Abstract
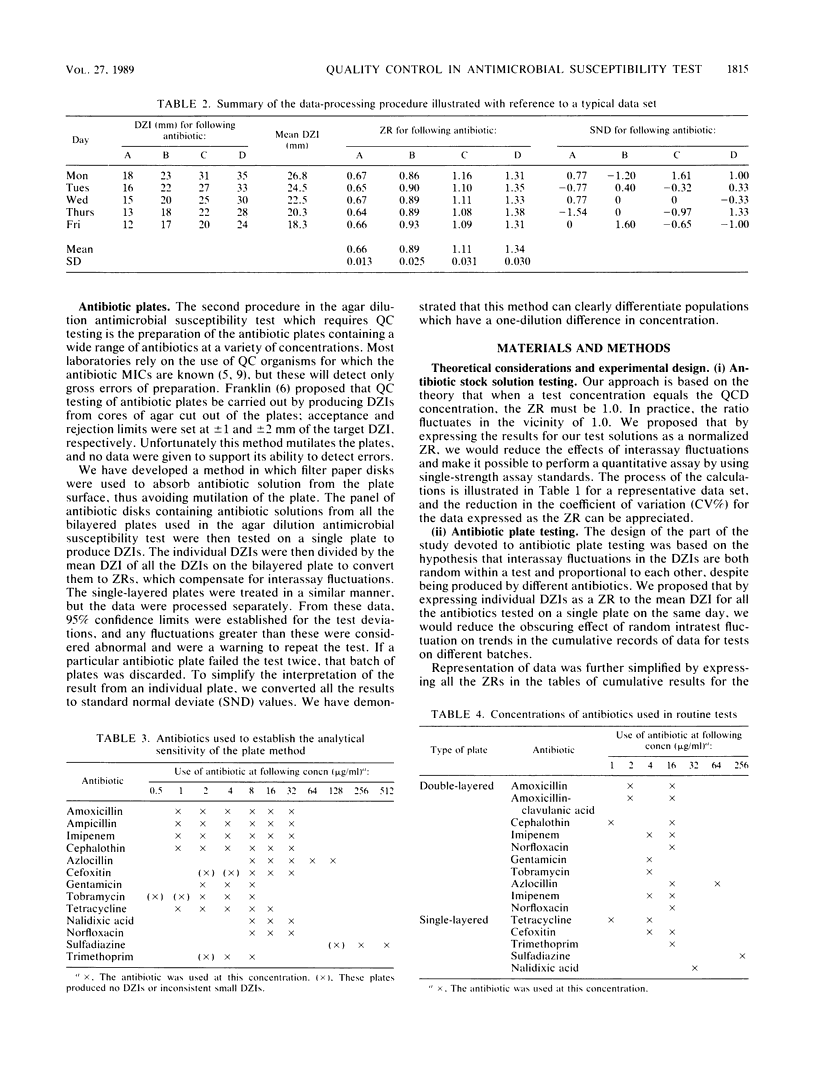
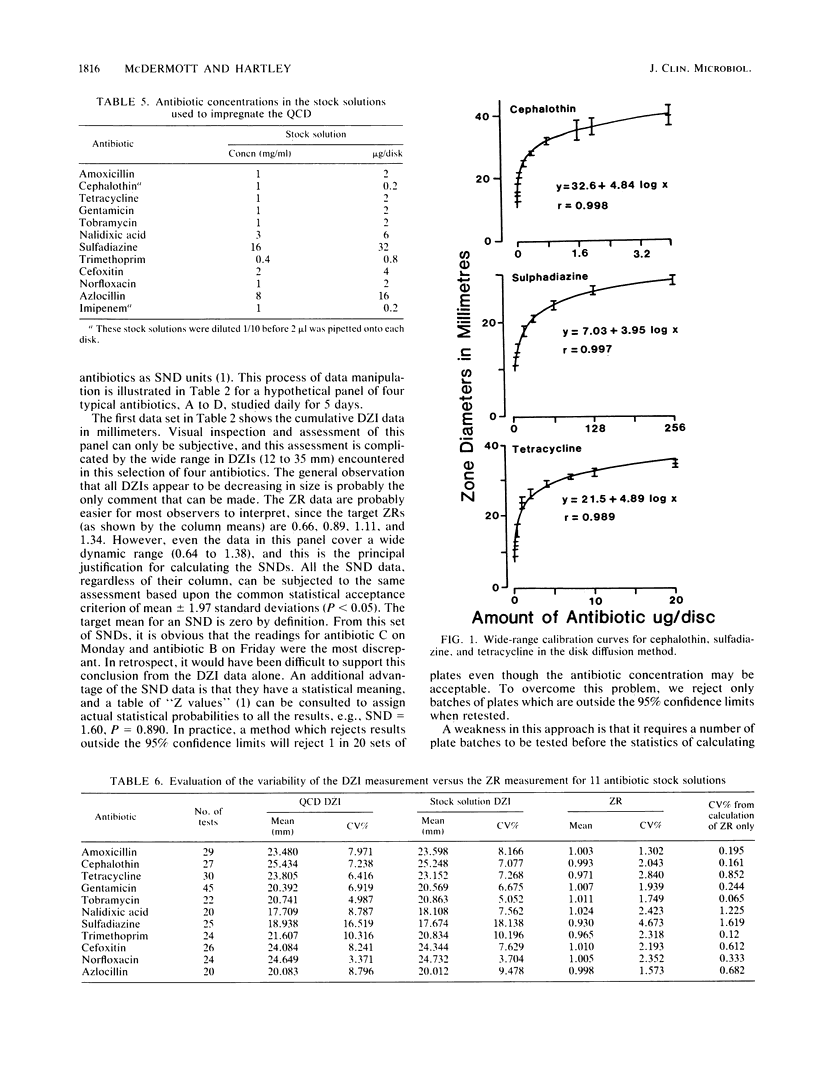
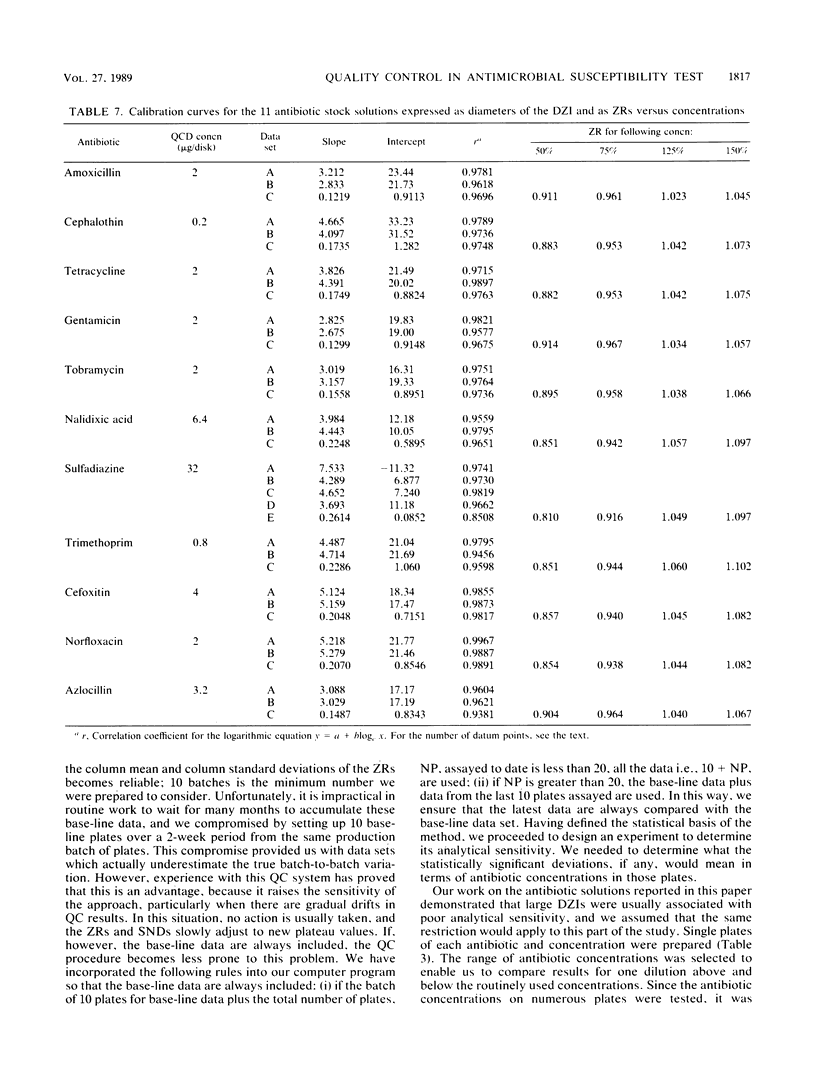
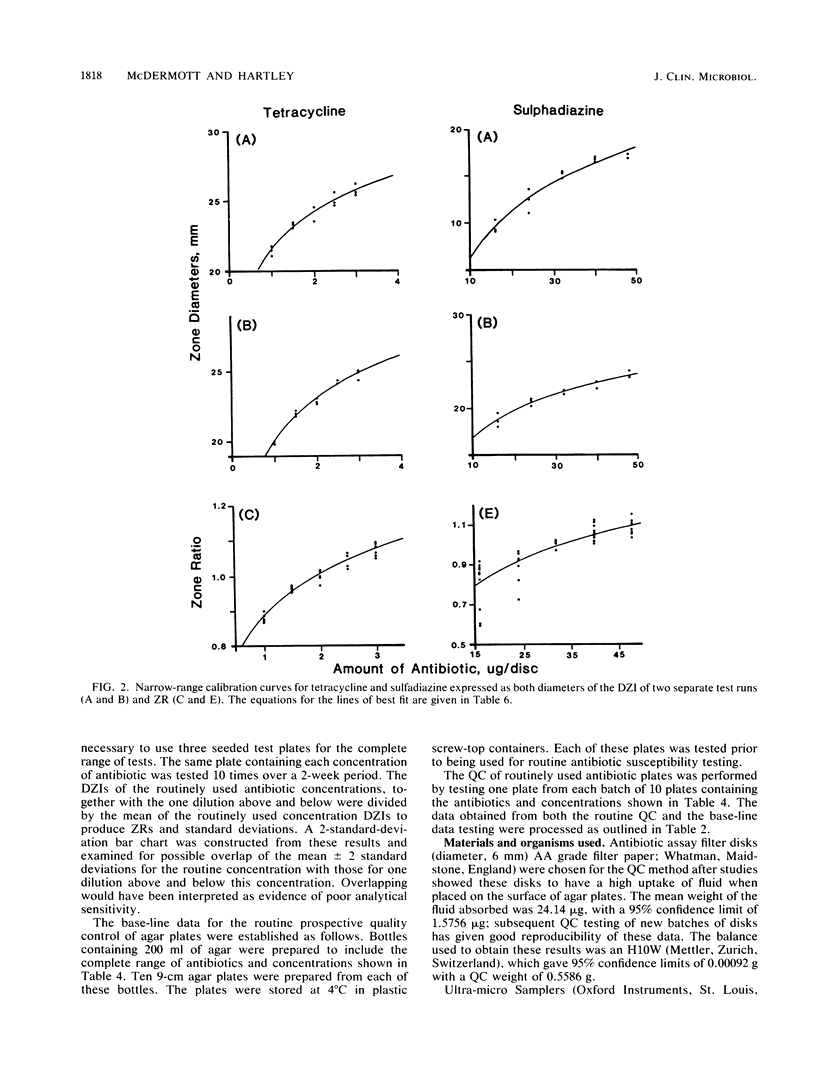
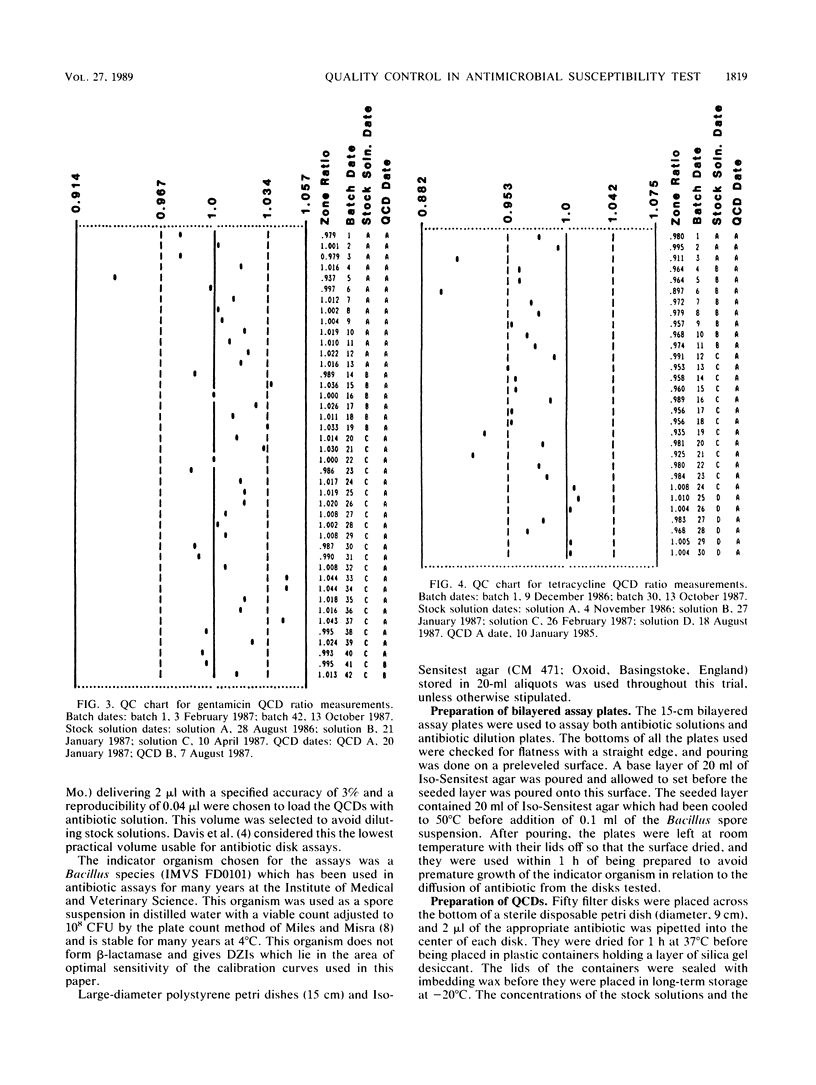
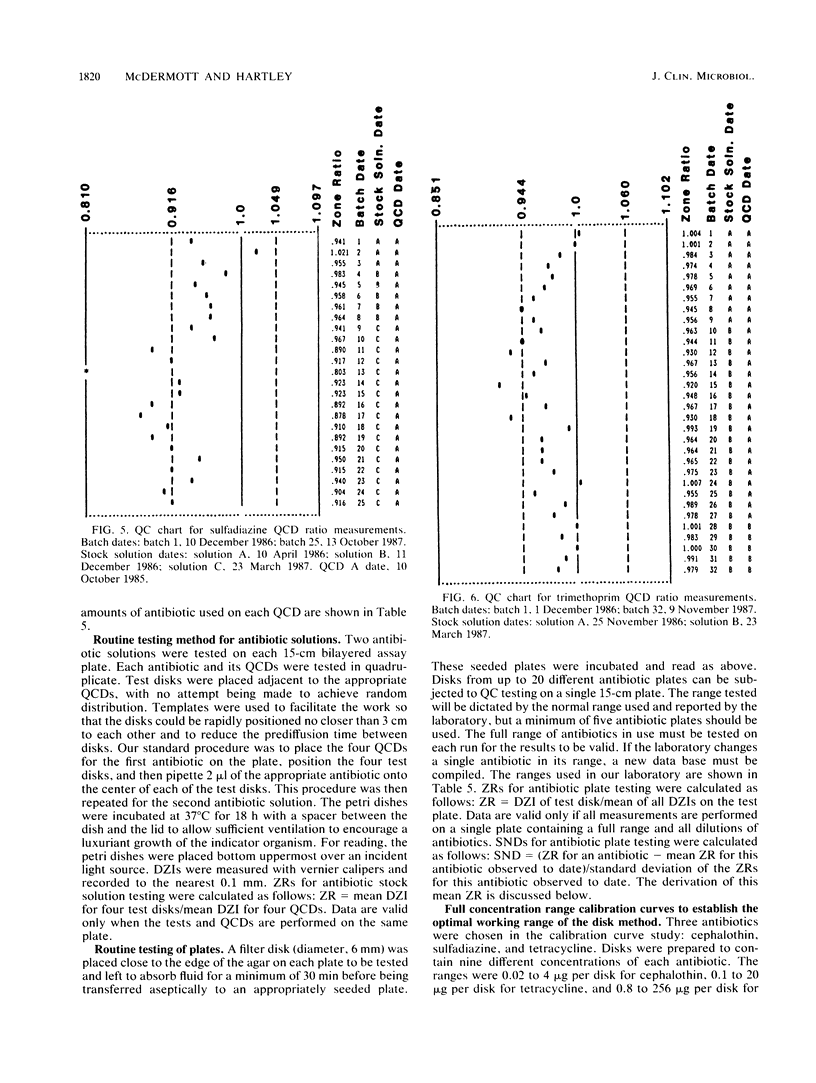
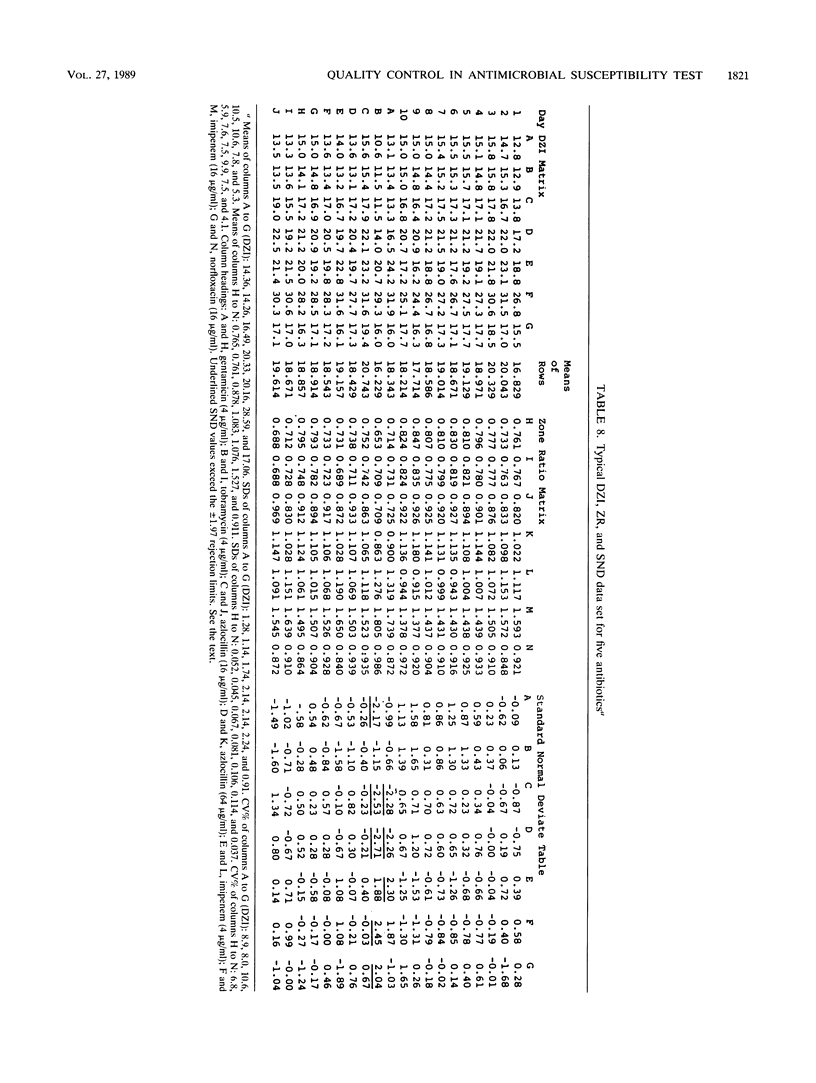
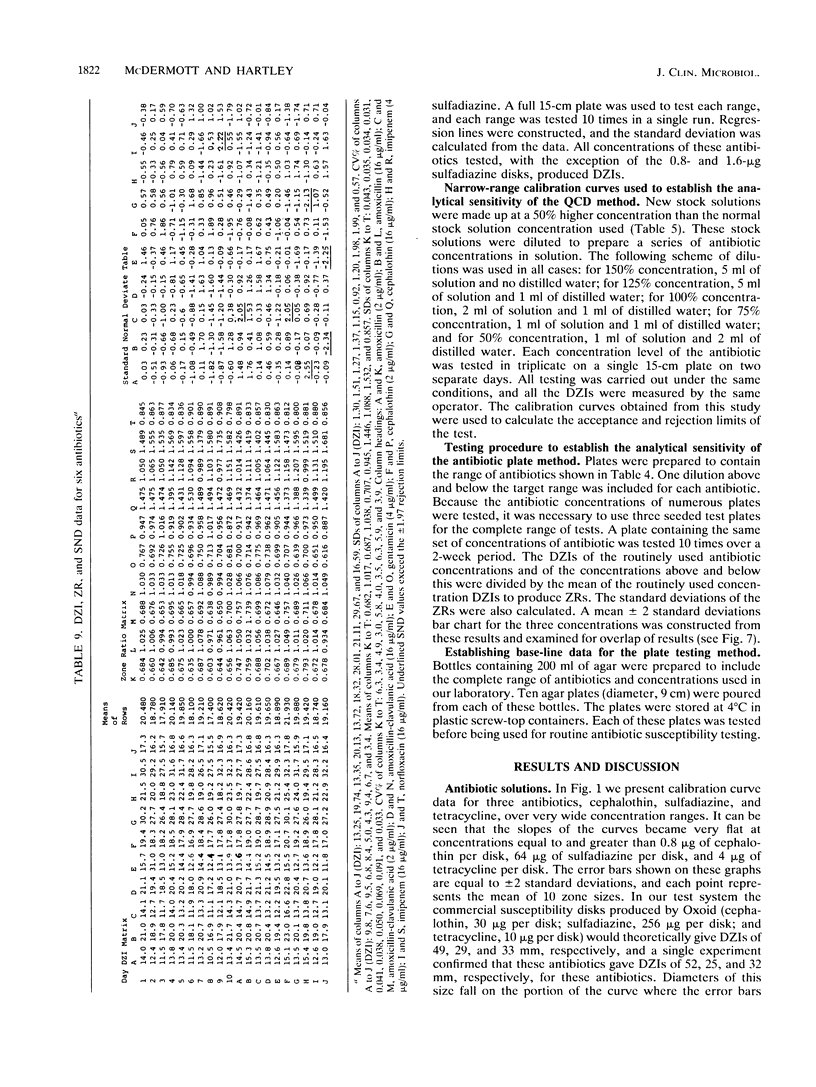
Quality control programs are described for monitoring both the antibiotic solutions and the plates used in the dilution antimicrobial