Abstract
In immuno-competent individuals, the natural course of chronic hepatitis C virus (HCV) infection is highly variable and 5%–30% of patients develop cirrhosis over 20 years. Co-infection with HCV and human immunodeficiency virus (HIV) is an important prognostic factor and associated with more frequent and accelerated progression to cirrhosis. Until recently HIV/AIDS-related complications were life limiting in patients co-infected with HCV; the introduction of highly active antiretroviral treatment (HAART) and the better prognosis of HIV infection has made HCV-related complications an emerging health problem in HCV/HIV co-infected individuals. Treatment of chronic HCV infection has also evolved since the introduction of interferon-alpha. Recently, introduction of pegylated interferon-alpha (peginterferon-alpha) has resulted in an increase in sustained virus clearance rates of up to 80% in selected genotypes and patient populations. The safety and efficacy of modern anti HCV treatment regimens – based on peginterferon-alpha in combination with ribavirin – was evaluated in 4 controlled trials. Sustained clearance of hepatitis C virus can be achieved in up to 35% of patients with HIV/HCV co-infection, and novel HCV treatment regimens based on peginterferon-alpha have no negative effect on the control of HIV disease. In conclusion, if HIV infection is well controlled and CD4+ cell counts >100/mm3, treatment of chronic hepatitis C with peginterferon in combination with ribavirin is safe and should be given for 48 weeks regardless of the HCV genotype. Introduction of peginterferon-alpha has significantly improved adherence to treatment and treatment efficacy; in particular sustained virologic response in patients with HCV genotype 1 or 4 infection improved, but sustained viral clearance in only 7%–38% of patients infected with genotype 1 and 4 cannot be the final step in development of effective treatments in patients with HCV/HIV co-infection.
Keywords: pegylated interferon-alpha, HIV, hepatitis C, ribavirin
Epidemiology
It is estimated that about 50 000 individuals in the United States are co-infected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) (Sherman et al 2002). The prevalence of HIV/AIDS in the US is about 800 000 (Sulkowski and Thomas 2003; Racial/ethnic disparities in diagnoses of HIV/AIDS 2006) compared with 4 million HCV infected people in the US (Alter 2006). The prevalence of chronic HCV infection among HIV-infected individuals varies between 15% and 90%, depending on the mode of viral transmission. HIV-infected individuals who received blood products or intravenous drug users who shared needles have a risk of HCV infection exceeding 90%. HCV/HIV co-infection rate among men having sex with men (MSM) is considerably lower (Terrault 2002), which is in accordance with data suggesting that the risk for sexual transmission of HCV is low (Neumayr et al 1999). The overall frequency of HCV infection in HIV-infected individuals was reported to be 16.4% in the Aids Clinical Trail Group (ACTG) paper (Sherman et al 2002), and about 30% in a European study (Rockstroh et al 2005).
Pathophysiology
Spontaneous clearance of the virus occurs in only about 15% of acute HCV infections, most of which are clinically asymptomatic (Lehmann et al 2004). If the virus cannot be cleared, progression of chronic hepatitis C to cirrhosis occurs in 5%– 30% of patients (Leone and Rizzetto 2005) over a period of 20 years (Seeff 2002). Risk factors associated with accelerated progression to fibrosis and cirrhosis include co-infection with HIV or hepatitis B virus (HBV), acquired infection after the age of 40, male sex, alcohol consumption of more than 50 g/day, hepatic steatosis, and immunosuppression (Chen and Morgan 2006). Interestingly, only 0%–2% of women show progression to cirrhosis 17–20 years after infection with anti-D-Rhesus immunoglobulin (Kenny-Walsh 1999), which illustrates the difficulties in comparing the prognosis of different cohorts; mode of transmission, co-morbidities, and life-style factors may also influence disease progression.
With ∼30% of transplant recipients having chronic hepatitis C, until recently, HCV-cirrhosis was the most frequent indication for liver transplantation in the US and in Europe (Belle et al 1996). The incidence of end stage liver disease caused by hepatitis C is expected to further increase over the next 10 years as a result of the “silent epidemic” of HCV infection. The calculated annual risk for the development of hepatocellular carcinoma in patients with chronic HCV infection is 1%–4%, attributable almost exclusively to patients in the cirrhotic stage of chronic HCV infection (Fattovich et al 1997). Besides hepatocellular carcinoma, decompensation of cirrhosis is another critical prognostic factor; 10-year survival after the first decompensation is 50%, compared with 80% in patients compensated cirrhosis (Fattovich et al 1997).
HCV has become a significant cause of morbidity and mortality in HCV/HIV co-infected individuals. Since the introduction of highly active antiretroviral therapy (HAART), prognosis of HIV infection has dramatically changed and chronic HCV infection with its associated complications is an emerging health problem (Lesens et al 1999; Darby et al 1997; Bica et al 2001). As discussed above, in immuno-competent patients, hepatitis C usually has a relatively mild course, and liver-related deaths are only slightly more frequent in HCV infected individuals (Hoofnagle 2002). In contrast, hepatitis C may take a more severe course in HIV infected patients; accordingly, liver-related deaths are 16.7 times more frequent in HIV/HCV co-infected people than in the general population and deaths due to liver cancer are 5.6 times more common (Darby et al 1997). Treatment of HCV infection is therefore of paramount importance in patients with HCV/HIV co-infection.
Treatment
It is well established that interferon-alpha therapy has favorable effects on liver-related morbidity and mortality in chronic hepatitis C (Akuta et al 2005); improvement of hepatic histology, ie, fibrosis and inflammation are used as surrogate markers for progression to cirrhosis in patients with chronic hepatitis C (Petrenkiene et al 2004). Favorable effects of interferon-alpha on disease progression were not limited to but most pronounced in patients with sustained virologic response (SVR), which is defined as serum HCV RNA concentration below the limit of detection (usually <50IU/mL) after 24 weeks after the end of treatment (Carlsson et al 2005; Ferenci et al 2005).
Decline in viral RNA during treatment with interferon-alpha follows several phases: During the first 2–3 days after initiation of treatment, hepatitis C virus RNA concentration in serum decreases rapidly (Jessner et al 2002, 2003). This early phase is followed by phases of less steep decline in viral load, which may reflect 2 different mechanisms of action of interferon-alpha. Lack of early virologic response is the single most important negative predictor for sustained virologic response, where a ≥2log10 reduction in HCV RNA or an HCV RNA <50U/mL after 12 weeks of therapy is associated with a sustained virologic response in 0%–2% of patients (Dahari et al 2005).
