Abstract
Background:
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative condition affecting the motor system, but recent work also shows more widespread cognitive impairment. This study examined performance on measures requiring knowledge of actions, and related performance to MRI cortical atrophy in ALS.
Methods:
A total of 34 patients with ALS performed measures requiring word-description matching and associativity judgments with actions and objects. Voxel-based morphometry was used to relate these measures to cortical atrophy using high resolution structural MRI.
Results:
Patients with ALS were significantly more impaired on measures requiring knowledge of actions than measures requiring knowledge of objects. Difficulty on measures requiring action knowledge correlated with cortical atrophy in motor cortex, implicating degraded knowledge of action features represented in motor cortex of patients with ALS. Performance on measures requiring object knowledge did not correlate with motor cortex atrophy. Several areas correlated with difficulty for both actions and objects, implicating these brain areas in components of semantic memory that are not dedicated to a specific category of knowledge.
Conclusion:
Patients with amyotrophic lateral sclerosis are impaired on measures involving action knowledge, and this appears to be due to at least two sources of impairment: degradation of knowledge about action features represented in motor cortex and impairment on multicategory cognitive components contributing more generally to semantic memory.
GLOSSARY
- ALS
= amyotrophic lateral sclerosis;
- ALSFRS-R
= ALS Functional Rating Scale–Revised;
- FTLD
= frontotemporal lobar degeneration;
- VBM
= voxel-based morphometry.
Semantic memory is the long-term representation of the meaning of objects, actions, and thoughts. Our model of semantic memory includes two components1: the sensory-motor features contributing to a concept, represented neuroanatomically near where they are otherwise processed2; and an amodal component supporting processes like selection from semantic memory and generalization from familiar to novel instances.3 From this perspective, an action concept like “typing” depends in part on the representation of finger movements in frontal motor-related regions, and amodal processes that allow us to recognize typing on a mobile phone keyboard.4
This study examined the neural basis for action knowledge in amyotrophic lateral sclerosis (ALS), a disorder of the motor system. About 10% of patients with ALS have frontotemporal lobar degeneration,5 perhaps related to histopathologic abnormalities shared across these conditions.6 Some nondemented patients with ALS also may have executive control7–9 and language10,11 impairments. Selected patients have action verb deficits,12 although it is unclear whether this is related to the grammatic properties of verbs, executive difficulties that limit verb processing, or some other cognitive disorder. There is cortical atrophy in motor-associated regions in ALS,13,14 and fMRI studies show frontal motor activation when listening to action names,15 but we are not aware of MRI studies directly correlating action verb difficulty with the neuroanatomic distribution of disease in ALS. If motor cortex disease degrades features of action concepts, we reasoned that patients with ALS should have difficulty on tasks requiring action knowledge, and action knowledge deficits should correlate with atrophy in motor-related cortical areas.
METHODS
Subjects.
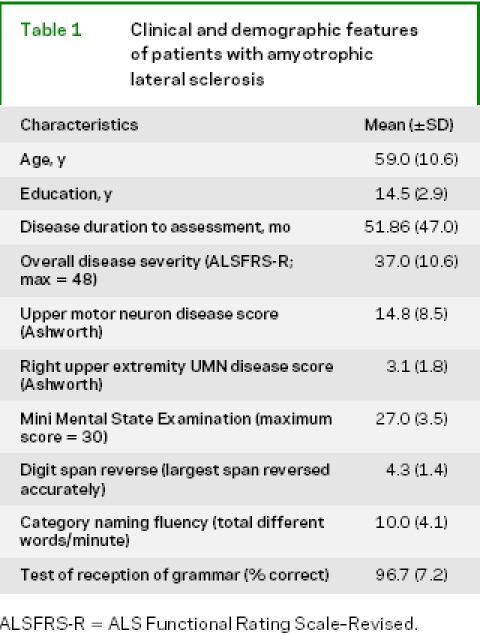
We studied 34 right-handed, high school educated, native English speakers with clinically definite ALS, diagnosed according to El Escorial criteria.16 Our initial cohort included 43 consecutively examined patients with ALS, but 9 were excluded because they did not have a score for both action knowledge and object knowledge (see below). These patients participated in an informed consent procedure approved by the University of Pennsylvania. Based on a screening battery involving abnormal performance (worse performance than 25 healthy matched controls, according to a z-score criterion of p < 0.05) on three of four tasks (reverse digit span, category naming fluency, an oral trails procedure, Frontal Behavioral Inventory) or the presence of a frontotemporal lobar degeneration (FTLD) syndrome, 14 (35.3%) of the 34 patients with ALS were thought to have a cognitive impairment. Exclusion criteria included evidence of another neurologic condition (e.g., trauma, hydrocephalus) or a medical condition that could explain their disorder. Participants were not taking sedating medications that could interfere with performance. Patients were evaluated by physicians who are experts in the diagnosis and care of patients with ALS (L.M., L.E.). Overall disease severity was assessed with the ALS Functional Rating Scale–Revised (ALSFRS-R),17 and the degree of upper motor neuron impairment across face, upper limbs, and lower limbs as well as right upper extremity disease were assessed with the Ashworth scale.18 Note should be made of the fairly high ALSFRS-R score relative to the fairly long duration of ALS. All patients did not perform all tasks listed below because of time limitations or inadvertent omission. Clinical and demographic features of the patients are summarized in table 1.
