using vaccinia vectors in Jurkat T cells, which do not express endogenous Btk (19, 39). A high proportion of virus-infected cells (≈90%) expressed exogenous Btk protein, as detected by immunostaining with an antibody (anti-Btk NT) recognizing the Btk amino-terminal region (Fig. 1 and data not shown). Btk 551Y site phosphorylation was specifically detected with 551PYmAb (Fig. 1, Upper) in cells overexpressing Lyn and Btk wild type (62% double positive) but not Lyn and Btk Y551F (0.6%). 551PYmAb also recognized cells expressing Lyn with Btk Y223F but not control cells expressing Lyn only, Btk isoforms coexpressed with kinase-inactive Lyn, or uninfected cells, (Fig. 1 and data not shown). Highly specific results also were obtained by using 223PYmAb. This antibody recognized cells expressing Lyn with Btk wild type (84% double positive) but not Lyn with BtkY223F (1%), Lyn-only, or uninfected controls (Fig. 1 Lower and data not shown). Addition of immunizing phosphopeptide (1 μM), but not the cognate peptide, during the primary antibody incubation completely inhibited immunostaining by 551PYmAb and 223PYmAb (data not shown). Thus, each phosphopeptide-specific antibody detects the relative abundance of one phosphorylated regulatory tyrosine residue of a single protein in the context of a full complement of cellular proteins and associated epitopes. In contrast with these results, a mAb (4G10) recognizing global tyrosine phosphorylation of cellular proteins stained the Lyn-alone infected cells (27%) better than the uninfected cells (13%) and did not distinguish significant variations in the levels of tyrosine phosphorylation among the cultures expressing Btk isoforms (data not shown).
Figure 1.
mAb specificity for intracellular detection of individual Btk tyrosine phosphorylation sites. Vaccinia viral stocks for Btk and Lyn proteins were produced and titered as described (19). Jurkat T cells (2 × 106 cells per ml) were infected by incubation with vaccinia virus stocks (multiplicity of infection = 5) for 1 hr at 4°C and grown for 8 hr at 37°C. Cells expressing Lyn wild type and/or Btk (wild type, Y551F, or Y223F) were fixed, permeabilized, and stained with antibodies (anti-Btk NT and either 551PYmAb or 223PYmAb) as indicated. The primary antibodies were detected with fluorescein isothiocyanate-labeled donkey anti-rabbit IgG and R-phycoerythrin-labeled donkey anti-mouse total IgG. Two-color fluorescence analysis was performed for each sample.
Kinetics of Btk Activation Measured in Situ.
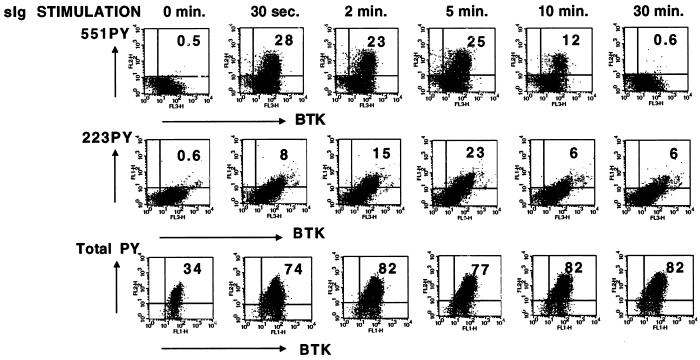
Genetic and biochemical evidence identify a critical role for Btk signaling function in the BCR intracellular signaling pathway during immune responses. Our goal was to evaluate the dynamic regulation of Btk signal transduction in individual B cells. Prior studies of Btk phosphorylation in receptor signaling pathways have utilized experimental methods (e.g., detergent lysis, immunoprecipitation, immunoblot) that average changes within a population and do not distinguish individual cell responses. Immunoblot analysis of human Ramos B cells demonstrates tyrosine phosphorylation of multiple proteins, including Btk, after BCR activation (19, 21). To evaluate variability in cell responses to surface IgM crosslinking, the kinetics of BCR-dependent protein tyrosine phosphorylation was measured with a general phosphotyrosine antibody (4G10) in an immunofluorescent flow cytometry assay. BCR-crosslinked Ramos cells had a rapid (within 30 seconds) and prolonged (at least 30 minutes) increase in total phosphotyrosine content among most of the cells (>75% positive), demonstrating a fairly uniform global response to BCR activation (Fig. 2, Bottom).