Treatment options for hepatitis C have significantly improved over recent years. Chronic infection with hepatitis C virus genotype 2 and 3 is now considered a curable disease in immuno-competent patients, and even patients with a more difficult to treat genotype (1 and 4) can achieve sustained clearance rates of HCV RNA in about 40%. Three major steps led to this significant improvement. First, the addition of ribavirin to interferon-alpha monotherapy increased response rates from less than 20% to over 40%; second, the development of pegylated interferon-alpha (peginterferon-alpha); and third, a better adherence to treatment (Abonyi and Lakatos 2005).
Pharmacology
After subcutaneous administration of unmodified interferon-alpha, which is rapidly absorbed, metabolized, and excreted by the kidney, maximum serum concentrations are observed after 8 hours (Chatelut et al 1999). Due to its short serum half-life, unmodified interferon-alpha is recommended to be administered 3–7 times weekly (de Ledinghen et al 2002). Thus, modified release of interferon-alpha was developed to meet patient expectations for less frequent parenteral drug administrations, and to increase sustained virologic response rates by improving patient adherence as well as more sustained drug concentrations with higher plasma concentrations.
Pegylation of proteins has become a widely used approach to optimize delivery and release of parenteral drugs. Pegylation is the covalent binding of a polyethylene glycol moiety to a protein. The size and structure of the polyethylene glycol (PEG) groups as well as the size and type of attachment determine the pharmacodynamic properties by interfering with receptor binding of interferon-alpha, where in vitro binding decreases with increasing size of the PEG moiety. In contrast, absorption half-life increases, whereas volume of distribution and renal elimination decrease with an increasing size of the PEG moiety (Veronese and Pasut 2005). The size and structure of the PEG moiety can be tailored to change pharmacokinetic and pharmacodynamic properties of interferon-alpha (Pedder 2003) (Figure 1).
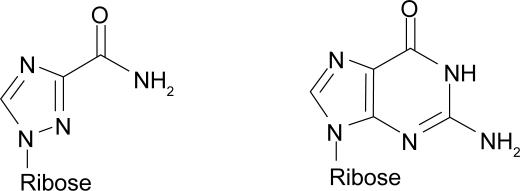
Figure 1.
Chemical structure of ribavirin (left) and guanosine (right)
Two different pegylated interferons have been approved for the treatment of chronic hepatitis C virus infection in HIV-infected individuals. They differ in size and type of PEG attachment, which translates into differences in pharmacological properties (Pedder 2003) (Table 1).
Table 1.
Pharmacokinetic parameters of interferons
| Peginterferon-alpha 2a | Peginterferon-alpha 2b | Interferon-alpha 2a | Interferon-alpha 2b | |
|---|---|---|---|---|
| Asorption half-life | 50 h | 4.6 h | 2.3 h | 2.3 h |
| Volume of distribution | 8–12 l | 0.99 L/kg | 31–73 L | 1.4 L/kg |
| Clearance | 60–100 mL/h | 22 mL h−1 kg−1 | 6600–29 200 mL/h | 231.2 mL h−1 kg−1 |
| Elimination half-life | 65 h | 40 h | 3–8 h | 4 h |
Adapted from Zeuzem S, Welsch C, Herrmann E. 2003. Pharmacokinetics of peginterferons. Semin Liver Dis, 23(Suppl 1):23–8. By permission.
Peginterferon-alpha 2a (40 kD)
Peginterferon-alpha 2a is pegylated with a branched 40 kD PEG moiety and is a mixture of 4 main isomers, involving a PEG moiety covalently bound to the charged side group of lysines at position 31, 121, 131, or 134. Once-weekly administration of 180 μg of peginterferon-alpha 2a by subcutaneous injection in patients with chronic hepatitis C produces a mean maximum serum concentration (Cmax) of 25.6 μg/L, which is reached in a mean time of 45 hours after injection (Zeuzem et al 2003). The steady state is reached after about 5–8 weeks after initiation of therapy and the ratio of peak to through is about 1–0.75, indicating minor variations in drug concentrations at steady state. The volume of distribution is 8–12 L, in contrast to 31–73 L of unmodified interferon-alpha 2a, suggesting a relatively high drug concentration in blood-rich organs such as in the liver (Harris et al 2001). Peginterferon-alpha 2a is excreted via the liver and kidney, where the renal clearance is 100-fold reduced compared with that of unmodified interferon-alpha. The serum concentration profile of peginterferon-alpha 2a is similar in patients with impaired renal function when compared with patients with normal renal function. In patients with end stage renal disease and chronic hemodialysis, dose reduction is recommended, because with 75% of the peginterferon-alpha 2a dose (135 μg once weekly) similar serum concentration profiles have been found when compared with individuals with unimpaired kidney function. Terminal half-life of peginterferon-alpha 2a is similar in cirrhotic patients and healthy volunteers (Zeuzem et al 2003).
Peginterferon-alpha 2b (12 kD)
Peginterferon-alpha 2b is a heterogeneous mixture of derivatized protein isoforms, where a linear PEG moiety is covalently attached to lysines, serines, threonines, histidines, or the C-terminal cysteine. The single most abundant positional isoform is an interferon-alpha poly-peptide, where the PEG molecule is bound to a histidine at position 34. This isoform has a high in vitro antiviral activity, but is also the isoform that is most susceptible to hydrolysis, because of the urethane bond which is the covalent bond conjugating the PEG to the polypeptide chain (Glue et al 2000). The relatively lower molecular weight and the more ready hydrolysis are the molecular basis for the shorter elimination half-life (∼40 h for peginterferon-alpha 2b versus >65 h for peginterferon-alpha 2a). The volume of distribution of unmodified interferon-alpha 2b is 1.4-fold that of peginterferon-alpha 2b; but the distribution volume of peginterferon-alpha 2b is generally higher than that of peginterferon-alpha 2a, which necessitates dosing adjusted to body weight (Glue et al 2000). Elimination routes are renal (30%) and hepatic, as well as intracellular degradation upon binding to the interferon-alpha receptor (Gupta et al 2002). Peginterferon-alpha 2b dose adjustment is recommended in patients with impaired renal function (creatinine clearance <50mL/min) (Patel and McHutchison 2001).
In summary, PEG modifies the pharmacokinetic and pharmacodynamic properties of the protein. The molecular weight of the PEG moiety is directly proportional to the serum half-life of the protein, and indirectly proportional to its half-life in the serum. The pharmacokinetics are further determined by the type and stability of PEG attachment to the protein.
