Abstract
Objective: To investigate the mechanism of carbapenem resistance and the occurrence of plasmid-mediated quinolone resistance determinants qnr and aac(6′)-Ib-cr in a clinical isolate of Enterobacter cloacae. Methods: An ertapenem-resistant E. cloacae ZY106, which was isolated from liquor puris of a female gastric cancer patient in a Chinese hospital, was investigated. Antibiotic susceptibilities were determined by agar dilution method. Conjugation experiments, isoelectric focusing, polymerase chain reaction (PCR), and DNA sequence analyses of plasmid-mediated carbapenemases and quinolone resistance determinants were preformed to confirm the genotype. Outer membrane proteins (OMPs) were examined by urea-sodium dodecyl sulfatepolyacrylamide gel electrophoresis (Urea-SDS-PAGE). Results: Minimum inhibitory concentrations (MICs) of imipenem, meropenem, and ertapenem for ZY106 were 2, 4, and 16 μg/ml, respectively. Conjugation studies with Escherichia coli resulted in the transfer of significantly reduced carbapenem susceptibility. ZY106 produced IMP-1 metallo-β-lactamase and CTX-M-3 extended-spectrum β-lactamase, and E. coli transconjugant produced IMP-1. Plasmid-mediated quinolone resistance determinant qnrS1 was detected in ZY106. Transfer of the qnrS1-encoding-plasmid into E. coli by conjugation resulted in intermediate resistance to ciprofloxacin in E. coli transconjugant. Urea-SDS-PAGE analysis of OMPs showed that ZY106 lacked an OMP of approximately 38 kDa. Conclusion: It is the first IMP-1-producing Enterobacteriaceae in China and the first report of a clinical isolate that harbors both blaIMP and qnrS genes as well. The blaIMP-1, blaCTX-M-3, and qnrS1 are encoded at three different plasmids. IMP-1 combined with the loss of an OMP possibly resulted in ertapenem resistance and reduced imipenem and meropenem susceptibility in E. cloacae.
Keywords: Antibiotic resistance, Carbapenem, Enterobacteriaceae, Outer membrane proteins (OMPs)
INTRODUCTION
Carbapenems are commonly used to treat serious infections caused by multi-resistant gram-negative bacilli, especially strains producing high-level of AmpC cephalosporinases or extended-spectrum β-lactamases (ESBLs) (Baldwin et al., 2008). However, the emergence of carbapenemase is becoming a therapeutic challenge. Carbapenemases involved in acquired resistance are of Ambler molecular classes A, B, and D (Walsh, 2008). The class B enzymes, metallo-β-lactamases (MBLs), are the most clinically threatening carbapenemases, as these enzymes are capable of hydrolyzing all β-lactams except monobactams, and are not susceptible to therapeutic β-lactamase inhibitors such as clavulanate, sulbactam, and tazobactam.
The most commonly acquired MBL families include the IMP, Verona integron-encoded metallo-β-lactamase (VIM), Sao Paulo metallo-β-lactamase (SPM), German imipenemase (GIM), and Seoul imipenemase (SIM). SPM, GIM, and SIM MBLs were detected in some specific regions of origins, while IMP and VIM, which are becoming common in Pseudomonas aeruginosa and other nonfermenting gram-negative bacteria, spread worldwide. IMP-1 was first detected in a P. aeruginosa isolate in Japan in 1990 (Watanabe et al., 1991). Soon afterward, IMP-1 was prevalent in Serratia marcescens and spread into several members of the family Enterobacteriaceae in Japan (Walsh et al., 2005; Queenan and Bush, 2007). There has been an increase in the detection and spread of the acquired MBLs in Enterobacteriaceae from Australia and Taiwan region of China, and IMP-4 and IMP-8 were the predominant MBLs (Walsh et al., 2005; Queenan and Bush, 2007; Peleg et al., 2005; Herbert et al., 2007; Wu et al., 2007; 2008; Liu et al., 2008). MBLs in Enterobacteriaceae have occurred sporadically in other countries. IMP-1 has been reported in Klebsiella pneumoniae from Singapore (Koh et al., 1999), Brazil (Lincopan et al., 2005; 2006), Turkey (Aktas et al., 2006), and the UK (Woodford et al., 2007), in Enterobacter cloacae from Turkey (Deshpande et al., 2006), and in Enterobacter aerogenes from France (Biendo et al., 2008). In China, there were only two reports on the production of IMP in Enterobacteriaceae. An IMP-4-producing Citrobacter youngae was isolated from Guangzhou in 2001 (Hawkey et al., 2001) and an IMP-4-producing K. pneumoniae from Wuhan in 2008 (Mendes et al., 2008).