Table 1 Clinical and demographic features of patients with amyotrophic lateral sclerosis
Cognitive materials and procedures.
Two core tasks were administered to these patients. These measures were selected because they are verbally mediated, do not involve gesture or action performance, are untimed, require minimal response effort in any modality of expression, and involve minimal task-related resources.
Associativity judgments of actions and objects19: Patients were given a target verb naming an action or a noun naming an object, and were asked to select the one of two available single-word choices that goes best with the target. The frequencies of the target stimuli and the choices were matched across categories using a frequency count sensitive to grammatic category.20
Word-description matching of actions and objects21: Patients were given a phrase describing an action or object, and then were asked to select the best of four available action verbs or object nouns that matches the described target. The frequencies of the target stimuli and the content words of the descriptions were matched across categories using a frequency count sensitive to grammatic category.20
All participants had a score on both a measure of actions and a measure of objects to achieve a within-patient design. When a score was available for two action measures or two object measures, we averaged these percent accuracy scores to yield a single measure for that category of knowledge.
We administered additional cognitive measures to assess executive functioning and grammatic comprehension (the number of participants performing each task is provided in parentheses). These materials were administered to patients as part of a larger cognitive protocol typically on the same day as the imaging protocol. Digit span reverse22: the longest number of digits correctly repeated in the reverse order (n = 27); Category naming fluency23: the number of unique words beginning with a specified target letter (“F”) produced in 1 minute (n = 27); Test of the Reception of Grammar24: a four-alternative, forced-choice sentence-picture matching task, where we administered the subset of items requiring agreements for gender and number, and appreciation of grammatically complex phrasing (n = 32).
Imaging methods and procedures.
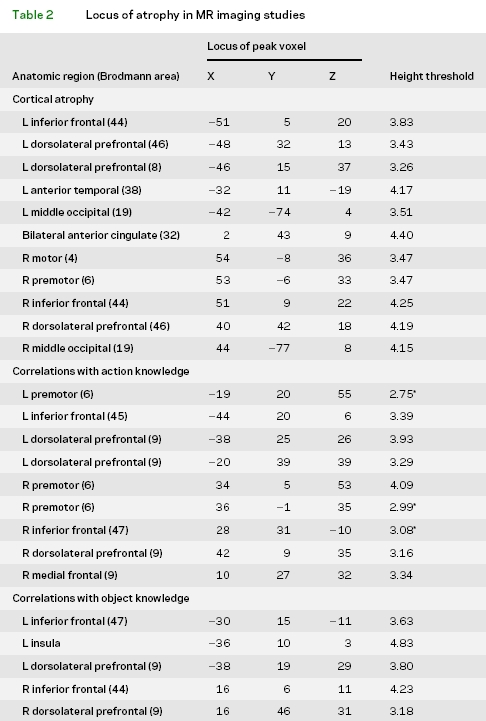
High resolution structural MRI scans were available in 26 patients with ALS to establish cortical volume using a modulated version of voxel-based morphometry (VBM). These patients were compared to 16 healthy, age-matched adults. Images were acquired by a Siemens Trio 3T MRI scanner. Each study began with a rapid sagittal T1-weighted image to determine patient position. Next, high resolution T1-weighted three-dimensional spoiled gradient echo images were acquired with repetition time = 1,620 msec, echo time = 3 msec, slice thickness 1.0 mm, flip angle 15º, matrix = 192 × 256, and in-plane resolution 0.9 × 0.9 mm. Brain volumes were registered in SPM2 using 12-parameter affine, nonlinear registration using 16 nonlinear iterations, and 7 × 8 × 7 basis functions. The brain volumes were then spatially normalized to the MNI coordinate system, and coordinates identified according to Talairach and Tournaux (www.mrc-cbu.cam.ac.uk/mni2tal). SPM2 was used