Pharmacodynamics
PEG has unfavorable effects on the binding of interferon-alpha to its target molecules (Grace et al 2005). However, pegylated interferons still have improved pharmacodynamic properties and the sustained virologic response rates are higher with pegylated than with unmodified interferon-alpha (Heathcote and Main 2005), which could be attributed to the altered pharmacokinetics. Interferon-alpha has two major routes of action: the direct antiviral effect of interferon-alpha is partly exerted by activation of double-stranded RNA-activated protein kinase (PKR) and with induction of the antiviral 2′5′-oligoadenlyate synthase (2′5′ OAS) pathway, which is used as a surrogate marker for interferon-alpha activity in vivo (Murashima et al 2000). The OAS pathway is induced by double stranded RNA; and represents an endogenous antiviral defense mechanism, which blocks viral replication by expression of an mRNA endonuclease. Additionally, viral life-cycle is inhibited directly (Chung et al 2001) by host immune modulation (Thomas et al 1999). Interferon-alpha induces activation of macrophages natural killer cells as well as cytotoxic T-cells (Dianzani 1993). In chronic hepatitis C, interferon-alpha produces a Th1-type immune response with induction and maintenance of an HCV-specific CD4+ T-helper cell response (Kamal et al 2002).
Riabvirin
Ribavirin (1-ß-D-ribofuranosyl-1,2,4-triazole) is a broad spectrum antiviral nucleoside analog, which is phosphorylated intracellularily to ribarivin monophosphate, ribavirin diphosphate and ribavirin triphosphate (Morse et al 1993). Although its precise mode of action in vivo is still unknown, in vitro evidence suggests that ribavirin monophosphate inhibits inosine monophosphate dehydrogenase which results in decreased synthesis of guanosine triphosphate (GTP), thereby blocking virus replication, because GTP is required for translation, virus transcription, and RNA synthesis. Alternatively, it has been suggested that ribavirin triphosphate is utilized by viral RNA-dependent RNA polymerase and causes lethal mutagenesis of the viral genome (Graci and Cameron 2006). The antiviral effect of ribavirin has further been ascribed to direct inhibition of viral RNA polymerase by ribavirin triphosphate. It is unlikely that the proposed inhibition of viral transcript capping by ribavirin is of great relevance in vivo because translation of HCV genotype 1b mRNA is CAP independent and mediated via an internal ribosomal entry site (IRES) (Hellen and Pestova 1999).
Another mode of action is that ribavirin modulates the immune response which results in a reduction of immune-mediated tissue damage via reduced interferon-gamma expression (Bergamini et al 2001) or reduction of IL-10 expression (Tam et al 1999) and thereby altering Th1/Th2 subset balance.
Despite the uncertainties about its exact mode of action, ribavirin significantly improves the efficacy of antiviral treatment as compared with interferon-alpha alone and has therefore evolved to be a cornerstone of antiviral treatment in chronic hepatitis C (Idea and Bellobuono 2002). In contrast, its widespread pharmacological effects have delayed the advance of ribavirin for hepatitis C in patients receiving antiretroviral treatment to control HIV infection.
Ribavirin/anti HIV drug interactions
In vitro, ribavirin inhibits intracellular phopsphorylation of nucleoside reverse transcriptase inhibitors (NTRIs) lamivudine (3TC), stavudine (d4T), and zidovudine (ZDV) used for treatment of HIV infection, thus potentially reducing their antiretroviral activity (De Clerq 2004). However, independent studies have confirmed that the pharmacokinetic parameters of NTRIs are not significantly different in patients receiving PEG-interferon-alpha monotherapy or a combination therapy with ribavirin (Margot and Miller 2005; Rodriguez-Torres et al 2005). The in vitro antagonism of ribavirin and NTRIs has delayed the use of ribavirin in HCV/HIV co-infected patients, but no negative impact of stavudine-ribavirin combination on anti-retoviral efficacy could be found in a recent randomized controlled trail (Salmon-Ceron et al 2003).
Pharmacokinetics of ribavirin
A recent study of mean steady state pharmacokinetic parameters of ribavirin in HCV/HIV /infected patients showed that potential drug interactions are of particular importance in the treatment of HIV infection (Rodriguez-Torres et al 2005). After oral administration, ribavirin is extensively and rapidly absorbed and transported actively by gastrointestinal N1 sodium-dependent nucleoside transporters in the duodenum and proximal jejunum (Glue 1999). Ribavirin is typically administered orally in doses of 800–1200 mg/day divided in 2–3 doses. A linear relationship between dose and AUC can be observed, but the bioavailability of ribavirin increases with high fat intake. The antiviral effect of ribavirin has been observed to depend significantly on plasma concentration and dosage (Kato et al 2005). Following a 600-mg dose, the mean maximum concentration in plasma, Cmax, was observed to be 782 ng/mL. The mean time to the maximum concentration, tmax was ∼1.5 h. The half-life of the distribution phase was ∼3.7 h. The terminal elimination phase was long with a mean final concentration time point following single dosing at ∼100 h. The elimination half-life was 79 h. The mean area under the curve, AUC, was 13394 ng mL−1 h−1 (Liu et al 1996).
Viral kinetics
The study of viral kinetics can give insight into the pathogenesis of viral disease and may allow prediction of treatment response early during treatment (Lee et al 1998; Tsubota et al 2005). Mathematical models of viral kinetics in patients with HIV infection have predicted that infected cells have an average life span of 2.2 days and plasma virions were estimated to have a mean life span of 0.3 days (Perelson et al 1996). In chronic HCV infection, the viral mRNA is relatively stable, where the rate of viral release from hepatocytes equals the infection rate of new hepatocytes. Although, without intervention, the mean serum concentration remains fairly constant, chronic hepatitis C is a highly dynamic process, where the half-life of virions has been estimated to be 3 h (Herrmann and Zeuzem 2006).
Hepatitis C viral dynamics change rapidly after administration of interferon-alpha. The study of viral kinetics in patients with chronic hepatitis C who show sustained virologic response to interferon-alpha treatment has shown that the decline in viral RNA follows at least two phases, where an initial steep decline in HCV RNA during the first weeks of treatment (immediate virologic response), is followed by a phase of less steep decline. According to one theory, is has been suggested that antiviral cytokines released from T cells can clear the cells of virus in a non cytopathic manner. This virus-clearing mechanism would have the advantage to the host that liver cells are lost at a slower rate. Regardless of the exact mode of action, the immediate effects of interferon-alpha are of prognostic value for the prediction of sustained virologic response (Pawlotksy 2006).