In this study, an ertapenem-resistant E. cloacae strain was collected from our hospital and its mechanisms of carbapenem resistance were analyzed. It is the first detection of IMP-1 in Enterobacteriaceae from China. A further study revealed that this E. cloacae strain lacked an outer membrane protein (OMP) and harbored a plasmid-mediated quinolone resistance determinant qnrS1.
MATERIALS AND METHODS
Bacterial strains
In August 2007, an E. cloacae strain (ZY106) was isolated from a purulent exudate sample from abdominal cavity of a patient in oncology ward in the Second Affiliated Hospital of Zhejiang University, China. The patient was diagnosed as gastric cancer and received surgery. During hospitalization, the patient was treated with cefoperazone/sulbactam and ciprofloxacin before the isolation of the ertapenem-resistant E. cloacae strain.
Antimicrobial susceptibility testing
Minimum inhibitory concentrations (MICs) were determined using the agar dilution method according to Clinical and Laboratory Standards Institute (2007) recommendations.
Conjugation experiments
Conjugal transfer experiments were carried out in mixed broth cultures (Zhang et al., 2008). Rifampin-resistant Escherichia coli EC600 and streptomycin-resistant E. coli C600 were used as recipients. IMP-producing E. coli transconjugants were selected on Muller-Hinton agar plates containing 400 μg/ml rifampin (or 400 μg/ml streptomycin) and 8 μg/ml ceftazidime, and qnr positive E. coli transconjugants were selected on plates containing 400 μg/ml rifampin (or 400 μg/ml streptomycin) and 0.25 μg/ml ciprofloxacin. The selected colonies were picked up and identified by Vitek system (bioMérieux, Hazelwood, MO, USA). Plasmids from E. cloacae ZY106 and E. coli transconjugants were extracted using AxyPrep Plasmid Miniprep Kit (Axygen Scientific, Union City, CA, USA) and examined by electrophoresis.
Isoelectric focusing (IEF) of β-lactamase
Crude β-lactamase preparations were obtained by ultrasonic method. IEF was carried out on PhastGel polyacrylamide gel (pH 3 to 9, Amersham Biosciences, Uppsala, Sweden) using the PhastSystem (Pharmacia Biotech, Uppsala, Sweden) by the method of Mathew et al.(1975). β-lactamase activity was visualized by staining the gel with Nitrocefin (Oxoid, Basingstoke, Hampshire, England). The isoelectric points (pIs) were determined as described previously (Zhang et al., 2008).
Polymerase chain reaction (PCR) amplification and DNA sequence analysis of bla and plasmid-mediated quinolone resistance genes
Plasmids from E. cloacae ZY106 and E. coli transconjugants were used as template. The primers used to amplify bla TEM, bla SHV, bla CTX-M (Yu et al., 2007), bla IMP-1, bla IMP-2 (Shibata et al., 2003), qnrA, qnrB, qnrS (Robicsek et al., 2006), and aac(6′)-Ib (Jiang et al., 2008) were described previously. Prepared bacterial DNA of the ZY106 was used as the template for PCR amplifications of gyrA, parC, and gene of chromosomal cephalosporinase (Deguchi et al., 1997; Conceição et al., 2004). The reaction was conducted in a GeneAmp PCR System 9600 thermal cycler (Applied Biosystems, Foster City, CA, USA). PCR amplification products were purified and sequenced using an ABI3730 Sequencer (Applied Biosystems). We determined partial sequences of the gyrA and parC genes of the ZY106, including the regions analogous to the quinolone resistance-determining region of the E. cloacae ATCC13047 gyrA and parC genes and the deduced amino acid sequences of the ZY106 gyrA and parC genes were compared with the corresponding regions of the E. cloacae ATCC13047 GyrA and ParC proteins. Detection of KPC-2 of the ZY106 was conducted according to our previous report (Zhang et al., 2008).