Figure 2.
Kinetics of Btk activation after BCR crosslinking measured by using intracellular immunofluorescent analysis. Ramos B cells were grown to a density of 0.5–1.5 × 106 cells per ml in RPMI medium 1640 supplemented with 5–10% fetal bovine serum, 2 mM glutamine, and 50 μM 2-mercaptoethanol. Cells were washed with serum-free RPMI, resuspended at 1 × 107 cells per ml, and incubated for 1 hr at 37°C. Goat F(ab′)2 anti-human IgM (10 μg/ml) was added for the indicated times at 37°C. Cells were fixed, permeabilized, and stained with biotinylated anti-Btk NT, 551PYmAb, and 223PYmAb (Top and Middle). The primary antibodies were detected with Tricolor-labeled streptavidin, R-phycoerythrin-labeled goat anti-mouse IgG2b, and fluorescein isothiocyanate-labeled monoclonal rat anti-mouse IgG1. Alternatively, cells were stained with anti-Btk NT and 4G10 anti-phosphotyrosine, followed by fluorescein isothiocyanate-labeled donkey anti-rabbit IgG and R-phycoerythrin-labeled donkey anti-mouse IgG. Three- or two-color fluorescence analysis was performed and data were plotted in two-dimensional formats (Top, 551PYmAb vs. anti-Btk NT; Middle, 223PYmAb vs. anti-Btk NT; Bottom, 4G10 anti-phosphotyrosine vs. anti-Btk NT).
To demonstrate cell-to-cell variation in Btk activation during receptor signaling pathways, control and BCR-crosslinked Ramos cells were immunofluorescently stained with a combination of 551PYmAb, 223PYmAb, and anti-Btk NT and evaluated by using flow cytometry. The data were plotted in two-dimensional graphs, comparing 551PYmAb or 223PYmAb with total Btk staining (Fig. 2, Top and Middle). Only 25–30% of the stimulated cells had detectably elevated levels of Btk phosphorylation. Among cells staining positive with the Btk phosphopeptide-specific antibodies, there was a continuous distribution of responses, indicating significant cell-to-cell variability in the activation of Btk. 551PYmAb staining was maximal within ≈30 seconds of receptor crosslinking and remained near that level until ≈5 minutes (Fig. 2, Top). 223PYmAb staining increased more slowly than 551PYmAb, reaching a peak of 15–20% positive cells at ≈5 minutes after stimulation (Fig. 2, Middle). The distinct phosphorylation kinetics of Btk 551Y and 223Y correspond well with results from immunoblot analysis of Btk activation (21). At the time of maximal 223PYmAb staining, more than 50% of these cells were also 551PYmAb positive (data not shown), in agreement with the observation that both phosphorylation events can occur simultaneously on individual Btk molecules (21). To understand why only a fraction of the total cell population responded to surface Ig crosslinking, Ramos cells were subcloned by using limiting dilution, and individual clonal lines were reanalyzed by using immunofluorescent flow cytometry for Btk phosphorylation with 551PYmAb. Clonal lines had significantly different reponses to BCR crosslinking, with some clones demonstrating almost no positive cells and other clones demonstrating a high percentage (>50%) of 551PYmAb positive staining (data not shown).