A poor interferon-alpha effect (<90% effectiveness; <1 log drop in virus RNA serum concentrations) after the first drug dose is predictive for non-SVR after interferon-alpha therapy for 48 weeks. This observation was made in patients treated with interferon-alpha monotherapy, combination therapy, and peginterferon-alpha therapy. In several studies, lack of a ≥1 log10 decline within 24 h or a less than 30% decrease in viral load was associated with failure to clear virus early in therapy as well as non-SVR (Ferenci et al 2005).
Although the immediate effect of interferon-alpha is of some predictive value, it is standard practice to assess early virologic response after 4 weeks of treatment for the prediction of sustained virologic response (Tsubota et al 2005). Accordingly, the pharmacokinetics of peginterferon-alpha are such that interferon-alpha serum concentrations are maintained after 4 weeks (Caliceti 2004). The predictive value of early virologic response after 4 weeks of treatment has now been demonstrated in several studies – also in the setting of HIV/HCV co-infection (Carrat et al 2004; Chung et al 2004; Torriani et al 2004).
According to changes in serum HCV RNA concentrations, within the first 4 weeks of treatment, patients have been grouped into flat partial responders, slow partial responders and rapid early responders (RER). In the latter group tests for HCV viral RNA in serum become negative after 4 weeks of treatment, whereas in slow partial responders a gradual second phase decline of virus mRNA follows an initial rapid 1–2 log drop in serum HCV mRNA concentrations. In flat partial responders, no further decline in HCV mRNA serum concentrations can be found after an initial rapid 1–2 log10 decline in serum HCV mRNA after 4 weeks of treatment. This response patterns are in contrast to the response observed in without a significant drop in viral load a relapse is observed under treatment and referred to as non-response (Ferenci 2003, 2004; Ferenci et al 2005).
In contemporary management of chronic hepatitis C monoinfection, prediction of treatment response is based on the HCV genotype, the initial serum virus RNA concentration and on the viral kinetics under treatment with peginterferon-alpha as measured at the start of treatment, after 4 weeks 12 weeks, and 24 weeks of treatment (Pawlotsky 2006). For HCV/HIV co-infected patients the predictive value of rapid early virologic response (4 weeks) and early virologic response (12 weeks) was assessed in a recent clinical trial (PRESCO trial) (Nunez et al 2005). The results of this trial demonstrate that lack of early virologic response (<1 log10 decline in serum HCV RNA concentrations) had a negative predictive value 63% for genotype 1 and 2 and 100% for genotype 2/3 that the patient would have no early virologic response after 12 weeks. Besides, HCV genotype ribavirin dose was the only factor independently associated with early virologic response (Nunez et al 2005). In larger trials, 0%–0.2% of HCV/HIV co-infected patients without early virologic response 12 weeks after the start of treatment achieved a sustained viral clearance (Chung et al 2004; Torriani et al 2004).
In summary, quantification of serum HCV mRNA before and during interferon-alpha treatment allows tailoring of treatment and prediction of treatment response and has therefore advanced to an important tool in contemporary patient management.
Effect of peginterferon-alpha on HIV disease
The safety and efficacy of peginterferon-alpha in HCV/HIV co-infected patients was the subject of 4 randomized controlled trials. In the ACTG trial (Chung et al 2004), where HIV mRNA levels at baseline and at 24 weeks were only available from only a subgroup of patients, 11% of patients treated with interferon-alpha and ribavirin became HIV RNA negative during treatment. In patients treated with peginterferon-alpha 2a and ribavirin 14% who had tested initially positive for HIV RNA became negative during the treatment period. In contrast 6% and 5% of patients initially tested negative for HIV RNA, but became positive during treatment with a combination of ribavirin and standard interferon-alpha or peginterferon-alpha respectively.
For the management of HIV infection CD4+ T cell counts are of greater prognostic value than HIV RNA levels, and patients enrolled in the ACTG trial on average showed a decrease in CD4+ cells by >100 cells/mm3 during the first 24 weeks of treatment with peginterferon-alpha 2a and ribavirin. No further decrease in CD4+ cells was observed after 24 weeks and after completion of treatment a return to baseline was observed. Since the risk of opportunistic infections and AIDS defining events occur more frequently when CD4+ cell counts fall, the question if peginterferon-alpha 2a treatment should be withheld from patients with a low CD4+ T cell count has been raised. Subgroup analysis of those patients enrolled in ACTG who had CD4+ T cell counts below 200/μm3 showed that baseline CD4+ cell count was not predictive of SVR – and SVR was in fact higher in those patients who had a CD4+ cell count of <200 cells/mm3. Further the overall incidence of AIDS defining events was 1% in the ACTG trial and not increased in those patients who had cell counts of <200 cells/mm3. Based on these data, the authors conclude that treatment of chronic HCV infection with peginterferon-alpha 2a and ribavirin should not be withheld from patients with low CD4+ cell counts; however, patients with CD4+ cell counts of less than 100 cells/mm3 were excluded from this and other studies.
The findings by Torriani et al (2004) regarding CD4+ cell counts were similar to the findings in the ACTG trial and a uniform decrease was observed in all three treatment groups during treatment, whereas the relative proportion of CD4+ cells increased. In a total of 10 patients AIDS-defining events occurred during the study, a calculated frequency of 1.2%, which is not significantly higher than expected and the relative frequency, which was equal in all three treatment arms. From study entry to week 24, the median CD4+ cell count decreased by 130 cells/mm3. However, the percentage of CD4+ cells increased in both groups and the absolute CD4+ cell count did not decrease further after week 24.
The effect of peginterferon-alpha 2b on HIV RNA was comparable with that of peginterferon-alpha 2a; however, the higher efficacy of peginterferon-alpha as compared with standard interferons also caused a more pronounced increase in HIV RNA, although the differences did not reach statistical significance (Carrat et al 2004).
In the study by Laguno et al (2004) CD4+ cell counts fell on average from 560 cells/mm3 to 331 cells/mm3 upon treatment with interferon-alpha, and no statistically significant differences between interferon-alpha 2b or peginterferon-alpha 2b could be identified. Regarding HIV viral load, “no meaningful” changes were found.
Based on these data, it is generally assumed that combination therapy with peginterferon-alpha in patients with controlled HIV disease and CD4+ cell counts of >100/mm3 is safe and not associated with loss of HIV disease control.
