Abstract
Bruton’s tyrosine kinase (Btk) plays pivotal roles in mast cell activation as well as in B cell development. Btk mutations lead to severe impairments in proinflammatory cytokine production induced by cross-linking of high-affinity IgE receptor on mast cells. By using an in vitro assay to measure the activity that blocks the interaction between protein kinase C and the pleckstrin homology domain of Btk, terreic acid (TA) was identified and characterized in this study. This quinone epoxide specifically inhibited the enzymatic activity of Btk in mast cells and cell-free assays. TA faithfully recapitulated the phenotypic defects of btk mutant mast cells in high-affinity IgE receptor-stimulated wild-type mast cells without affecting the enzymatic activities and expressions of many other signaling molecules, including those of protein kinase C. Therefore, this study confirmed the important roles of Btk in mast cell functions and showed the usefulness of TA in probing into the functions of Btk in mast cells and other immune cell systems. Another insight obtained from this study is that the screening method used to identify TA is a useful approach to finding more efficacious Btk inhibitors.
Cross-linking of high-affinity IgE receptor (FcɛRI) found predominantly on mast cells and basophils stimulates signaling cascades that lead to exocytosis of inflammatory mediators of the allergic response (1). FcɛRI consists of an IgE-binding α subunit, a β subunit with four transmembrane domains, and a disulfide-bonded pair of γ subunits (2). Similar to the signaling subunits of the T cell receptor and B cell receptor (BCR) systems, the β and γ subunits have immunoreceptor tyrosine-based activation motif (ITAM) sequences in their cytoplasmic portions (3). A β subunit-associated Src family protein-tyrosine kinase (PTK), Lyn, is activated on FcɛRI cross-linking (4) and phosphorylates tyrosine residues in the ITAM sequences. Phosphorylated ITAM sequences in β and γ subunits recruit Lyn and Syk, another PTK with two tandemly arranged Src homology 2 (SH2) domains, respectively, in a phosphotyrosine-SH2 interaction-dependent manner (5–8). Phospho-ITAM-bound Lyn and Syk then are activated and phosphorylate their target proteins, such as phospholipase C (PLC)-γ, Vav, HS-1, etc. Downstream of these early tyrosine phosphorylation events, activation of several signaling pathways follows: PLC activation leads to the activation of protein kinase C (PKC) and an increased [Ca2+]i, both of which are required for the optimal degranulation response (9). All three major subfamilies of mitogen-activated protein (MAP) kinases, i.e., ERKs, JNKs, and p38, also are activated to exert their functions in mast cell activation (10–15).
Bruton’s tyrosine kinase (Btk) belongs to the Tec subfamily of PTKs activated on FcɛRI cross-linking (16, 17). Btk is known to be mutated in human (X-linked agammaglobulinemia) and murine [X-linked immunodeficiency (xid)] inherited immunodeficiencies (18–21). Btk, which can be phosphorylated and activated by Lyn (22), recently was shown to be required for the full activation of mast cells. In particular, secretion of cytokines including tumor necrosis factor (TNF) α and interleukin (IL) 2 is severely defective in btk mutant mast cells (23). This defect is accounted for by the defective transcription of the cytokine genes, which is at least partly caused by an impairment of the JNK/c-Jun signaling pathway in these cells (24, 25). Thus, Btk regulates JNK whereas the activity of ERKs is largely independent of Btk (14).
In our previous studies, we demonstrated that Btk is physically associated with various isoforms of PKC through interactions between the N-terminal pleckstrin homology (PH) domain of Btk and the phorbol ester-binding C1 region of PKC (26, 27). Furthermore, PKC phosphorylates Btk and down-regulates the catalytic activity of the latter in mast cells (26). In search of inhibitors that block the interaction between PKC and the Btk PH domain, we have found a quinone epoxide antibiotic, terreic acid (TA), as an effective inhibitor. In the present study we have characterized TA as a selective inhibitor of Btk in mast cells and other immune cells.
MATERIALS AND METHODS
Antibodies.
Sources of commercial antibodies are as follows: antiphosphotyrosine mAb 4G10 from Upstate Biotechnology, Lake Placid, NY; anti-Btk (M-138), anti-Lyn (44), anti-Syk (C-20), anti-JNK1 (C-17), anti-PKC (MC5), anti-PKCβII (C-18), anti-ERK1 (C-16), and anti-p38 (C-20) from Santa Cruz Biotechnology; antiphospho-MAPK and antiphospho-p38 from New England Biolabs; and anti-mouse IgM F(ab′)2 from Southern Biotechnology Associates.
In Vitro PH Domain Binding Assay.
A human mast cell line, HMC-1 (28), was cultured in Iscove’s medium supplemented with 10% fetal bovine serum and 1.2 mM α-thioglycerol (Sigma). Nonidet P-40 lysates of HMC-1 cells were incubated with glutathione S-transferase (GST)-BtkPH beads (26) in the absence or presence of acetone extracts of actinomycetes or fungi. The Btk PH domain-bound PKC was detected by SDS/PAGE followed by immunoblotting with anti-PKC (MC5) that reacts with conventional PKC isoforms (α, βI, βII, and γ).
Purification of TA.
Fermented broths of a fungus sample dg2 were filtered. The filtrate adjusted to pH 2 with 1 N HCl was extracted twice with ethyl acetate. Dried extracts were purified by successive chromatography with silica gel (Merk) and Sephadex LH-20 columns (Amersham Pharmacia). The active fractions from the second column gave a pure sample of TA. The physical property of TA was confirmed by UV spectrum, 1H NMR spectrum, and 13C NMR spectrum.