Analysis of outer membrane proteins (OMPs)
OMPs of E. cloacae ZY106 and E. cloacae ATCC13047 were isolated and separated on 11.6%(w/v) acrylamide-0.4% (w/v) bisacrylamide-0.1% (w/v) sodium dodecyl sulfate gel containing 20% (w/v) urea (urea-sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Urea-SDS-PAGE) as described previously (Zhang et al., 2008).
RESULTS
Antibiotics susceptibility
E. cloacae ZY106 showed reduced susceptibility to imipenem and meropenem with MICs of 2 and 4 μg/ml, and resistance to ertapenem with MIC of 16 μg/ml. The isolate was highly resistant to β-lactams except piperacillin/tazobactam and aztreonam. MICs of carbapenems significantly decreased (≥4-fold) in the presence of ethylenediaminetetraacetic acid (EDTA) (a constant concentration of 320 μg/ml) (Table 1). These results suggest the production of MBL. Similarly, MIC of ceftazidime was reduced from >256 μg/ml to 1 μg/ml, and that of cefotaxime was reduced from 256 μg/ml to 64 μg/ml, suggesting the production of CTX-M ESBL. E. cloacae ZY106 was also resistant to quinolones and aminoglycosides.
Table 1.
Antimicrobial susceptibility results of E. cloacae ZY106 and E. coli transconjugants
| Antimicrobial agents |
MIC (μg/ml) |
|||||
| E. cloacae ZY106 | E. coli C600 | Transconjugant A | E. coli EC600 | Transconjugant B1 | Transconjugant B2 | |
| Imipenem | 2 | ≤0.0625 | 0.5 | 0.125 | 0.125 | 0.125 |
| Imipenem+EDTA* | 0.5 | ≤0.0625 | ≤0.0625 | 0.125 | ||
| Meropenem | 4 | ≤0.0625 | 0.25 | ≤0.0625 | ≤0.0625 | ≤0.0625 |
| Meropenem+EDTA | 0.25 | ≤0.0625 | ≤0.0625 | ≤0.0625 | ||
| Ertapenem | 16 | ≤0.0625 | 0.5 | ≤0.0625 | ≤0.0625 | ≤0.0625 |
| Ertapenem+EDTA | 0.5 | ≤0.0625 | ≤0.0625 | ≤0.0625 | ||
| Ceftazidime | >256 | ≤0.125 | 256 | ≤0.125 | ≤0.125 | ≤0.125 |
| Ceftazidime+EDTA | 1 | ≤0.125 | ≤0.125 | ≤0.125 | ||
| Cefotaxime | 256 | ≤0.125 | 32 | ≤0.125 | ≤0.125 | ≤0.125 |
| Cefotaxime+EDTA | 64 | ≤0.125 | 0.25 | ≤0.125 | ||
| Cefepime | 128 | ≤0.125 | 8 | ≤0.125 | ≤0.125 | ≤0.125 |
| Cefoperazone/sulbactam | >256 | ≤0.125 | 128 | ≤0.125 | ≤0.125 | ≤0.125 |
| Ampicillin | >256 | 1 | 128 | 8 | 8 | 8 |
| Piperacillin | 256 | 0.25 | 4 | 1 | 1 | 1 |
| Piperacillin/tazobactam | 16 | 0.25 | 2 | 1 | 1 | 1 |
| Cefoxitin | >256 | >256 | 4 | 4 | 4 | |
| Aztreonam | 32 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 |
| Ciprofloxacin | >32 | ≤0.125 | ≤0.125 | ≤0.125 | 2 | 2 |