Results of clinical trials
The safety and efficacy of peginterferon-alpha in HIV/HCV co-infected individuals was tested in 4 randomized controlled trials (Charrat et al 2004; Chung et al 2004; Torriani et al 2004). The key characteristics of these trials are summarized in Table 2. The rate of sustained virologic response after treatment with either peginterferon-alpha 2a or peginterferon-alpha 2b in combination with ribavirin was significantly lower in HCV/HIV co-infected as compared with HCV monoinfected patients in all trials. This is of particular interest in the light of the well compensated HIV infection in all patients included in the studies, in whom CD4+ cell counts were relatively high (>100 cells/μL).
Table 2.
Key characteristics of clinical trials on safety and efficacy of peginterferon-alpha in HIV/HCV co-infected individuals
| Study | Chung et al 2004 (ACTG 5071) | Torriani et al 2004 (APRICOT) | Carrat et al 2004 (RIBAVIC) | Laguno et al 2004 (Barcelona) |
|---|---|---|---|---|
| N | 66 | 868 | 412 | 95 |
| Design (weekly interferon-alpha dose) | peginterferon-alpha 2a
vs interferon-alpha 2a |
peginterferon-alpha 2
vs interferon-alpha 2aa |
peginterferon-alpha 2b
vs standard interferon-alpha 2b |
peginterferon-alpha 2b
vs standard interferon-alpha 2b |
| Duration | 48 weeks | 48 weeks | 48 weeks | 48 weeks (genotype 2/3 24 weeks) |
| Ribavirin dose | ≤1000 mg (escalating) | 800 mg | 800 mg | 1200 mg (adjusted to body weight) |
| Peginterferon-alpha dose per week | 180 μg peginterferon-alpha 2a | 180 μg peginterferon-alpha 2a | 1.5 μg/kg peginterferon-alpha 2b | 100–150 μg peginterferon-alpha 2b |
| Standard interferon-alpha dose per week | 3 × 6 million units | 3 × 3 million units | 3 × 3 million units | 3 × 3 million units |
| Therapy completed | 88% | 61% | 57% | 81% |
| Treatment discontinued due to side-effects | 11% | 13% | 17% | 16% |
| Dose reduction in peginterferon-alpha group | 6% | 13%b | 16% | 25% |
| SVR (week 72) genotype 1 (or 4) in peginterferon-alpha group | 14% | 29% | 17% | 38% |
| SVR (week 72) genotype 1 (or 4) in standard interferon-alpha group | 6% | 7% | 6% | 7% |
| SVR (week 72) genotype 2 or 3 in peginterferon-alpha group | 11% | 62% | 44% | 53% |
| SVR (week 72) genotype 2 or 3 in standard interferon-alpha group | 5% | 17% | 43% | 47% |
| Antiretroviral therapy | 86% | 84% | 83% | 88% |
| Cirrhosis or advanced fibrosis | 10% | 14% | 39% | 30% |
3rd treatment arm peginterferon-alpha 2b w/o ribavirin.
treatment with granulocyte colony stimulating factor and erythropoietin was permitted if leucopenia and anemia occurred during treatment.
Abbreviation: SVR, sustained virologic response.
Efficacy
In the European multicenter study, 416 patients with HIV/HCV co-infection (39% of patients had cirrhosis or bridging fibrosis) were randomized to receive either interferon-alpha-2b (3 million units 3 times a week) or peginterferon-alpha 2b (1.5 μg/kg once weekly) plus 800 mg/day ribavirin. SVR rates in the group who had received Peginterferon-alpha were 27% compared with 20% of patients receiving standard interferon-alpha (Carrat et al 2004).
In the Adult AIDS Clinical Trials Group trial 5071, 133 patients were randomized to receive 48 weeks of combination therapy with either interferon-alpha 2a 3 million units 3 times a week or peginterferon-alpha 2a 180 μg once a week plus ribavirin 600 mg/day initially, which was increased if tolerated. Peginterferon-alpha 2a was superior to standard interferon-alpha, and the frequency of SVR in the peginterferon-alpha ribavirin group was 14% for genotype 1 infected patients. In contrast, for genotype 2 and 3 the frequency of SVR was 73%, in patients receiving a 48-week treatment course. In the PEG-interferon-alpha group, 27% achieved SVR compared with only 12% in the standard interferon-alpha group (Chung et al 2004).
In the international AIDS Pegasys Ribavirin International Co-infection Trial, 868 HCV/HIV co-infected patients were randomized in 3 groups to receive 48 weeks of treatment with either standard interferon-alpha 2a (3 million units 3 times a week) plus ribavirin, Peginterferon-alpha 2a (180 μg/week) plus placebo or plus ribavirin, respectively. Peginterferon-alpha 2a in combination with ribavirin proved superior (SVR 40% for all genotypes) to peginterferon-alpha 2a without ribavirin (SVR 12% for all genotypes). In patients with genotype 1 infection treated with peginterferon-alpha 2a 29% achieved SVR, whereas 62% in patients with genotypes 2 or 3 had a sustained virus clearance after treatment with Peginterferon 2a plus rivabirin (Torriani et al 2004).
Finally, the Barcelona single center randomized controlled trial included 95 patients; 30% of whom had bridging fibrosis or cirrhosis upon liver biopsy before treatment. Patients were randomized to receive either peginterferon-alpha 2b or interferon-alpha 2b, both with ribavirin. Peginterferon-alpha doses were adjusted to body weight with 100 μg/week for patients <75 kg or 150 μg/week for patients ≥75 kg; ribavirin doses were 800 mg, 1000 mg, or 1200 mg for body weights <60 kg, 60–75 kg, and >75 kg, respectively. In accordance with treatment protocols for HCV mono-infected patients, genotype 1 infections were treated for 48 weeks, while patients with genotypes 2 or 3 were treated for 24 weeks. In patients with genotypes 1 and 4, SVR was achieved in 38% of peginterferon-alpha group versus 7% in the conventional interferon-alpha group. In patients with genotype 2 and 3 infection SVR was achieved in 53% for the peginterferon-alpha group versus 47% for the standard interferon-alpha group (Laguno et al 2004).
In summary, all 4 trials support a recommendation of PEG-interferon-alpha/ribavirin combination therapy for patients with HCV/HIV co-infection. Detailed analysis of the studies suggests that the dose of ribavirin should be adjusted to body weight and a correlation between ribavirin dose and sustained virologic response rate can be observed; however this has yet to be formally tested in patients with HIV/HCV co-infection. The results of one trial suggests that a 24-week treatment regimen for genotypes 2 and 3 may be sufficient for patients with genotypes 2 and 3, but this has not yet entered the canon of medicine and international expert panels still suggest a full 48-week treatment regimen for should be given in patients with HCV/HIV co-infection regardless of the genotype. The majority of patients do not respond to therapy, but the high mortality from liver diseases in HIV-infected patients supports the urgent introduction of HCV treatment. Antiviral treatment for hepatitis C in HIV co-infected patients should be introduced only after stabilization of HIV by antiviral treatment for HIV. In patients in whom no anti-viral treatment for HIV is required, treatment for HCV should be introduced immediately.