to segment the brain volumes into four tissue types (gray matter, white matter, CSF, and other). Gray matter volume was smoothed with a 12 mm full-width half-maximum Gaussian filter. A threshold was used to include only voxels with >40% gray matter. Implicit masking was used to ignore zeros, and global calculation was omitted. The cortical volumes of patients with ALS were contrasted with controls using a two-sample t test to identify the anatomic distribution of significant cortical atrophy. The statistical threshold for significant atrophy included voxel height corrected for multiple comparisons by false discovery rate at p< 0.05, and cluster volume corrected for multiple comparisons at p< 0.05. SPM2 was used to perform a regression analysis relating cortical volume in patients with ALS to performance accuracy on measures of action knowledge and object knowledge. We used the percent correct accuracy scores computed for average performance on measures involving action knowledge and object knowledge. Because of the very small size of the voxels in the VBM analysis, we accepted a voxel height threshold of p < 0.001 uncorrected, and a cluster consisting of >100 adjacent, significantly correlated voxels, except where indicated by an asterisk in table 2.
Table 2 Locus of atrophy in MR imaging studies
RESULTS
Cognitive studies.
Patients with ALS were more impaired on measures requiring action knowledge than object knowledge [t(33) = 3.05; p < 0.005] (figure 1). The pattern of worse performance with measures requiring action knowledge compared to object knowledge was present in 24 (72.7%) of 33 individuals (one tied score) [χ2 (1) = 6.82; p < 0.01]. The effect of cognitive difficulty was evaluated with an analysis of variance, using a group (2) × category (2) design. We found a main effect for group [F(1,32) = 5.79; p < 0.02], where patients with ALS with cognitive difficulty (mean [±SD] = 88.6% [±9.1] correct) were significantly more impaired than patients with ALS without cognitive difficulty (mean [±SD] = 93.9% [±6.7] correct). There was also a main effect for category [F(1,32) = 11.03; p < 0.005], with measures requiring action knowledge worse than object knowledge. However, there was no interaction effect for group × category (p > 0.2), suggesting that difficulty on measures requiring action knowledge is not determined by the presence of overall cognitive impairment.
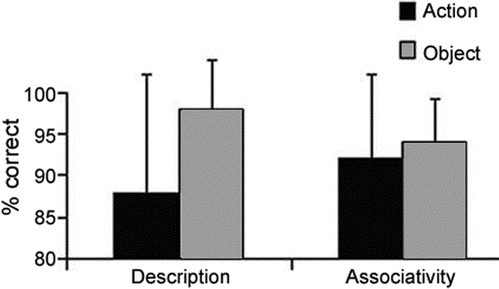
Figure 1 Mean (±SD) percent accuracy for actions and objects on word-description matching and associativity judgment tasks in amyotrophic lateral sclerosis
Performance on executive and grammatic measures is summarized in table 1. Performance on measures requiring action knowledge correlated with executive measures [category naming fluency: r(27) = 0.57; p < 0.005; digit span reverse: r(27) = 0.56; p < 0.005, two-tailed], while performance on measures of both action knowledge and object knowledge correlated with grammatic comprehension [action: r(32) = 0.58; p < 0.001; object: r(32) = 0.53; p < 0.005]. Grammatic comprehension thus does not appear to explain the greater impairment for action knowledge than object knowledge in ALS. We found no significant correlations between the cognitive measures and the demographic and clinical features.
Imaging studies.