| Gentamicin | 16 | 0.5 | 0.5 | 0.5 | 1 | 16 |
| Amikacin | 16 | 0.5 | 0.5 | 0.5 | 1 | 16 |
The final concentration of EDTA was fixed at 320 μg/ml
Transfer of antibiotics resistance and plasmid analysis
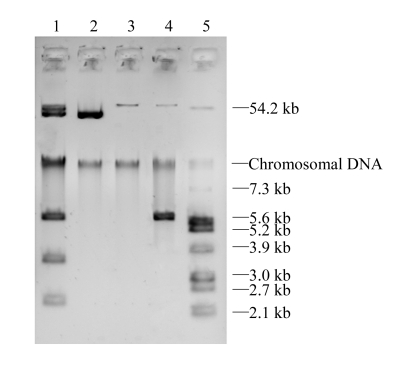
E. cloacae ZY106 failed to transfer carbapenem resistance to E. coli EC600 by conjugation, but it was successful for E. coli C600. The bacteria grew on plates containing streptomycin and ceftazidime were named as E. coli transconjugant A. The plasmid profiles indicated that ZY106 harbored several plasmids and E. coli transconjugant A acquired a plasmid with a size of approximately 40 kb (Fig.1). Transfer of ciprofloxacin resistance from E. cloacae ZY106 to E. coli EC600 was successful. Two different E. coli transconjugants were obtained. The one with a 60-kb plasmid was named E. coli transconjugant B1, and the other with an extra 6-kb plasmid was named E. coli transconjugant B2.
Fig. 1.
Plasmid profiles of E. cloacae ZY106 and E. coli transconjugants. Lane 1: E. cloacae ZY106; Lanes 2~4: E. coli transconjugants A, B1 and B2; Lane 5: E. coli V517
As shown in Table 1, MICs of imipenem, meropenem, and ertapenem for E. coli transconjugant A were 0.5, 0.25, and 0.5 μg/ml, respectively, which increased at least 4-fold compared with those for E. coli C600 (MICs of ≤0.0625 μg/ml), although E. coli transconjugant A remained susceptible to carbapenems according to the Clinical and Laboratory Standards Institute (CLSI) breakpoints. E. coli transconjugant A was resistant to several β-lactams but was susceptible to piperacillin, piperacillin/tazobactam, and aztreonam. E. coli transconjugant A had a dramatic decrease in the MICs of carbapenems and cephalosporins in the presence of EDTA. E. coli transconjugant B1 was intermediately resistant to ciprofloxacin (MIC of 2 μg/ml) and was susceptible to other tested antibiotics. Conjugation experiments and antimicrobial susceptibility results of E. coli transconjugant B suggest the presence of plasmid-mediated quinolone resistance determinants. The antimicrobial susceptibility patterns of E. coli transconjugant B2 were similar to those of B1, except its resistance to aminoglycosides.
IEF analysis
IEF analysis demonstrated that both E. cloacae ZY106 and E. coli transconjugant A had a band with β-lactamase activity with a pI of approximately 9.0 (Fig.2).
Fig. 2.