Predictors of response
As discussed above, HCV genotype is an important prognostic factor in HCV mono-infected individuals, and the disparity in response rates between different genotypes is even more pronounced in patients with HIV/HCV co-infection, where highest relapse rates in genotype 1 infected patients account for this difference. For genotypes 2 and 3 a 24-week combination therapy was sufficient to achieve sustained virologic response in 35% of patients with genotype 3; SVR was higher (52%) in patients treated for 48 weeks. Based on these data a 48-week treatment regimen was suggested also for patients with genotype 2 or genotype 3 infection (Laguno et al 2004).
Remarkably, neither African race nor high body mass index, which are well established negative predictors of SVR in patients with chronic hepatitis C alone, have been associated with decreased SVR in APRICOT.
In HCV mono-infected patients and HCV/HIV co-infection, prediction of sustained virologic response is further made on the basis of viral load; the prognosis to clear the infection is worst in genotype 1 infected individuals with high viral load (>800 000 IU/mL), where sustained virologic response rates of only 18% were achieved. Interestingly the HIV factors, such as CD4+ cell count at baseline and the non-use of antiretroviral therapy, did not affect SVR rates. In non-responders it has been suggested that peginterferon-alpha/ribavirin combination treatment may still have favorable effects on disease progression, as suggested by the observed improvement of fibrosis scores which were found in a subgroup of patients who were biopsied before and after treatment (Torriani et al 2004). However, if this histological improvement translates to an improved clinical outcome is still unclear.
A careful risk to benefit analysis in HCV/HIV co-infected patients requires prediction of treatment response by evaluating early virologic response by at week 12. It has been shown that early virologic response assessed after 12 weeks of treatment is the strongest predictive factor. Patients who do not show a 2 log10 decline or undetectable serum HCV RNA at week 12 when compared with baseline viral load, are highly unlikely to achieve sustained virologic response (Chung et al 2004). In such patients discontinuation of treatment may be considered if the tolerability of treatment is poor.
It should be noted that adherence to treatment is a major factor determining treatment response. Psychiatric adverse events including depression are well known side-effects of interferon-alpha; patients should therefore be managed in an optimal setting of experts in different fields to improve adherence to treatment. Consultation of a psychiatrist before and during treatment as well as antidepressants have been shown to ameliorate side-effects and improve treatment adherence (Musselman et al 2001; Schaefer et al 2003).
Safety and tolerability
Ribavirin causes dose dependent hemolysis, which is often dose limiting in HCV mono-infection. Studies have shown that a sufficient ribavirin dose is crucial for achieving optimal SVR (Manns et al 2001; Hadziyannis et al 2004). Since hemolysis is a significant problem for HIV-infected patients receiving HAART, the ribavirin doses were relatively low in all 4 randomized trials, and in the trial published by Chung et al (2004) dose reduction was allowed of ribavirin if hematological side-effects occurred. This partly explains the relatively low rate of SVR in HCV/HIV co-infected patients and future studies will show is new drugs such as viramidine whose potency to induce hemolysis is lower, will result in an improvement of SVR rates in this difficult to treat patient population (Gish 2006). Further the use of erythropoietin may be an alternative to maintain ribavirin in some pateints (Lebray et al 2005).
Tolerability data for peginterferons are also available from these trials and treatment interruption due to side-effects or abnormal laboratory findings was reported in 11%–17% (Table 2); a statistically significant difference in side-effects between peginterferon-alpha and standard interferon-alpha could not be found in any of the trials. The most common side-effects apart from anemia included neutropenia, thrombocytopenia, influenza like symptoms, asthenia, anorexia, headache, myalgia, depression, insomnia, alopecia, and injection site reactions, as well as altered thyroid function. Since some of the side-effects are dose dependent, doses of peginterferon-alpha and/or ribavirin were reduced if necessary. The frequencies of dose reduction were higher in patients receiving peginterferon-alpha preparations and are shown in Table 2. Although tolerability of peginterferon-alpha 2a appears slightly superior, treatment was discontinued in 29% in one trial, despite the use of hematopoietic growth factors (granulocyte colony stimulating factor and erythropoietin) being allowed in this trial (Chung et al 2004).
Pancreatitis was another complication observed during peginterferon-alpha/ribavirin treatment in HIV patients receiving HAART. Inhibition of inosine-5′-monophosphate dehydrogenase by ribavirin facilitates conversion of didanosine to its active metabolite and thus causes potential mitochondrial toxicity, for which hyperlactatemia is a surrogate marker. The occurrence of pancreatitis in a total of 24 patients in all multicenter trials together (Charrat et al 2004; Chung et al 2004; Torriani et al 2004) in patients receiving peginterferon-alpha/ribavirin and a didanosine based HART regimen led to the recommendation to change the HAART regimen if didanosine is used, before peginterferon-alpha/ribavirin treatment is started in HCV/HIV co-infected patiens (Manns and Wedermeyer 2004).
Recommendations
Based on the existing body of evidence and in accordance with published management guidelines, individuals co-infected with HCV and HIV should be evaluated for the need for treatment of HIV first and, if anti-retroviral therapy is not required, treatment for HCV should be introduced. If anti-retroviral treatment is required, this should be started before any anti-viral therapy for hepatitis C is given and the HIV disease should be stabilized before introducing treatment with peginterferon-alpha and ribavirin (Heathcote and Main 2005) for 48 weeks. The results from clinical trials show that response rates increase with increasing ribavirin doses; ribavirin doses should therefore be adjusted to body weight and severity of side-effects (anemia and leucopenia). The use of hematopoietic growth factors may help to maintain patients on sufficiently high ribavirin doses when anemia of leucopenia develops. Other side-effects such as depression, flu-like symptoms, and thyroid dysfunction must be monitored carefully and treated symptomatically or treatment stopped if required. When HIV treatment is started the use of didanosine should be avoided because of an increased risk of pancreatitis.
Although introduction of peginterferon-alpha and ribavirin have both significantly advanced the treatment of hepatitis C in HIV-infected patients, it will be essential to view peginterferon-alpha as “a significant progress but not the final step” (Manns and Wedemeyer (2004).