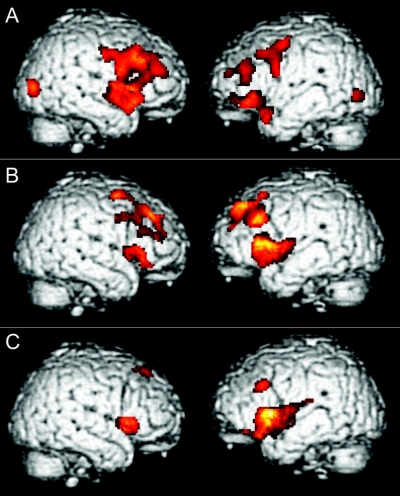
Figure 2A illustrates significant cortical atrophy in ALS relative to controls in the frontal lobes bilaterally. Table 2 summarizes the anatomic location of the peak voxels in the clusters showing significant cortical atrophy. We found significant cortical atrophy in motor and premotor cortex. We also saw significant atrophy in prefrontal and anterior temporal cortices.
Figure 2 (A) Cortical atrophy in amyotrophic lateral sclerosis; (B) correlation of cortical atrophy with performance on tasks requiring action knowledge; (C) correlation of cortical atrophy with performance on tasks requiring object knowledge
Figure 2B and table 2 show that cortical atrophy in premotor areas associated with the representation of the face, arm, and leg correlated with performance on measures requiring action knowledge. These areas overlapped in part with the distribution of cortical atrophy in these patients (see figure 2A). Nonmotor cortical regions associated with action knowledge included bilateral dorsolateral prefrontal cortex and inferior frontal cortex, also overlapping with cortical atrophy in ALS. Dorsolateral prefrontal and inferior frontal areas correlated significantly with performance on measures requiring object knowledge as well (see figure 2C and table 2). However, performance on measures requiring object knowledge did not correlate with motor cortex atrophy.
DISCUSSION
Patients with a motor disorder due to ALS were significantly impaired in their performance on measures requiring action knowledge. Correlation studies relating this deficit to cortical atrophy implicated two components of action concepts in ALS: The motor system that presumably supports the representation of knowledge of action features and an amodal system involved in processing actions as well as objects. We discuss these findings below.
ALS has long been characterized as a neurodegenerative disorder affecting the motor system, and patients with ALS were thought to have minimal cognitive difficulty. The exception appeared to include a handful of patients who had a co-occurring dementia with a phenotype resembling FTLD.5 Recent studies demonstrate instead that cognitive difficulties are not uncommon in ALS.7–11 Patients with ALS also have significant cortical atrophy that affects motor-associated brain regions, consistent with the upper motor neuron component of their disease. Moreover, cortical atrophy in ALS extends into prefrontal and anterior temporal regions.13,14 This is the anatomic distribution of disease in patients with FTLD, including those who have FTLD-U pathology.25,26 The unifying feature appears to be the underlying histopathology in these conditions: patients with ALS and patients with FTLD both show FTLD-U pathology,27,28 with ubiquitin inclusions that are immunoreactive for a TAR DNA protein of 43 kDa (TDP-43) thought to be the disease protein responsible for both conditions.29,30 One report implicates TDP-43 in the cognitive difficulties of FTLD-U patients.31
In this context, we found significant difficulty on measures requiring action knowledge compared to object knowledge in ALS. Although the discrepancy between actions and objects may appear somewhat greater for the word-description matching task than the associativity judgment task, the pattern of worse performance for actions than objects was present in a significant majority of individual patients with ALS. Greater difficulty on tasks requiring action knowledge than object knowledge emphasizes that the impairment cannot be explained entirely by a nonspecific semantic memory deficit. We hypothesize instead that the deficit in ALS is related at least in part to the degradation of action feature knowledge associated with a neurodegenerative condition that compromises motor-related cortical regions. Direct evidence consistent with the degradation of action knowledge comes from the correlation of performance on measures requiring action knowledge with cortical atrophy in motor regions. Since this correlation overlaps with the anatomic distribution of cortical atrophy in ALS, it appears that the large-scale neural network underlying action concepts is interrupted in these patients. The selective nature of this correlation is emphasized by the fact that performance on measures of object knowledge does not correlate with motor cortex atrophy. Sensory-motor theories of semantic memory postulate that the motor features of action concepts are represented in brain regions close to where the motor processes are implemented; namely, motor-associated cortex.2 fMRI studies of healthy adults show activation of motor and premotor cortex during tasks challenging action knowledge.4 For example, the leg region of primary motor cortex is activated when reading or silently naming verbs that involve a leg action, and the face region of primary motor cortex is recruited when reading and naming verbs that involve a facial action.15
While evidence in this study suggests that degraded action feature knowledge contributes to a semantic memory deficit in ALS, this may not entirely explain their difficulty. Performance on measures requiring action knowledge also correlated with executive control tasks. This correlation was not seen for measures of object knowledge. This is consistent with previous work showing that verbs are more difficult to process than nouns.32 We assessed other possible accounts for action verb difficulty. The sets of action and object words were matched for frequency, so this cannot explain the verb deficit. Performance on a grammatic comprehension task was correlated with measures involving both action verbs and object nouns. A comparison of subsets of patients with ALS with differing levels of cognitive difficulty found that both cognitively intact and cognitively impaired groups have greater difficulty with actions than objects, and that the discrepancy between these categories of knowledge is equivalent across groups. There are other differences between action verbs and object nouns that may contribute to the action verb deficit in ALS, and these should be investigated in future work.