Btk activation varied significantly in response to BCR stimulation in a population of cultured B cells, even though the cells responded to receptor activation with increased global protein tyrosine phosphorylation. This result suggests that transduction of an intracellular signal from receptor to Btk is critically dependent on intrinsic cell features, such as cell cycle stage and/or the level of other signaling proteins (e.g., PI 3-kinase, Src kinases, lipid and protein phosphatases, docking proteins, etc). Analysis with 551PYmAb and 223PYmAb could potentially identify individual cells within complex lymphoid populations where Btk is actively signaling. For instance, marrow, spleen, and lymph node B cell populations could be analyzed to identify developmental stages with high levels of activated Btk before or after an immune stimulus or cytokine treatment.
Subcellular Location of Activated Btk Molecules.
Aggregation of BCR molecules and associated transducing proteins forms a macromolecular signaling complex with high levels of protein tyrosine phosphorylation, high affinity binding interactions, and activation of critical signal-transducing enzymes. Genetic, biochemical, and structural evidence indicate that Btk signaling corresponds with plasma membrane association, but no direct evidence exists for the presence of Btk molecules in the BCR signaling complex.
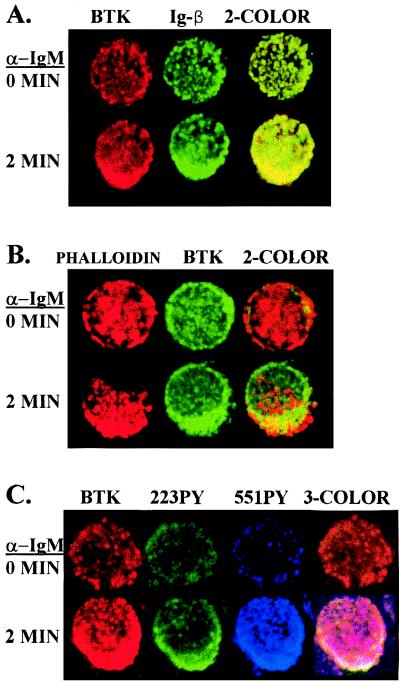
To test the hypothesis that Btk molecules associate with the BCR signaling complex, cells were stained with anti-Btk NT and anti-Igβ before and after receptor crosslinking. Analysis of representative cells with fluorescence confocal microscopy demonstrated colocalization of both Btk and Igβ in a focal region within the stimulated cells compared with a diffuse distribution of these proteins in the control cells, as depicted in Fig. 3A. Aggregation of cell surface receptors after crosslinking also corresponds with a restructuring of cytoskeletal components, such as actin, in the cytosolic region beneath the receptor complex. Control and BCR-crosslinked cells were stained with the actin probe phalloidin and anti-Btk NT. Focal actin accumulation in a juxtamembrane location and colocalization with Btk can be observed in stimulated cells as demonstrated in Fig. 3B. To determine the subcellular location of activated Btk molecules, control and stimulated Ramos B cells were stained with anti-Btk NT antibody, 551PYmAb, and 223PYmAb. Simultaneous three-channel fluorescence analysis revealed focal accumulation of activated Btk molecules after BCR crosslinking, as depicted in Fig. 3C for a representative cell. These data demonstrate Btk molecules associate with the BCR signaling complex at a time point of maximal Btk phosphorylation, suggesting that Btk signaling is spatially constrained to the region of the receptor signaling complex.
Figure 3.
Activated Btk molecules colocalize with the aggregated BCR. Cells were treated without or with anti-IgM for 2 min, fixed, permeabilized, and stained as indicated. In A, cells were stained with anti-Btk NT (secondary reagent Texas Red-labeled donkey anti-rabbit IgG) and anti-human Igβ (secondary reagent fluorescein isothiocyanate-labeled rat anti-mouse IgG1). In B, cells were stained with anti-Btk NT (secondary reagent fluorescein isothiocyanate-labeled donkey anti-rabbit IgG) and Texas Red X-labeled phalloidin. In C, cells were stained with anti-Btk NT (secondary reagent Texas Red-labeled donkey anti-rabbit IgG), 55IPYmAb (secondary reagent Cy5-labeled goat anti-mouse IgG2b), and 223PYmAb (secondary reagent fluorescein isothiocyanate-labeled rat anti-mouse IgG1). Confocal microscopic analysis was used to visualize Btk and the other proteins in representative cells.