Isoelectric focusing patterns of crude β-lactamase extracts from E. cloacae ZY106 and E. coli transconjugant A. Lane 1:, E. cloacae ZY106; Lane 2: E. coli transconjugant A; Lane 3: strains producing TEM-28 (pI of 6.1), SHV-7 (pI of 7.6) and ACT-1 (pI of 9.0)
PCRs and DNA sequence analysis
The above-mentioned antibiotic resistance genes were analyzed by PCR and DNA sequencing. E. cloacae ZY106 was positive for bla IMP-1, bla CTX-M, qnrS, aac(6′)-Ib genes and chromosomal AmpC lactamase gene, while E. coli transconjugant A was only positive for the bla IMP-1 gene, B1 was positive for the qnrS gene, and B2 was positive for qnrS and aac(6′)-Ib genes. DNA sequencing results identify the genes as bla IMP-1, bla CTX-M-3, qnrS1, and aac(6′)-Ib. These results indicate that the four genes were carried on separated plasmids. The sizes of plasmids encoding IMP-1, qnrS1, and aac(6′)-Ib were approximately 40, 60, and 6 kb, respectively, and CTX-M-3 was presumed to be encoded on a 50-kb plasmid (Fig.1). AAC(6′)-Ib-cr, a variant aminoglycoside acetyltransferase capable of modifying ciprofloxacin and reducing its activity, was not found. The aac(6′)-Ib conferred the resistance to gentamicin and amikacin but did not elevate the ciprofloxacin MIC for E. coli transconjugant B2 compared with that for B1. Although E. cloacae ZY106 produced two β-lactamases, only one band with β-lactamase activity was observed in IEF. It was because that IMP-1 and CTX-M-3 had an overlapping band at the same pI of approximately 9.0 (Baldwin et al., 2008; Jeong et al., 2005).
Since the KPC-2 carbapenemase was often detected in carbapenem-resistant Enterobacteriaceae from our hospital (Zhang et al., 2007; 2008; Cai et al., 2008), we had also analyzed the KPC-2 carbapenemase in ZY106. However, we confirmed that this isolate did not produce KPC-2 carbapenemase.
Detection of mutations in gyrA and parC genes
As shown in Table 1, transconjugants B1 and B2 exhibited rather high ciprofloxacin MICs. Usually transconjugants containing qnrS determinants only show ciprofloxacin MICs of less than 0.5 mg/L. To investigate whether other determinants had been contributing to the high ciprofloxacin resistance, we analyzed the partial sequences of the gyrA and parC genes of ZY106. ZY106 had two mutations generating Ser(S)83→Phe(F) and Asp(D)87→Ala(A) changes in GyrA and a Ser(S)83→Ile(I) mutation in ParC (data not shown). Therefore, the resistance to ciprofloxacin of ZY106 is attributed to the mutations in gyrA and parC in the quinolone resistance-determining region (Deguchi et al., 1997).
Urea-SDS-PAGE analysis of OMPs
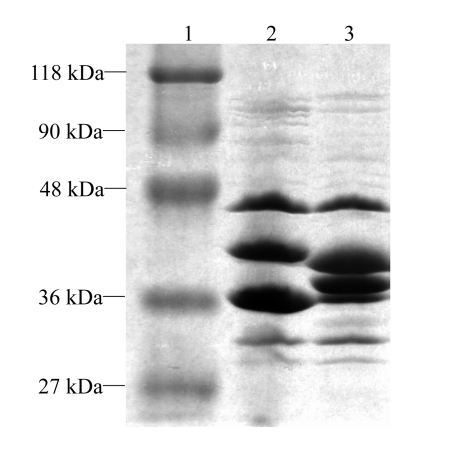
Urea-SDS-PAGE analysis showed that E. cloacae ATCC13047 expressed four major OMPs, with molecular weights of 47, 41, 38, and 36 kDa. The four OMPs were analogous to E. coli LamB, OmpC, OmpF, and OmpA, respectively (Szabó et al., 2006). The 41- and 38-kDa proteins were considered to be porins, and the later was absent in E. cloacae ZY106 (Fig.3).
Fig. 3.