Cells, Stimulation, and Biological Consequences.
Bone marrow cells from femur of CBA/J or 129/C57BL F2 mice were cultured in IL-3-containing RPMI medium 1640 as described (29). More than 95% pure mast cells were obtained after 4 weeks of culture. These cells were incubated overnight with dinitrophenyl-specific monoclonal IgE antibody and stimulated with a multivalent antigen, dinitrophenyl conjugates of human serum albumin for the indicated intervals. Cell suspensions were briefly centrifuged to separate culture media from cell pellets. Histamine, leukotriene, and cytokines secreted into culture media were measured as described (23, 29). Cell pellets were lysed in 1% Nonidet P-40 lysis buffer (30) and centrifuged, and supernatants were used for immunoprecipitation.
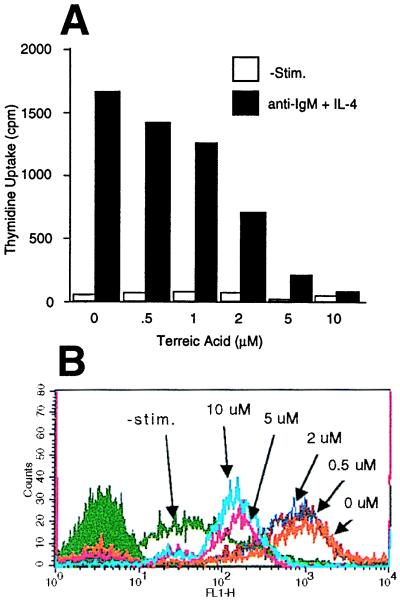
Spleen cells from BALB/c mice were cultured in RPMI medium 1640 supplemented with 10% fetal bovine serum. Cells were stimulated via BCR with F(ab′)2 fragments of anti-IgM antibody and IL-4 (Kirin Brewery, Tokyo) for 72 hr in the presence or absence of TA. [3H]thymidine was present during the last 4 hr. Thymidine incorporation into the acid-insoluble fraction was counted. In the experiments shown in Fig. 6B, B cells were negatively purified from spleen cells of BALB/c mice with CD90 (Thy 1.2) Microbeads (Miltenyi Biotec, Auburn, CA). Cells were stimulated with anti-IgM F(ab′)2 and IL-4 in the presence of TA for 48 hr. Expression of major histocompatibility complex class II on the gated live cells was measured by a FACSCalibur (Becton-Dickinson) after staining cells with fluosceinated anti-I-Ad antibody (PharMingen).
Figure 6.
Effects of TA on B cell activation. (A) Spleen cells were stimulated with IL-4 and anti-IgM F(ab′)2 for 72 hr in the presence of TA and the indicated concentrations of [3H]thymidine during the last 4 hr. Thymidine incorporation into the acid-insoluble fraction was counted. Representative of two experiments with similar results. (B) Splenic B cells were stimulated with IL-4 and anti-IgM F(ab′)2 in the presence of the indicated concentrations of TA for 48 hr. Expression of major histocompatibility complex class II was measured by cytofluorometry. Representative of three similar experiments.
Immune Complex Kinase Assays.
Immunoprecipitates were washed five times in lysis buffer and once with kinase buffer without ATP. Washed immune complexes were incubated with individual kinase buffers with or without exogenous substates (Btk without exogenous substrate; Lyn with 2 μg of the FcɛRI β peptide, KVPDDRLYEELHVYSPIYSALEDTR, ref. 31; Syk with 2 μg of GST-HS1 containing the sequence from position 352 to position 486 of the human HS1 protein, ref. 32; JNK1 with GST-c-Jun(1–79) in the presence of [γ-32P]ATP (DuPont/NEN). Reaction products were analyzed by SDS/PAGE followed by electroblotting onto poly(vinylidene difluoride) membranes (DuPont/NEN) and autoradiography, except for Lyn kinase assays in which the phosphorylated β peptide was detected by autoradiography of dried gels. Phosphorylated protein bands were quantified by densitometry.
Recombinant Btk Expressed in Sf9 Cells and in Vitro Kinase Reactions.
Mouse btk cDNA was cloned into pVL1392 vector (Invitrogen) and transfected into Sf9 insect cells. Btk was partially purified by DEAE-Sepharose CL-6B and Heparin Sepharose CL-6B (both from Pharmacia). Btk-containing fractions were identified by immunoblotting with anti-BtkC antibody (26). Peak fractions were incubated in a [γ-32P]ATP-containing kinase buffer in the presence or absence of TA. Btk autophosphorylation was analyzed as above.
In Vitro Assays of PKC and Other Kinases.
Kinase activities of PKCα, PKCβI, and PKCβII (all recombinant human isoforms from Panvera, Madison, WI) in the absence or presence of TA were measured by using the PKC “pseudosubstrate” peptide PKC(19–36) in a PKC assay kit (Life Technologies). Assays on protein kinase A (catalytic subunit from bovine heart, from Sigma) and casein kinase I (recombinant rat, from Calbiochem) in the absence or presence of TA were performed by using GST-VavSH2 (a kind gift from Amnon Altman, La Jolla Institute for Allergy and Immunology) and casein as substrate, respectively.
Immunoblotting.