In situ confocal microscopic analysis of Btk signaling could potentially identify dynamic changes in the subcellular localization of activated Btk molecules in relation to other signaling molecules. Immunofluorescence imaging of spleen or lymph node tissue sections with 551PYmAb and 223PYmAb may reveal the spatial distribution of germinal center B cells containing activated Btk, changes in the distribution of these cells after antigen stimulation, and the relationship of Btk signaling to other critical events such as antigen presentation, recombination, proliferation, or apoptosis.
PI 3-Kinase Activity in Btk Activation.
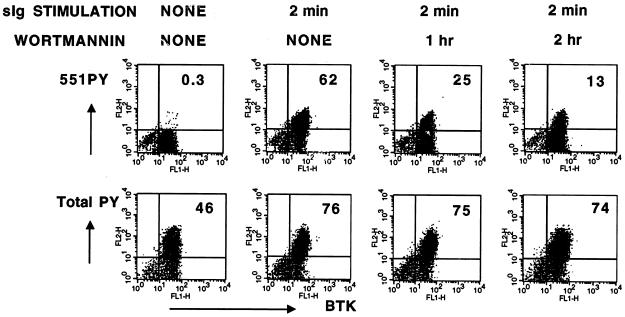
Inhibition of PI 3-kinase with wortmannin decreases the capacity of BCR to signal to downstream targets, including Btk, by blocking formation of the plasma membrane lipid phosphatidylinositol 3,4,5-trisphoshpate (1, 2, 27, 34, 40, 41). To test the influence of PI 3-kinase activity in receptor-dependent Btk activation as measured with in situ immunofluorescence analysis, Ramos B cells were incubated with wortmannin for increasing periods of time before crosslinking of BCR and fixed and stained with either 551PYmAb or a general phosphotyrosine antibody. Wortmannin treatment (10nM) induced a time-dependent decrease in BCR-induced Btk activation from ≈62% positive cells to ≈13% (Fig. 4). In contrast, BCR-crosslinked cells had similar elevated levels of total phosphotyrosine content detected with the 4G10 antibody, regardless of the duration of wortmannin treatment. This data reveals the capacity of the 551PYmAb to detect a selective decrease in receptor activation of Btk resulting from inhibition of an important upstream signaling pathway.
Figure 4.
Selective inhibition of BCR-dependent Btk activation by the PI 3-kinase inhibitor wortmannin. Ramos B cells, grown as described above, were incubated in serum-free RPMI at 1 × 107 cells per ml for 2 hr at 37°C in the absence or presence of 10nM wortmannin or 0.1% DMSO for the indicated times. Goat F(ab′)2 anti-human IgM (10 μg/ml) was added for 2 min at 37°C. Cells were fixed, permeabilized, and stained with anti-Btk NT and either 551PYmAb or 4G10. The primary antibodies were detected with fluorescein isothiocyanate-labeled donkey anti-rabbit IgG and R-phycoerythrin-labeled donkey anti-mouse IgG. Two-color fluorescence analysis was performed and the data plotted in 2-dimensional formats (Upper, 551PYmAb vs. anti-Btk NT; Lower, 4G10 vs. anti-Btk NT).
551PYmAb and 223PYmAb should be useful in demonstrating changes in Btk signaling in relation to the function of other intracellular signaling pathways. By modulation of signaling pathways using costimulation of multiple receptors and/or pharmacological agonists or inhibitors, pleiotropy in cell activation of Btk could be evaluated in natural populations or cell lines to evaluate synergy or antagonism in signal transduction.
Because natural cell populations in the immune system are heterogeneous mixtures of cells with varying potential based on intrinsic (e.g., developmental stage) or extrinsic (e.g., presence of multiple receptor ligands) factors, it is critical to define cell-specific and pathway-specific responses to immune stimuli. The phosphopeptide-specific antibodies recognizing regulatory phosphorylated Btk tyrosine residues enable in situ immunofluorescent analysis of Btk signaling in developmental, spatial, and kinetic contexts. In situ detection of activated Btk should have research and clinical diagnostic utility in the evaluation of Btk function in immunodeficiency and autoimmunity syndromes and possibly in lymphoid or myeloid leukemias.