Figure 1A.
Ethyleneglycol monomer
Figure 1B.
Polyethylene glycol
References
- Abonyi ME, Lakatos PL. Ribavirin in the treatment of hepatitis C. Anticancer Res. 2005;25:1315–20. [PubMed] [Google Scholar]
- Akuta N, Suzuki F, Suzuki Y, et al. Long-term follow-up of interferon monotherapy in 454 consecutive naive patients infected with hepatitis C virus: multi-course interferon therapy may reduce the risk of hepatocellular carcinoma and increase survival. Scand J Gastroenterol. 2005;40:688–96. doi: 10.1080/00365520510015467. [DOI] [PubMed] [Google Scholar]
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Belle SH, Beringer KC, Detre KM. Recent findings concerning liver transplantation in the United States. In: Terasaki PI, Cecka JM, editors. Clinical transplants. Los Angeles: UCLA Tissue Typing Laboratory; 1996. pp. 15–30. [PubMed] [Google Scholar]
- Bergamini A, Bolacchi F, Cepparulo M, et al. Treatment with ribavirin and interferon-alpha reduces interferon-gamma expression in patients with chronic hepatitis C. Clin Exp Immunol. 2001;123:459–64. doi: 10.1046/j.1365-2249.2001.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- Caliceti P. Pharmacokinetics of pegylated interferons: what is misleading? Dig Liver Dis. 2004;36(Suppl 3):S334–9. doi: 10.1016/s1590-8658(04)80002-1. [DOI] [PubMed] [Google Scholar]
- Carlsson T, Reichard O, Norkrans G, et al. Hepatitis C virus RNA kinetics during the initial 12 weeks treatment with pegylated interferon-alpha 2a and ribavirin according to virological response. J Viral Hepat. 2005;12:473–80. doi: 10.1111/j.1365-2893.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–48. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- Chatelut E, Rostaing L, Gregoire N, et al. A pharmacokinetic model for alpha interferon administered subcutaneously. Br J Clin Pharmacol. 1999;47:365–71. doi: 10.1046/j.1365-2125.1999.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung RT, He W, Saquib A, et al. Hepatitis C virus replication is directly inhibited by IFN-alpha in a full-length binary expression system. Proc Natl Acad Sci U S A. 2001;98:9847–52. doi: 10.1073/pnas.171319698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahari H, Major M, Zhang X, et al. Mathematical modeling of primary hepatitis C infection: noncytolytic clearance and early blockage of virion production. Gastroenterology. 2005;128:1056–66. doi: 10.1053/j.gastro.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Darby SC, Ewart DW, Giangrande PL, et al. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. UK Haemophilia Centre Directors’ Organisation. Lancet. 1997;350:1425–31. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–33. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- de Ledinghen V, Trimoulet P, Winnock M, et al. Daily or three times per week interferon alpha-2b in combination with ribavirin or interferon alone for the treatment of patients with chronic hepatitis C not responding to previous interferon alone. J Hepatol. 2002;36:819–26. doi: 10.1016/s0168-8278(02)00071-5. [DOI] [PubMed] [Google Scholar]
- Dianzani F. Biological basis for the clinical use of interferon. Gut. 1993;34:S74–6. doi: 10.1136/gut.34.2_suppl.s74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- Ferenci P.2003. More effective by design. Hoffmann-La Roche Ltd.
- Ferenci P. Predictors of response to therapy for chronic hepatitis C. Semin Liver Dis. 2004;24(Suppl 2):25–31. doi: 10.1055/s-2004-832925. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–33. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Gish RG. Treating HCV with ribavirin analogues and ribavirin-like molecules. J Antimicrob Chemother. 2006;57:8–13. doi: 10.1093/jac/dki405. [DOI] [PubMed] [Google Scholar]
- Grace MJ, Lee S, Bradshaw S, et al. Site of pegylation and polyethylene glycol molecule size attenuate interferon-alpha antiviral and antiproliferative activities through the JAK/STAT signaling pathway. J Biol Chem. 2005;280:6327–36. doi: 10.1074/jbc.M412134200. [DOI] [PubMed] [Google Scholar]
- Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis. 1999;19:17–24. [PubMed] [Google Scholar]
- Glue P, Fang JW, Rouzier-Panis R, et al. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin Pharmacol Ther. 2000;68:556–67. doi: 10.1067/mcp.2000.110973. [DOI] [PubMed] [Google Scholar]
- Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Pittenger AL, Swan SK, et al. Single-dose pharmacokinetics and safety of pegylated interferon-alpha2b in patients with chronic renal dysfunction. J Clin Pharmacol. 2002;42:1109–15. doi: 10.1177/009127002401382713. [DOI] [PubMed] [Google Scholar]
- Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- Harris JM, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet. 2001;40:539–51. doi: 10.2165/00003088-200140070-00005. [DOI] [PubMed] [Google Scholar]
- Heathcote J, Main J. Treatment of hepatitis C. J Viral Hepat. 2005;12:223–35. doi: 10.1111/j.1365-2893.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- Hellen CU, Pestova TV. Translation of hepatitis C virus RNA. J Viral Hepat. 1999;6:79–87. doi: 10.1046/j.1365-2893.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Zeuzem S. The kinetics of hepatitis C virus. Eur J Gastroenterol Hepatol. 2006;18:339–42. doi: 10.1097/00042737-200604000-00006. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–9. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- Ideo G, Bellobuono A. New therapies for the treatment of chronic hepatitis C. Curr Pharm Des. 2002;8:959–66. doi: 10.2174/1381612024607009. [DOI] [PubMed] [Google Scholar]
- Jessner W, Stauber R, Hackl F, et al. Early viral kinetics on treatment with pegylated interferon-alpha-2a in chronic hepatitis C virus genotype 1 infection. J Viral Hepat. 2003;10:37–42. doi: 10.1046/j.1365-2893.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- Jessner W, Watkins-Riedel T, Formann E, et al. Hepatitis C viral dynamics: basic concept and clinical significance. J Clin Virol. 2002;25(Suppl 3):S31–9. doi: 10.1016/s1386-6532(02)00194-4. [DOI] [PubMed] [Google Scholar]
- Kamal SM, Fehr J, Roesler B, et al. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology. 2002;123:1070–83. doi: 10.1053/gast.2002.36045. [DOI] [PubMed] [Google Scholar]