Cleared cell lysates or immunoprecipitates were separated by SDS/PAGE and blotted electrophoretically. Poly(vinylidene difluoride) membranes were blocked and incubated with a primary antibody and then with a horseradish peroxidase-conjugated secondary antibody. Visualization of immunoreactive proteins was performed with enhanced chemiluminescence reagents (NEN).
Transcription Assays.
Mouse mast cells cultured as above were transiently transfected by electroporation with reporter constructs, IL-2Luc or TNF-αLuc (24). Twenty-four hours later the cells were sensitized with IgE and stimulated by antigen in the absence or presence of TA after another 20 hr. Luciferase activity was measured as described (24).
RESULTS
TA Inhibits the Interaction between PKC and the PH Domain of Btk in Vitro and in Vivo.
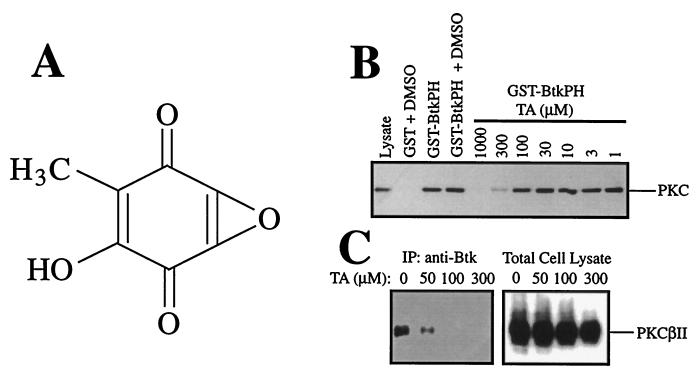
By using the in vitro binding assay described (26), we screened some 500 extracts of various actinomycetes and fungi for the activity that inhibits the PKC-PH domain interaction. TA was purified and identified as an effective inhibitor (Fig. 1A). TA inhibited the binding of GST-BtkPH to PKC in lysates of HMC-1 human mast cells with an IC50 of ≈100 μM (Fig. 1B).
Figure 1.
TA as an inhibitor of the PKC-Btk PH domain interaction. (A) The chemical structure of TA. (B) In vitro binding assays using GST-BtkPH beads and HMC-1 cell lysates. GST-BtkPH beads were incubated with HMC-1 cell lysates in the absence or presence of various concentrations of TA. PKC in HMC-1 lysates, which bound to the Btk PH domain beads, was detected by immunoblotting with anti-PKC (MC5). Similar experiments with similar TA effects were done by using lysates of mouse bone marrow-derived mast cells (results not shown). (C) Effects of TA on the physical association between Btk and PKCβII in mouse bone marrow-derived mast cells. Cell lysates were immunoprecipitated with anti-Btk, and coprecipitated PKCβII was detected by immunoblotting with anti-PKCβII. As a control, total cell lysates also were immunoblotted with anti-PKCβII. A representative result of two similar experiments is shown.
Next, we examined whether TA inhibits the physical association of the intact Btk molecule with PKC. Because βII is one of the most abundant PKC isoforms and the major Btk-associated isoform in bone marrow-derived mouse mast cells (26), we compared the amounts of PKCβII coimmunoprecipitated with Btk in the absence or presence of TA. The results show that TA inhibits the association of Btk with PKCβII in mouse mast cells at lower concentrations (IC50 of ≈30 μM, Fig. 1C). When the same blot was reprobed with anti-PKC (MC5) that reacts with all of the conventional PKC isoforms (α, βI, βII, and γ), the intensity of the PKC band coprecipitated with Btk was similarly reduced by TA (data not shown).
TA Inhibits the Catalytic Activity of Btk But Not PKC.
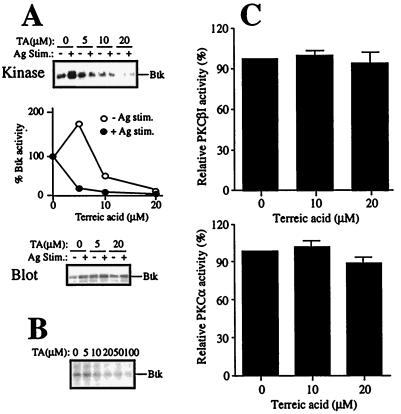
Next, we examined effects of TA on the catalytic activities of Btk and PKC. When IgE-sensitized mouse mast cells were pretreated with TA for 30 min before stimulation by antigen for 3 min, TA inhibited both the basal and activation levels of Btk autophosphorylating activity in a dose-dependent manner (Fig. 2A). IC50s for these activities were 10 μM and 3 μM, respectively. Further, TA inhibited the autophosphorylating activity of Btk purified partially from recombinant baculovirus-infected Sf9 insect cells in a dose-dependent manner (Fig. 2B). The IC50 for the in vitro inhibition of the baculovirus-derived Btk was similar to that for the inhibition of the basal Btk activity in mast cells, probably reflecting the fact that the baculovirus-derived Btk is in a resting state. TA also inhibited the kinase activity of Emt/Itk, a T cell homologue of Btk (17, 33), immunoprecipitated from Jurkat T cells at similar doses (data not shown).
Figure 2.