Urea-SDS-PAGE analysis of outer membrane proteins (OMPs) of E. cloacae ZY106. Lane 1: protein molecular weight standard (MBI Fermentas); Lane 2: E. cloacae ZY106; Lane 3: E. cloacae ATCC13047
DISCUSSION
IMP-type MBLs were most frequently detected among gram-negative nonfermenters. IMP-producing Enterobacteriaceae were mainly reported from Japan, Australia, and Taiwan region of China. Production of IMP in Enterobacteriaceae was rare in mainland China, including IMP-4 in C. youngae and K. pneumoniae (Hawkey et al., 2001; Mendes et al., 2008). In this study, we detected bla IMP-1 and qnrS1 genes along with alternations in gyrA and parC in a clinical isolate of ertapenem-resistant E. cloacae. To our knowledge, no other Enterobacteriaceae harboring such genes were recovered so far. In addition, further studies revealed the loss of an OMP, which enhanced the carbapenem resistance in this isolate. However, for E. cloacae ZY106, the MIC of ertapenem (16 μg/ml) was much higher than those of imipenem (2 μg/ml) and meropenem (4 μg/ml). E. coli transconjugant A that produced IMP-1 exhibited significantly reduced susceptibility to carbapenems (MICs of 0.25 to 0.5 μg/ml). However, it was not sufficient to explain the relatively high carbapenem resistance in E. cloacae ZY106 with imipenem MIC of 2 μg/ml, meropenem MIC of 4 μg/ml, even ertapenem MIC of 16 μg/ml. Therefore, other mechanisms such as deficiency in the OMPs (Bush et al., 1985; Lee et al., 1991; 1992) and existence of efflux pump might also be contributing to the ertapenam resistance (Szabó et al., 2006), although the efflux mechanism is not common in the Enterobacteriaceae.
The Urea-SDS-PAGE analysis of OMPs proved that ZY106 lacked the OMP (38 kD), which suggests that at least reduced outer membrane permeability might be involved. However, it requires further investigation to prove whether the efflux mechanism was also involved in the ertapenam resistance in ZY106. These results suggest that production of IMP combined with the OMP deficiency and possibly efflux mechanism had more effect on susceptibility to ertapenem than on that to imipenem and meropenem in E. cloacae, and that ertapenem may be the preferred antibiotic for screening such isolate.
IMP alone does not confer high-level carbapenem resistance in Enterobacteriaceae, and it might spread without attracting attention. Microbiologists should be aware of suspicious gram-negative bacteria with reduced susceptibility to carbapenems, and examine their carbapenemase activity.
References
- 1.Aktas Z, Bal C, Midilli K, Poirel L, Nordmann P. First IMP-1-producing Klebsiella pneumoniae isolate in Turkey. Clin Microbiol Infect. 2006;12(7):695–696. doi: 10.1111/j.1469-0691.2006.01480.x. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs. 2008;68(6):803–838. doi: 10.2165/00003495-200868060-00006. [DOI] [PubMed] [Google Scholar]
- 3.Biendo M, Canarelli B, Thomas D, Rousseau F, Hamdad F, Adjide C, Laurans G, Eb F. Successive emergence of extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacter aerogenes isolates in a university hospital. J Clin Microbiol. 2008;46(3):1037–1144. doi: 10.1128/JCM.00197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Tanaka SK, Bonner DP, Sykes RB. Resistance caused by decreased penetration of beta-lactam antibiotics into Enterobacter cloacae . Antimicrob Agents Chemother. 1985;27(4):555–560. doi: 10.1128/aac.27.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai JC, Zhou HW, Zhang R, Chen GX. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob Agents Chemother. 2008;52(6):2014–2018. doi: 10.1128/AAC.01539-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Wayne, PA: CLSI; Clinical and Laboratory Standards Institute (CLSI) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, M7-A7. (17th Ed) 2007