- Kato T, Date T, Miyamoto M, et al. Detection of anti-hepatitis C virus effects of interferon and ribavirin by a sensitive replicon system. J Clin Microbiol. 2005;43:5679–84. doi: 10.1128/JCM.43.11.5679-5684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–33. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- Laguno M, Murillas J, Blanco JL, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. Aids. 2004;18:F27–36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- Lebray P, Nalpas B, Vallet-Pichard A, et al. The impact of haematopoietic growth factors on the management and efficacy of antiviral treatment in patients with hepatitis C virus. Antivir Ther. 2005;10:769–76. [PubMed] [Google Scholar]
- Lee WM, Reddy KR, Tong MJ, et al. Early hepatitis C virus-RNA responses predict interferon treatment outcomes in chronic hepatitis C. The Consensus Interferon Study Group. Hepatology. 1998;28:1411–5. doi: 10.1002/hep.510280533. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Meyer MF, Monazahian M, et al. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. J Med Virol. 2004;73:387–91. doi: 10.1002/jmv.20103. [DOI] [PubMed] [Google Scholar]
- Leone N, Rizzetto M. Natural history of hepatitis C virus infection: from chronic hepatitis to cirrhosis, to hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2005;51:31–46. [PubMed] [Google Scholar]
- Lesens O, Deschenes M, Steben M, et al. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999;179:1254–8. doi: 10.1086/314720. [DOI] [PubMed] [Google Scholar]
- Liu XD, Xie L, Han KQ, Liu GQ. Weibull function fits to pharmacokinetic data of ribavirin in man. Eur J Drug Metab Pharmacokinet. 1996;21:227–31. doi: 10.1007/BF03189718. [DOI] [PubMed] [Google Scholar]
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- Manns MP, Wedemeyer H. Treatment of hepatitis C in HIV-infected patients: significant progress but not the final step. JAMA. 2004;292:2909–13. doi: 10.1001/jama.292.23.2909. [DOI] [PubMed] [Google Scholar]
- Margot NA, Miller MD. In vitro combination studies of tenofovir and other nucleoside analogues with ribavirin against HIV-1. Antivir Ther. 2005;10:343–8. [PubMed] [Google Scholar]
- Morse GD, Shelton MJ, O’Donnell AM. Comparative pharmacokinetics of antiviral nucleoside analogues. Clin Pharmacokinet. 1993;24:101–23. doi: 10.2165/00003088-199324020-00002. [DOI] [PubMed] [Google Scholar]
- Murashima S, Kumashiro R, Ide T, et al. Effect of interferon treatment on serum 2’,5’-oligoadenylate synthetase levels in hepatitis C-infected patients. J Med Virol. 2000;62:185–90. doi: 10.1002/1096-9071(200010)62:2<185::aid-jmv9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Neumayr G, Propst A, Schwaighofer H, et al. Lack of evidence for the heterosexual transmission of hepatitis C. QJM. 1999;92:505–8. doi: 10.1093/qjmed/92.9.505. [DOI] [PubMed] [Google Scholar]
- Nunez M, Camino N, Ramos B, et al. Impact of ribavirin exposure on early virological response to hepatitis C therapy in HIV-infected patients with chronic hepatitis C. Antivir Ther. 2005;10:657–62. [PubMed] [Google Scholar]
- Patel K, McHutchison J. Peginterferon alpha-2b: a new approach to improving response in hepatitis C patients. Expert Opin Pharmacother. 2001;2:1307–15. doi: 10.1517/14656566.2.8.1307. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43:S207–20. doi: 10.1002/hep.21064. [DOI] [PubMed] [Google Scholar]
- Pedder SC. Pegylation of interferon alfa: structural and pharmacokinetic properties. Semin Liver Dis. 2003;23(Suppl 1):19–22. doi: 10.1055/s-2003-41635. [DOI] [PubMed] [Google Scholar]
- Perelson AS, Neumann AU, Markowitz M, et al. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–6. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- Petrenkiene V, Gudinaviciene I, Jonaitis L, et al. Improvement of liver histopathology in patients with hepatitis C after interferon and ribavirin combination therapy. Medicina (Kaunas) 2004;40:962–8. [PubMed] [Google Scholar]
- Racial/ethnic disparities in diagnoses of HIV/AIDS–33 states , 2001–20042006MMWR Morb Mortal Wkly Rep 55121–5. [PubMed] [Google Scholar]
- Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Torres M, Torriani FJ, Soriano V, et al. Effect of ribavirin on intracellular and plasma pharmacokinetics of nucleoside reverse transcriptase inhibitors in patients with human immunodeficiency virus-hepatitis C virus coinfection: results of a randomized clinical study. Antimicrob Agents Chemother. 2005;49:3997–4008. doi: 10.1128/AAC.49.10.3997-4008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon-Ceron D, Lassalle R, Pruvost A, et al. Interferon-ribavirin in association with stavudine has no impact on plasma human immunodeficiency virus (HIV) type 1 level in patients coinfected with HIV and hepatitis C virus: a CORIST-ANRS HC1 trial. Clin Infect Dis. 2003;36:1295–304. doi: 10.1086/374837. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443–51. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- Sherman KE, Rouster SD, Chung RT, et al. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Thomas DL. Hepatitis C in the HIV-Infected Person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- Tam RC, Lim C, Bard J, Pai B. Contact hypersensitivity responses following ribavirin treatment in vivo are influenced by type 1 cytokine polarization, regulation of IL-10 expression, and costimulatory signaling. J Immunol. 1999;163:3709–17. [PubMed] [Google Scholar]
- Thomas HC, Torok ME, Forton DM, et al. Possible mechanisms of action and reasons for failure of antiviral therapy in chronic hepatitis C. J Hepatol. 1999;31(Suppl 1):152–9. doi: 10.1016/s0168-8278(99)80393-6. [DOI] [PubMed] [Google Scholar]
- Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99–105. doi: 10.1053/jhep.2002.36797. [DOI] [PubMed] [Google Scholar]
- Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- Tsubota A, Arase Y, Someya T, et al. Early viral kinetics and treatment outcome in combination of high-dose interferon induction vs. pegylated interferon plus ribavirin for naive patients infected with hepatitis C virus of genotype 1b and high viral load. J Med Virol. 2005;75:27–34. doi: 10.1002/jmv.20232. [DOI] [PubMed] [Google Scholar]
- Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–8. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Welsch C, Herrmann E. Pharmacokinetics of peginterferons. Semin Liver Dis. 2003;23(Suppl 1):23–8. doi: 10.1055/s-2003-41631. [DOI] [PubMed] [Google Scholar]