Effects of TA on the enzymatic activities of Btk and PKC. (A) Mouse bone marrow-derived mast cells were left unstimulated (−) or stimulated with IgE/antigen (+) for 3 min in the presence of the indicated concentrations of TA. Btk was immunoprecipitated from cell lysates and subjected to in vitro kinase assays without substrate. Autophosphorylated Btk bands were detected by autoradiography (Top). Shown is a representative result of three experiments with similar results. The intensities of the autophosphorylated Btk bands were measured by densitometry and plotted with the value without TA being 1 (Middle). Expression of Btk protein was measured by immunoblotting of total cell lysates with anti-Btk (Bottom). (B) Btk partially purified from recombinant baculovirus-infected Sf9 cells were incubated with [γ-32P]ATP in the presence of the indicated concentrations of TA. Autophosphorylated Btk was detected as above. Essentially the same results were obtained in another experiment (data not shown). (C) Recombinant human PKCβI and PKCα (Panvera) were incubated with a peptide substrate and [γ-32P]ATP in the presence of the indicated concentrations of TA. 32P incorporated into the peptide was measured. Results were normalized to the counts without TA.
Effects on recombinant human PKCα, PKCβI, and PKCβII were measured in in vitro kinase assays. In contrast with the inhibition of Btk, phosphorylation of a peptide substrate by PKCα, PKCβI, or PKCβII was not significantly affected by TA (Fig. 2C and data not shown).
Effects of TA on Early Activation Events in Mast Cells.
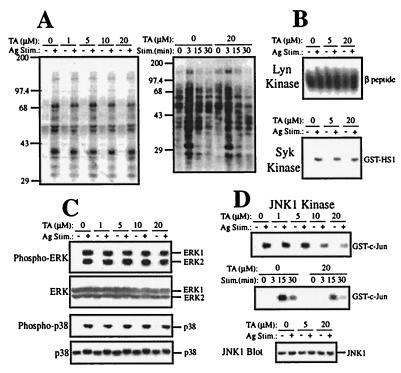
Given that TA inhibits the Btk kinase activity, we examined its effects on mast cell activation. As early activation events, tyrosine phosphorylation of cellular proteins and activation of Lyn, Syk, and MAP kinases were examined. TA did not significantly affect the IgE/antigen-induced tyrosine phosphorylation pattern, as revealed by immunoblotting of total cell lysates (Fig. 3A). Correspondingly, TA did not affect the kinase activities of Lyn and Syk in in vitro kinase assays on the respective immunoprecipitates from TA-pretreated, IgE/antigen-stimulated mast cells (Fig. 3B). These results indicate that TA inhibits Btk selectively among the major PTKs that are activated on FcɛRI cross-linking. The selectivity of TA for Btk was further demonstrated by the findings that it did not affect the catalytic activities of protein kinase A or casein kinase I in in vitro kinase assays (data not shown).
Figure 3.
Effects of TA on early mast cell activation events. Mouse bone marrow-derived mast cells were stimulated with IgE and antigen. Cell lysates were either directly analyzed by SDS/PAGE and immunoblotting (A and C) or by immune complex kinase assays (B and D). (A) Immunoblotting of total cell lysates with antiphosphotyrosine mAb 4G10 was performed with mast cells left unstimulated (−) or stimulated with IgE/antigen for 3 min (+) or the indicated intervals in the presence of the indicated concentrations of TA. Lack of the TA effect on total tyrosine phosphorylation represented by the results shown in A was confirmed in at least 10 experiments. (B) Cells were left unstimulated (−) or stimulated with IgE/antigen for 3 min (+). In vitro kinase assays were done on immune complexes precipitated with anti-Lyn or anti-Syk by using a FcɛRI β peptide or GST-HS1(352–486), respectively, as an exogenous substrate. (C) Immunoblotting of total cell lysates with anti-phospho-MAPK, anti-ERK, anti-phospho-p38, or anti-p38 (New England Biolabs). Cells stimulated for 3 min (for ERK probing) or 10 min (for p38 probing) in the presence of the indicated concentrations of TA. The data shown in B and C were confirmed in another experiment. (D) Cells were left unstimulated (−) or stimulated for 15 min (+) or the indicated intervals in the absence or presence of the indicated concentrations of TA. In vitro kinase assays were performed on anti-JNK1 immunoprecipitates by using GST-c-Jun(1–79) as a substrate. (Bottom) An immunoblot of total cell lysates with anti-JNK1. Representative of three independent experiments.
The effects of TA on MAP kinases were interesting. TA did not exhibit significant effects on the activities of ERK1, ERK2, and p38, as revealed by immunoblotting cell lysates with antibodies that specifically detect the phosphorylated, activated forms of these MAP kinases (Fig. 3C). On the other hand, JNK1 activity was inhibited by TA in a dose-dependent manner (IC50 = ≈10 μM, Fig. 3D), although the direct incubation of JNK with TA did not affect the JNK activity (data not shown). However, the kinetics of JNK1 activation by FcɛRI was not influenced by TA (Fig. 3D). The reduced JNK activities in the presence of TA were similar to those in btk mutant mast cells (14). During the incubation periods, expression of these MAP kinases was not changed (Fig. 3 C and D).
Effects of TA on Late Activation Events in Mast Cells.