- 7.Conceição T, Faria N, Lito L, Melo Cristino J, Salgado MJ, Duarte A. Diversity of chromosomal AmpC beta-lactamases from Enterobacter cloacae isolates in a Portuguese hospital. FEMS Microbiol Lett. 2004;230(2):197–202. doi: 10.1016/S0378-1097(03)00891-7. [DOI] [PubMed] [Google Scholar]
- 8.Deguchi T, Yasuda M, Nakano M, Ozeki S, Kanematsu E, Nishino Y, Ishihara S, Kawada Y. Detection of mutations in the gyrA and parC genes in quinolone-resistant clinical isolates of Enterobacter cloacae . J Antimicrob Chemother. 1997;40(4):543–549. doi: 10.1093/jac/40.4.543. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande LM, Jones RN, Fritsche TR, Sader HS. Occurrence and characterization of carbapenemase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program (2000-2004) Microb Drug Resist. 2006;12(4):223–230. doi: 10.1089/mdr.2006.12.223. [DOI] [PubMed] [Google Scholar]
- 10.Hawkey PM, Xiong J, Ye H, Li H, M′Zali FH. Occurrence of a new metallo-β-lactamase IMP-4 carried on a conjugative plasmid in Citrobacter youngae from the People’s Republic of China. FEMS Microbiol Lett. 2001;194(1):53–57. doi: 10.1016/S0378-1097(00)00506-1. [DOI] [PubMed] [Google Scholar]
- 11.Herbert S, Halvorsen DS, Leong T, Franklin C, Harrington G, Spelman D. Large outbreak of infection and colonization with gram-negative pathogens carrying the metallo-β-lactamase gene bla IMP-4 at a 320-bed tertiary hospital in Australia. Infect Control Hosp Epidemiol. 2007;28(1):98–101. doi: 10.1086/508841. [DOI] [PubMed] [Google Scholar]
- 12.Jeong SH, Bae IK, Kwon SB, Lee JH, Song JS, Jung HI, Sung KH, Jang SJ, Lee SH. Dissemination of transferable CTX-M-type extended-spectrum beta-lactamase-producing Escherichia coli in Korea. J Appl Microbiol. 2005;98(4):921–927. doi: 10.1111/j.1365-2672.2004.02526.x. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Zhou Z, Qian Y, Wei Z, Yu Y, Hu S, Li L. Plasmid-mediated quinolone resistance determinants qnr and aac(6′)-Ib-cr in extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in China. J Antimicrob Chemother. 2008;61(5):1003–1006. doi: 10.1093/jac/dkn063. [DOI] [PubMed] [Google Scholar]
- 14.Koh TH, Babini GS, Woodford N, Sng LH, Hall LM, Livermore DM. Carbapenem-hydrolysing IMP-1 β-lactamase in Klebsiella pneumoniae from Singapore. Lancet. 1999;353(9170):2162. doi: 10.1016/S0140-6736(05)75604-X. [DOI] [PubMed] [Google Scholar]
- 15.Lee EH, Nicolas MH, Kitzis MD, Pialoux G, Collatz E, Gutmann L. Association of two resistance mechanisms in a clinical isolate of Enterobacter cloacae with high-level resistance to imipenem. Antimicrob Agents Chemother. 1991;35(6):1093–1098. doi: 10.1128/aac.35.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EH, Collatz E, Trias J, Gutmann L. Diffusion of beta-lactam antibiotics into proteoliposomes reconstituted with outer membranes of isogenic imipenem-susceptible and -resistant strains of Enterobacter cloacae . J Gen Microbiol. 1992;138(11):2347–2351. doi: 10.1099/00221287-138-11-2347. [DOI] [PubMed] [Google Scholar]
- 17.Lincopan N, McCulloch JA, Reinert C, Cassettari VC, Gales AC, Mamizuka EM. First isolation of metallo-β-lactamase-producing multiresistant Klebsiella pneumoniae from a patient in Brazil. J Clin Microbiol. 2005;43(1):516–519. doi: 10.1128/JCM.43.1.516-519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lincopan N, Leis R, Vianello MA, de Araújo MR, Ruiz AS, Mamizuka EM. Enterobacteria producing extended-spectrum β-lactamases and IMP-1 metallo-β-lactamases isolated from Brazilian hospitals. J Med Microbiol. 2006;55(11):1611–1613. doi: 10.1099/jmm.0.46771-0. [DOI] [PubMed] [Google Scholar]
- 19.Liu YF, Yan JJ, Ko WC, Tsai SH, Wu JJ. Characterization of carbapenem-non-susceptible Escherichia coli isolates from a university hospital in Taiwan. J Antimicrob Chemother. 2008;61(5):1020–1023. doi: 10.1093/jac/dkn049. [DOI] [PubMed] [Google Scholar]