susceptibility test. For the quality control of antibiotic solutions, a simple comparative disk diffusion assay is used in which the data are expressed as zone ratios calculated from the following formula: zone ratios = mean zones of inhibition for disks impregnated with antibiotic under test/mean zones of inhibition of previously prepared quality control disks impregnated with the same antibiotic. The rejection limits set for this method are twice as stringent as for MIC estimation, and its precision is four times greater. For quality control of the antibiotic agar plates, fluid was absorbed directly into filter paper disks placed on the surface of the medium. These disks were then transferred to a seeded plate to produce zones of inhibition. These zones of inhibition were then converted to zone ratios by dividing the zone of inhibition for the individual antibiotics by the mean of the total zones of inhibition for all the antibiotics tested on the same plate. We further normalized these data by expressing each entry as a standard normal deviate calculated from the mean and standard deviation of the cumulative data for each individual antibiotic. The use of zone ratios reduced batch-to-batch variation, and standard normal deviate values gave a uniform result for convenience. The method has a sensitivity equal to that of an MIC estimation if it were possible to estimate the MICs of antibiotics at these low levels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOPER K. E., LINTON A. H. The importance of the temperature during the early hours of incubation of agar plates in assays. J Gen Microbiol. 1952 Aug;7(1-2):8–17. doi: 10.1099/00221287-7-1-2-8. [DOI] [PubMed] [Google Scholar]
- Davis W. W., Stout T. R. Disc plate method of microbiological antibiotic assay. I. Factors influencing variability and error. Appl Microbiol. 1971 Oct;22(4):659–665. doi: 10.1128/am.22.4.659-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin J. C. Quality control in agar-dilution sensitivity-testing by direct assay of the antibiotic in the solid medium. J Clin Pathol. 1980 Jan;33(1):93–95. doi: 10.1136/jcp.33.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSHBAUM A., KRAMER J., ARRET B. The assay and control of antibiotic discs. Antibiot Chemother (Northfield) 1960 Apr;10:249–258. [PubMed] [Google Scholar]