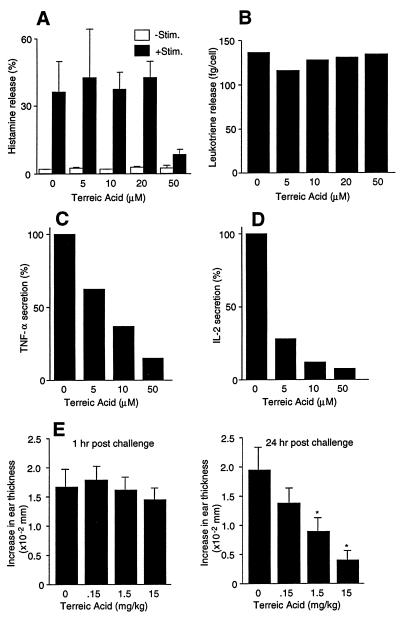
As representative biological consequences of FcɛRI stimulation, histamine release (as an indicator of degranulation), leukotriene release, and secretion of TNF-α and IL-2 were examined for the TA effects. Neither histamine release nor leukotriene release was affected significantly except for a reduction in histamine release at the highest tested dose (50 μM) of TA (Fig. 4 A and B). In contrast, cytokine secretion was severely impaired by TA in a dose-dependent manner (IC50 = ≈7 μM for TNF-α and ≈3 μM for IL-2, Fig. 4 C and D).
Figure 4.
Effects of TA on mast cell activation events in vitro and in vivo. Mouse bone marrow-derived mast cells were stimulated with IgE/antigen for 45 min (histamine release and leukotriene release) or 24 hr (cytokine secretion). Histamine (A), leukotriene (B), TNF-α (C), and IL-2 (D) secreted into the culture media were measured. Results of histamine release and leukotriene release are representative of two independent experiments and those of cytokine secretion of at least five independent experiments. (E) Mice were primed with antidinitrophenyl IgE for 24 hr and challenged by epicutaneous administration of dinitrofluorobenzene (hapten) on both sides of the ear. TA was injected i.p. 1 hr before hapten challenge. Ear thicknesses were measured at 1 hr and 24 hr. Each value represents the mean ± SEM of six mice. ∗, P < 0.05; ∗∗, P < 0.01 vs. control.
Because the defect in cytokine production in btk mutant mast cells is at least partly caused by the defective transcription of cytokine genes (23, 24), we examined whether TA affects the promoter activity of the mouse IL-2 and TNF-α genes. Transcriptional activity of the IL-2Luc and TNF-αLuc constructs was reduced by 98% and 70%, respectively, in cells pretreated with 20 μM TA, compared with nonpretreated, FcɛRI-stimulated mast cells (Table 1). These data suggest that the inhibitory effect of TA on the secretion of the TNF-α and IL-2 is at least partly the result of its effect on the transcription of these cytokine genes, consistent with the above observation that TA inhibits JNK activity (Fig. 3D). Greater sensitivity of the IL-2 promoter to TA compared with the TNF-α promoter is consistent with greater effects of TA on IL-2 vs. TNF-α secretion (Fig. 4 C and D). Furthermore, the btk mutations also are known to have more severe effects on IL-2 production than on TNF-α production in mast cells (23).
Table 1.
Effects of TA on transcriptional activities of the mouse IL-2 and TNF-α promoters
| TA, μM | Relative activity, % | |
|---|---|---|
| IL-2Luc | TNFαLuc | |
| 0 | 100 (3) | 100 (2) |
| 10 | 75.2 ± 9.2 (3) | ND |
| 20 | 1.9 ± 0.0 (2) | 30.1 ± 1.4 (2) |
Mouse mast cells were transfected with IL-2Luc or TNF-αLuc reporter constructs. Twenty-four hours later cells were sensitized with IgE and stimulated by antigen. Luciferase activities were measured. Results were normalized against the values of a vehicle (dimethyl sulfoxide) control. Numbers in parentheses indicate numbers of independent experiments. ND, not done.
Effects of TA on in Vivo Allergic Cutaneous Reactions.
The effects of TA on IgE/antigen-mediated cutaneous reactions in BALB/c mice also were examined (23). Mice received i.v. injection of antidinitrophenyl IgE antibody followed by epicutaneous administration of 0.15% dinitrofluorobenzene 24 hr later. TA was injected i.p. 1 hr before antigen challenge. The increased ear thickness resulting from an edematous allergic reaction occurs from 2 hr after antigen challenge and continues for at least 48 hr (23, 34). The early response, as examined 1 hr after antigen challenge, was not affected by TA. In contrast, TA inhibited the late-phase (24 hr) response in a dose-dependent manner (Fig. 4E). This result is consistent with the TA-mediated inhibition of TNF-α secretion from activated mast cells, because late-phase reactions are in part caused by TNF-α secreted from activated mast cells (34).
Effects of TA on Mast Cell Proliferation.
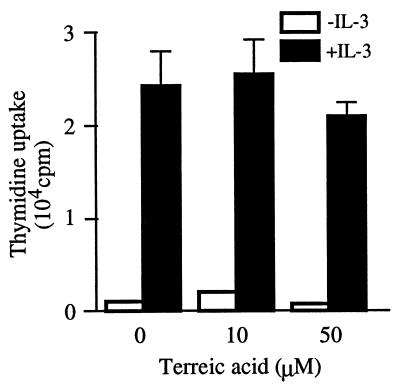
Selective inhibition of cytokine production, but not degranulation or lipid mediator release, seems to be caused by a specific inhibition of Btk, but not by a general inhibitory effect by this compound. To further address this issue, we tested whether TA affects IL-3-induced DNA synthesis in mouse mast cells. As shown in Fig. 5, TA at 10 μM did not affect thymidine uptake at all and even at 50 μM suppressed it by less than 20% compared with that in the absence of TA, consistent with the minimal effect of btk mutations on IL-3 induction of DNA synthesis (14). These and the dose-response data on mast cell activation support the notion that TA mediates its effects through specific inhibition of the catalytic activity of Btk.
Figure 5.