- 20.Mathew A, Harris AM, Marshall MJ, Ross GW. The use of analytical isoelectric focusing for detection and identification of β-lactamase. J Gen Microbiol. 1975;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 21.Mendes RE, Bell JM, Turnidge JD, Yang Q, Yu Y, Sun Z, Jones RN. Carbapenem-resistant isolates of Klebsiella pneumoniae in China and detection of a conjugative plasmid (bla KPC-2 plus qnrB4) and a bla IMP-4 gene. Antimicrob Agents Chemother. 2008;52(2):798–799. doi: 10.1128/AAC.01185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peleg AY, Franklin C, Bell JM, Spelman DW. Dissemination of the metallo-β-lactamase gene bla IMP-4 among gram-negative pathogens in a clinical setting in Australia. Clin Infect Dis. 2005;41(11):1549–1556. doi: 10.1086/497831. [DOI] [PubMed] [Google Scholar]
- 23.Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob Agents Chemother. 2006;50(8):2872–2874. doi: 10.1128/AAC.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata N, Doi Y, Yamane K, Yagi T, Kurokawa H, Shibayama K, Kato H, Kai K, Arakawa Y. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J Clin Microbiol. 2003;41(12):5407–5413. doi: 10.1128/JCM.41.12.5407-5413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabó D, Silveira F, Hujer AM, Bonomo RA, Hujer KM, Marsh JW, Bethel CR, Doi Y, Deeley K, Paterson DL. Outer membrane protein changes and efflux pump expression together may confer resistance to ertapenem in Enterobacter cloacae . Antimicrob Agents Chemother. 2006;50(8):2833–2835. doi: 10.1128/AAC.01591-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh TR. Clinically significant carbapenemases: an update. Curr Opin Infect Dis. 2008;21(4):367–371. doi: 10.1097/QCO.0b013e328303670b. [DOI] [PubMed] [Google Scholar]
- 28.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005;18(2):306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa . Antimicrob Agents Chemother. 1991;35(1):147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodford N, Dallow JW, Hill RL, Palepou MF, Pike R, Ward ME, Warner M, Livermore DM. Ertapenem resistance among Klebsiella and Enterobacter submitted in the UK to a reference laboratory. Int J Antimicrob Agents. 2007;29(4):456–459. doi: 10.1016/j.ijantimicag.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Wu JJ, Ko WC, Tsai SH, Yan JJ. Prevalence of plasmid-mediated quinolone resistance determinants QnrA, QnrB, and QnrS among clinical isolates of Enterobacter cloacae in a Taiwanese hospital. Antimicrob Agents Chemother. 2007;51(4):1223–1227. doi: 10.1016/j.ijantimicag.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu JJ, Ko WC, Wu HM, Yan JJ. Prevalence of Qnr determinants among bloodstream isolates of Escherichia coli and Klebsiella pneumoniae in a Taiwanese hospital. 1999-2005. J Antimicrob Chemother. 2008;61(6):1234–1239. doi: 10.1093/jac/dkn111. [DOI] [PubMed] [Google Scholar]
- 33.Yu YS, Ji SJ, Chen YG, Zhou WL, Wei ZQ, Li LJ, Ma YL. Resistance of strains producing extended-spectrum β-lactamases and genotype distribution in China. J Infect. 2007;54(1):53–57. doi: 10.1016/j.jinf.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Zhou HW, Cai JC, Chen GX. Plasmid-mediated carbapenem-hydrolysing β-lactamase KPC-2 in carbapenem-resistant Serratia marcescens isolates from Hangzhou, China. J Antimicrob Chemother. 2007;59(3):574–576. doi: 10.1093/jac/dkl541. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, Yang L, Cai JC, Zhou HW, Chen GX. High-level carbapenem resistance in a Citrobacter freundii clinical isolate is due to a combination of KPC-2 production and decreased porin expression. J Med Microbiol. 2008;57(Pt 3):332–337. doi: 10.1099/jmm.0.47576-0. [DOI] [PubMed] [Google Scholar]