Acknowledgments
We thank Dr. Susan Ou for help in generation of hybridomas, Dr. Chris Turck for synthesis of peptides, J. Johnson for technical assistance, and J.C. for help in the preparation of this manuscript. M.I.W. is supported by the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation (Fellowship DRG-086). S.N. is an Associate of the Howard Hughes Medical Institute. This work was supported by Grant CA12800, Immune Functions and Cancer (Randy Wall, Principal Investigator) from the National Cancer Institute. D.J.R. is a recipient of the McDonnell Scholar Award. O.N.W. is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- BCR
B cell receptor
- Btk
Bruton’s tyrosine kinase
- Cy5
indodicarbocyanine
- PI 3-kinase
phosphatidylinositol 3-kinase
- xid
X linked immunodeficiency
- XLA
X linked agammaglobulinemia
References
- 1.Fluckiger A C, Li Z, Kato R M, Wahl M I, Ochs H D, Longnecker R, Kinet J P, Witte O N, Scharenberg A M, Rawlings D J. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawlings D J, Kinet J P. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takata M, Kurosaki T. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigley K P, Harnett M M, Phillips R J, Klaus G G B. Eur J Immunol. 1989;19:2081–2086. doi: 10.1002/eji.1830191117. [DOI] [PubMed] [Google Scholar]
- 5.Satterthwaite A B, Li Z, Witte O N. Semin Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- 6.Thomas J D, Sideras P, Smith C I E, Vorechovsky I, Chapman V, Paul W E. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 7.Kurosaki T. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 8.Aoki Y, Isselbacher K J, Pillai S. Proc Natl Acad Sci USA. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Weers M, Brouns G S, Hinshelwood S, Kinnon C, Schuurman R K B, Hendriks R W, Borst J. J Biol Chem. 1994;269:23857–23860. [PubMed] [Google Scholar]
- 10.Santos-Argumedo L, Lund F E, Heath A W, Solvason N, Wu W W, Grimaldi J C, Parkhouse R M E, Howard M. Int Immunol. 1995;7:163–170. doi: 10.1093/intimm/7.2.163. [DOI] [PubMed] [Google Scholar]
- 11.Hasbold J, Klaus G G B. Eur J Immunol. 1994;24:152–157. doi: 10.1002/eji.1830240123. [DOI] [PubMed] [Google Scholar]
- 12.Koike M, Kikuchi Y, Tominaga A, Takaki S, Akagi K, Miyazaki J I-I, Yamamura K I-I, Takatsu K. Int Immunol. 1995;1:21–30. doi: 10.1093/intimm/7.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Go N F, Castle B E, Barret R, Kastelein R, Dang W, Mosmann T R, Moore K W, Howard M. J Exp Med. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hata D, Kawakami Y, Inagaki N, Lantz C S, Kitamura T, Khan W N, Maeda-Yamamoto M, Miura T, Han W, Hartman S E, et al. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quek L S, Bolen J, Watson S P. Curr Biol. 1998;8:1137–1140. doi: 10.1016/s0960-9822(98)70471-3. [DOI] [PubMed] [Google Scholar]
- 16.Satterthwaite A B, Cheroutre H, Khan W N, Sideras P, Witte O N. Proc Natl Acad Sci USA. 1997;94:13152–13157. doi: 10.1073/pnas.94.24.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingjan G M, Maas A, Nawijn M C, Smit L, Voerman J S, Grosveld F, Hendriks R W. EMBO J. 1998;17:5309–5320. doi: 10.1093/emboj/17.18.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurosaki T, Kurosaki M. J Biol Chem. 1997;272:15595–15598. doi: 10.1074/jbc.272.25.15595. [DOI] [PubMed] [Google Scholar]