Effects of TA on IL-3-induced DNA synthesis. Mouse bone marrow-derived mast cells were depleted of IL-3 for 12 hr and preincubated with various concentrations of TA for 30 min. Then cells were cultured with 1 ng/ml of mouse recombinant IL-3 and TA for ≈18 hr in the presence of [3H]thymidine during the last 4 hr. Radiolabel incorporated into the acid-insoluble fraction was measured. Representative of two independent experiments.
Effects of TA on B Cell Activation.
We also examined effects of TA on B cell activation induced by antigen receptor stimulation. Mouse spleen B cells were stimulated by anti-IgM F(ab′)2 in the absence or presence of TA. DNA synthesis was inhibited by TA in a dose-dependent manner with an IC50 of ≈1.5 μM (Fig. 6A). Autophosphorylating activity of Btk immunoprecipitated from BCR-stimulated spleen cells exhibited a similar dose-response to TA (data not shown). BCR-induced expression of major histocompatibility complex class II molecules also was inhibited in a similar dose-dependent manner (Fig. 6B). These results are consistent with the crucial role of Btk in BCR signaling.
DISCUSSION
In this study TA was identified as an inhibitor that blocks the PKC-Btk PH domain interaction in vitro. Experiments using mast cells and partially purified recombinant Btk showed that TA inhibits the kinase activity of Btk. Importantly, TA treatment of wild-type mast cells precisely recapitulated the phenotypes of mast cells with xid or btk null mutations: (i) TA severely inhibited the production of TNF-α and IL-2 whereas degranulation was only marginally affected by TA. Consistent with the defective cytokine production, TA inhibited the late-phase response of in vivo allergic cutaneous reactions. (ii) TA inhibited the FcɛRI-induced activation of JNK in a dose-dependent manner whereas it did not affect the ERK activity. In accord with the reduced JNK activation, the transcriptional activation of TNF-α and IL-2 promoters was also strongly inhibited by TA. (iii) TA did not affect IL-3-mediated DNA synthesis. Importantly, the doses of TA required to exert these inhibitory effects are similar (IC50 = 3–15 μM), suggesting that the TA effects originate from the specific inhibition of Btk by TA. This notion is further supported by little, if any, influence by TA on the activities of various enzymes, including Lyn, Syk, PKC, PKA, casein kinase I, etc. As shown for btk null mast cells (23), neither tyrosine phosphorylation of the FcɛRI β subunit nor expression levels of several signaling proteins such as FcɛRIβ, Lyn, Syk, Btk, Vav, phospholipase C (PLC)-γ1 and PLC-γ2, Shc, Grb-2, and H-Ras were affected by TA treatment of mast cells (data not shown). On the other hand, TA inhibited degranulation at very high concentrations whereas degranulation in xid and btk null mast cells were modestly defective in our previous studies (23). However, our recent unpublished data show that btk null mast cells exhibited only slightly reduced histamine release compared with wild-type mast cells of the same genetic background. This finding may suggest that Btk plays a minimal or dispensable role in degranulation.
TA originally was detected in and isolated from a culture of Aspergillus terreus because of its antibiotic properties (35). As a mechanism for its antibiotic action, it inhibits protein synthesis by blocking the formation of leucyl-tRNA in sensitive bacteria (36). This activity might contribute to relatively high toxicity to mouse mast cells at high TA doses (Table 2). Although we cannot exclude the possibility that the same mechanism of the TA effect might work in mast cells, it should have little, if any, effect on early activation events such as activation of PTKs and MAP kinases. Thus, levels of these proteins in mast cells were unchanged for at least 30 min after FcɛRI cross-linking (Figs. 2 and 3 and data not shown). Furthermore, TA did not affect IL-3-mediated DNA synthesis at low doses that inhibit Btk activity and cytokine production.
Table 2.
Half-maximal effective lethal concentrations (LC50) of TA on mouse mast cells
| Incubation time, hr | LC50, μM |
|---|---|
| 24 | 70.0 |
| 48 | 28.9 |
| 72 | 27.7 |
| 96 | 26.2 |
Mouse mast cells were cultured in duplicate in growth media in 0, 5, 10, 15, 20, 25, 30, 40, 45, 50, 60, 80, or 100 μM TA for the indicated periods. Numbers of live cells that exclude Trypan blue dye were counted.
TA inhibits the catalytic activity of Btk without affecting those of α, βI, and βII isoforms of PKC (Fig. 2). TA inhibits the interaction between PKC and Btk PH domain in vitro and dissociates Btk from PKCβII (and other conventional PKC isoforms) in mast cells. Therefore, it seems that TA binds to the PH domain of Btk and induces a conformational change that prevents PKC from interacting with the Btk PH domain, because our previous study showed that the PKC-PH domain interaction is direct (26, 27). Indeed, TA inhibited the physical association between purified recombinant human PKCβI and partially purified recombinant mouse Btk (data not shown). Because the inhibition of the association of Btk with PKC required similar concentrations of TA as the inhibition of the Btk catalytic activity did, both of these TA effects may be the result of a conformational change of the PH domain of Btk. For the same reason it was impossible to assign these two TA effects to individual activation events as a basis of inhibition. These observations implicate the PH domain in the regulation of kinase activities. There is circumstantial evidence for this notion. Thus, a point mutation resulting in a glutamic acid-to-lysine substitution at position 41 of Btk generates a constitutively active mutant with a propensity for membrane localization (37). Similarly, regulation of the catalytic activity of Akt (also known as protein kinase B) involves the interaction between the N-terminal PH domain and the C-terminal kinase domain (reviewed in ref. 38). Binding of phosphatidylinositol 3,4,5-trisphosphate to the PH domain may lead to an open conformation that allows protein kinases, PDK1 and PDK2, to phosphorylate the sites on Akt to activate it. Similar activation mechanisms may operate for Btk, because phosphatidylinositol 3,4,5-trisphosphate together with an activated Src family PTK activates Btk to a full extent (39–41). A mutagenesis study showed that the PH domain of protein kinase D plays a negative regulatory role (42). However, mechanistic details of the inhibition of Btk by TA must await structural analysis of Btk-TA cocrystals.
TA inhibits not only Btk but also Emt/Itk, probably indicating its potential as a general, but selective, inhibitor of Tec subfamily PTKs. Emt/Itk is implicated in T cell receptor and CD28 signaling (43, 44). Indeed, TA inhibited the proliferation of antigen-specific T cells stimulated by antigen-presenting cells in the presence of specific peptide antigen (data not shown). This data and the possible conformational effects on the TA-bound PH domains may raise the possibility of broader effects of TA on PH domain-containing proteins. This concern is reasonable because numerous signaling proteins have one or more PH domains (45). However, TA may target few PH domains other then those of Tec subfamily PTKs at the concentrations that are effective for Btk inhibition for the following reasons. First, different PH domains generally are very divergent (45). However, the PH domains of Tec subfamily PTKs are highly conserved, consistent with the TA effects on both Btk and Emt/Itk. Second, the effects of TA on mast cell activation are so similar to those of btk mutations. If TA affects other PH domains, it should affect many other activation events; for example, activation of Ras is regulated by a PH domain-containing guanine nucleotide exchange factor, Sos. But apparently Ras activity was not affected by TA, as measured by the activity of downstream MAP kinases, ERK1 and ERK2 (Fig. 3C).
In summary, we identified and characterized TA as a selective inhibitor of the PKC-PH domain interaction and the Btk catalytic activity. Because treatment of mast cells with this small-molecule compound faithfully recapitulates the phenotypes of btk mutations, TA will be a useful tool to probe into the functions of Btk (and other Tec PTKs) not only in mast cells but also in other immune cells. TA also will be useful for understanding roles of Btk (and other Tec PTKs) in disease states such as allergy and autoimmune diseases. Although TA itself might not be therapeutically useful because of its cytotoxicity, modification of the TA structure may be undertaken to design improved Btk inhibitors with lower toxicity. Another insight obtained from this study is that it is a reasonable approach to use the same screening principle, i.e., inhibition of the PKC-Btk PH domain interaction, to find other efficacious anti-Btk compounds.
Acknowledgments
We thank Drs. Kimishige Ishizaka, Howard Grey, and Tadashi Sudo for encouragement and support. We also thank Dr. Amnon Altman for his critical reading of the manuscript. Dr. Katsuji Sugie is appreciated for his kind help in performing flow cytomeric analysis. Helpful discussions by the faculty members of La Jolla Institute for Allergy and Immunology are greatly appreciated. We thank Dr. J. H. Butterfield of the Mayo Clinic for his kind gift of the HMC-1 cells. This study was supported in part by National Institutes of Health Grants AI33617 and AI38348 and an institutional grant (T.K.). This is publication 224 from the La Jolla Institute for Allergy and Immunology.
ABBREVIATIONS
- BCR
B cell receptor
- Btk
Bruton’s tyrosine kinase
- FcɛRI
high-affinity IgE receptor
- GST
glutathione S-transferase
- IL
interleukin
- MAP
mitogen-activated protein
- PH
pleckstrin homology
- PKC
protein kinase C
- PTK
protein-tyrosine kinase
- TA
terreic acid
- TNF
tumor necrosis factor
References
- 1.Beaven M A, Metzger H. Immunol Today. 1993;14:222–226. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- 2.Blank U, Ra C, Miller L, White K, Metzger H, Kinet J-P. Nature (London) 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 3.Cambier J C. Immunol Today. 1995;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 4.Eiseman E, Bolen J B. Nature (London) 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 5.Jouvin M-H E, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet J-P. J Biol Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- 6.Kihara H, Siraganian R P. J Biol Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]
- 7.Wilson B S, Kapp N, Lee R J, Pfeiffer J R, Martinez A M, Platt Y, Letourneur F, Oliver J M. J Biol Chem. 1995;270:4013–4022. doi: 10.1074/jbc.270.8.4013. [DOI] [PubMed] [Google Scholar]
- 8.Shiue L, Green J, Green O M, Karas J L, Morgenstern J P, Ram M K, Taylor M K, Zoller M J, Zydowsky L D, Bolen J B, Brugge J S. Mol Cell Biol. 1995;15:272–281. doi: 10.1128/mcb.15.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozawa K, Szallasi Z, Kazanietz M G, Blumberg P M, Mischak H, Mushinski J F, Beaven M A. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 10.Fukamachi H, Takei M, Kawakami T. Int Arch Allergy Immunol. 1993;102:15–25. doi: 10.1159/000236546. [DOI] [PubMed] [Google Scholar]
- 11.Hirasawa N, Santini F, Beaven M A. J Immunol. 1995;154:5391–5402. [PubMed] [Google Scholar]