- 19.Rawlings D J, Scharenberg A M, Park H, Wahl M I, Lin S, Kato R M, Fluckiger A C, Witte O N, Kinet J P. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 20.Mahajan S, Fargnoli J, Burkhardt A L, Kut S A, Saouaf S J, Bolen J B. Mol Cell Biol. 1995;15:5304–5311. doi: 10.1128/mcb.15.10.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahl M I, Fluckiger A-C, Kato R M, Park H, Witte O N, Rawlings D J. Proc Natl Acad Sci USA. 1997;94:11526–11533. doi: 10.1073/pnas.94.21.11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, Wahl M I, Afar D E, Turck C W, Rawlings D J, Tam C, Scharenberg A M, Kinet J-P, Witte O N. Immunity. 1996;4:515–525. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 23.Takeshita H, Taniuchi I, Kato J, Watanabe T. Int Immunol. 1998;10:435–444. doi: 10.1093/intimm/10.4.435. [DOI] [PubMed] [Google Scholar]
- 24.Satterthwaite A B, Lowell C A, Khan W N, Sideras P, Alt F W, Witte O N. J Exp Med. 1998;188:833–844. doi: 10.1084/jem.188.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roulier E M, Panzer S, Beckendorf S K. Mol Cell. 1998;1:819–829. doi: 10.1016/s1097-2765(00)80081-7. [DOI] [PubMed] [Google Scholar]
- 26.Guarnieri D J, Dodson G S, Simon M A. Mol Cell. 1998;1:831–840. doi: 10.1016/s1097-2765(00)80082-9. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Wahl M I, Eguinoa A, Stephens L R, Hawkins P T, Witte O N. Proc Natl Acad Sci USA. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao L, Kawakami Y, Kawakami T. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 30.Tsukada S, Simon M, Witte O, Katz A. Proc Natl Acad Sci USA. 1994;91:11256–11260. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langhans-Rajasekaran S A, Wan Y, Huang X-Y. Proc Natl Acad Sci USA. 1995;92:8601–8605. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bence K, Ma W, Kozasa T, Huang X-Y. Nature (London) 1997;389:296–299. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Tsukada S, Satterthwaite A, Havlik M H, Park H, Takatsu K, Witte O N. Immunity. 1995;2:451–460. doi: 10.1016/1074-7613(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 34.Bolland S, Pearse R N, Kurosaki T, Ravetch J V. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 35.Rameh L E, Arvidsson A-k, Carraway K L, III, Couvillon A D, Rathbun G, Crompton A, VanRenterghem B, Czech M P, Ravichandran K S, Burakoff S J, et al. J Biol Chem. 1997;272:22059–22066. doi: 10.1074/jbc.272.35.22059. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda M, Kojima T, Kabayama H, Mikoshiba K. J Biol Chem. 1996;271:30303–30306. doi: 10.1074/jbc.271.48.30303. [DOI] [PubMed] [Google Scholar]
- 37.Salim K, Bottomley M J, Querfurth E, Zvelebil M J, Gout I, Scaife R, Margolis R L, Gigg R, Smith C I E, Driscoll P C, et al. Oncogene. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 38.Li T, Rawlings D J, Park H, Kato R M, Witte O N, Satterthwaite A B. Oncogene. 1997;15:1375–1383. doi: 10.1038/sj.onc.1201308. [DOI] [PubMed] [Google Scholar]
- 39.Smith C I E, Baskin B, Humire-Greiff P, Zhou J-N, Olsson P G, Maniar H S, Kjellen P, Lambris J D, Christensson B, Hammarstrom L, et al. J Immunol. 1994;152:557–565. [PubMed] [Google Scholar]
- 40.Powis G, Bonjouklian R, Berggren M M, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter W F, Dodge J, Grindey G, et al. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 41.Beckwith M, Fenton R G, Katona I M, Longo D L. Blood. 1996;87:202–210. [PubMed] [Google Scholar]