- 12.Tsai M, Chen R-H, Tam S-Y, Blenis J, Galli S J. Eur J Immunol. 1993;23:3286–3291. doi: 10.1002/eji.1830231234. [DOI] [PubMed] [Google Scholar]
- 13.Offermanns S, Jones S V P, Bombien E, Schultz G. J Immunol. 1994;152:250–261. [PubMed] [Google Scholar]
- 14.Kawakami Y, Miura T, Bissonnette R, Hata D, Khan W N, Kitamura T, Maeda-Yamamoto M, Hartman S E, Yao L, Alt F W, Kawakami T. Proc Natl Acad Sci USA. 1997;94:3938–3942. doi: 10.1073/pnas.94.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizuka T, Oshiba A, Sakata N, Terada N, Johnson G L, Gelfand E W. J Biol Chem. 1996;271:12762–12766. doi: 10.1074/jbc.271.22.12762. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami Y, Yao L, Tsukada S, Witte O N, Kawakami T. Mol Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami Y, Yao L, Tashiro M, Gibson S, Mills G B, Kawakami T. J Immunol. 1995;155:3556–3562. [PubMed] [Google Scholar]
- 18.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, et al. Nature (London) 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 19.Tsukada S, Saffran D, Rawlings D J, Parolini O, Allen R C, Klisak I, Sparkes R S, Kubagawa H, Mohandas T, Quan S, et al. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 20.Thomas J D, Sideras P, Smith C I E, Vorechovsky I, Chapman V, Paul W E. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 21.Rawlings D J, Saffran D C, Tsukada S, Largaespada D A, Grimaldi J C, Cohen L, Mohr R N, Bazan J F, Howard M, Copeland N G, et al. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 22.Rawlings D J, Scharenberg A M, Park H, Wahl M I, Lin S, Kato R M, Fluckiger A-C, Witte O N, Kinet J-P. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 23.Hata D, Kawakami Y, Inagaki N, Lantz C S, Kitamura T, Khan W N, Maeda-Yamamoto M, Miura T, Han W, Hartman S E, et al. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hata D, Kitaura J, Hartman S E, Kawakami Y, Yokota T, Kawakami T. J Biol Chem. 1998;273:10979–10987. doi: 10.1074/jbc.273.18.10979. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami Y, Hartman S E, Hollans P M, Cooper J A, Kawakami T. J Immunol. 1998;161:1795–1802. [PubMed] [Google Scholar]
- 26.Yao L, Kawakami Y, Kawakami T. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao L, Suzuki H, Ozawa K, Deng J, Lehel C, Fukamachi H, Anderson W B, Kawakami Y, Kawakami T. J Biol Chem. 1997;272:13033–13039. doi: 10.1074/jbc.272.20.13033. [DOI] [PubMed] [Google Scholar]
- 28.Butterfield J H, Weiler D, Dewald G, Gleich G J. Leukemia Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami T, Inagaki N, Takei M, Fukamachi H, Coggeshall K M, Ishizaka K, Ishizaka T. J Immunol. 1992;148:3513–3519. [PubMed] [Google Scholar]
- 30.Tashiro M, Kawakami Y, Abe R, Han W, Hata D, Sugie K, Yao L, Kawakami T. J Immunol. 1997;158:2382–2389. [PubMed] [Google Scholar]
- 31.Kimura T, Kihara H, Bhattacharyya S, Sakamoto H, Appella E, Siraganian R P. J Biol Chem. 1996;271:27962–27968. doi: 10.1074/jbc.271.44.27962. [DOI] [PubMed] [Google Scholar]
- 32.Fukamachi H, Yamada N, Miura T, Kato T, Ishikawa M, Gulbins E, Altman A, Kawakami Y, Kawakami T. J Immunol. 1994;152:642–652. [PubMed] [Google Scholar]
- 33.Yamada N, Kawakami Y, Kimura H, Fukamachi H, Baier G, Altman A, Kato T, Inagaki Y, Kawakami T. Biochem Biophys Res Commun. 1993;192:231–240. doi: 10.1006/bbrc.1993.1404. [DOI] [PubMed] [Google Scholar]
- 34.Wershil B K, Wang Z-S, Gordon J R, Galli S J. J Clin Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkins W H, Harris G C M. Br J Exp Pathol. 1942;23:166–169. [Google Scholar]
- 36.Basu S, Bose S K, Jr, Bose S K. J Appl Bacteriol. 1989;67:191–200. doi: 10.1111/j.1365-2672.1989.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 37.Li T, Tsukada S, Satterthwaite A, Havlik M H, Park H, Takatsu K, Witte O N. Immunity. 1995;2:451–460. doi: 10.1016/1074-7613(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 38.Downward J. Science. 1998;279:673–674. doi: 10.1126/science.279.5351.673. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Wahl M I, Eguinoa A, Stephens L R, Hawkins P T, Witte O N. Proc Natl Acad Sci USA. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawlings D J, Kinet J-P. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fluckiger A-C, Li Z, Kato R M, Wahl M I, Ochs H D, Longnecker R, Kinet J-P, Witte O N, Scharenberg A M, Rawlings D J. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iglesias T, Rozengurt E. J Biol Chem. 1998;273:410–416. doi: 10.1074/jbc.273.1.410. [DOI] [PubMed] [Google Scholar]
- 43.August A, Gibson S, Kawakami Y, Kawakami T, Mills G B, Dupont B. Proc Natl Acad Sci USA. 1994;91:9347–9351. doi: 10.1073/pnas.91.20.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson S, August A, Kawakami Y, Kawakami T, Dupont B, Mills G B. J Immunol. 1996;156:2716–2722. [PubMed] [Google Scholar]
- 45.Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]