We also found overlapping correlations between measures of both action and object knowledge, on the one hand, and cortical atrophy in inferior frontal and dorsolateral prefrontal cortex in ALS. fMRI studies in healthy adults show inferior frontal and dorsolateral prefrontal activation during semantically related executive processes. For example, interpretation of word meaning requires cognitive components beyond category-specific feature knowledge such as selection from semantic memory33,34 and categorization of stimuli into a meaningful concept.35,36 Executive components of semantic memory such as these are shared by action and object concepts, although this may affect verbs more than nouns because of the greater executive demands associated with verbs. The correlations in left inferior frontal and dorsolateral prefrontal regions also may be related to grammatic processing that allows verbs and nouns to be used correctly in speech. fMRI studies in healthy adults show inferior frontal and dorsolateral prefrontal activation during probes of lexical grammatic subcategory,37,38 and grammatic processing also utilizes executive resources.39,40
Several caveats must be kept in mind. We studied small numbers of patients on a limited number of tasks, and difficulty on measures involving action knowledge in ALS must be confirmed. We may not have seen a correlation between action knowledge and upper motor neuron disease because we did not assess actions involving specific body parts or study patients with disease involving only one body segment, and it would be useful to confirm involvement of specific motor cortex regions by relating impaired knowledge of actions involving specific body parts with disease involving the corresponding portion of motor cortex in patients with selective motor impairments. With these caveats in mind, we found a deficit on measures of comprehension in patients with ALS that compromises action knowledge significantly more than object knowledge. This impairment appears to reflect disease in motor cortex that interferes with tasks requiring knowledge of action features. The deficit also may be due in part to prefrontal disease associated with an amodal semantic impairment.
Address correspondence and reprint requests to Dr. Murray Grossman, Department of Neurology–2 Gibson, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA 19104-4283 mgrossma@mail.med.upenn.edu
Editorial, page 1388
e-Pub ahead of print on September 10, 2008, at www.neurology.org.
Supported in part by National Institutes of Health (AG17586, AG15116, NS44266, and NS53488) and the Dana Foundation.
Disclosure: The authors report no disclosures.
Received January 4, 2008. Accepted in final form May 1, 2008.
REFERENCES
- 1.Koenig P, Smith EE, Glosser G, et al. Categorization of novel animals by patients with Alzheimer’s disease and corticobasal degeneration. Neuropsychology 2007;21:195–206. [DOI] [PubMed] [Google Scholar]
- 2.Martin A. The representation of object concepts in the brain. Annu Rev Psychol 2007;58:25–45. [DOI] [PubMed] [Google Scholar]
- 3.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 2007;8:976–987. [DOI] [PubMed] [Google Scholar]
- 4.Pulvermuller F. Brain mechanisms linking language and action. Nat Rev Neuroscience 2005;6:576–582. [DOI] [PubMed] [Google Scholar]
- 5.Bak T, Hodges JR. Cognition, language and behavior in Motor Neurone disease: evidence of frontotemporal dysfunction. Dement Geriatr Cogn Disord 1999;10:29–32. [DOI] [PubMed] [Google Scholar]
- 6.Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol 2007;6:994–1003. [DOI] [PubMed] [Google Scholar]
- 7.Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 2000;38:734–747. [DOI] [PubMed] [Google Scholar]
- 8.Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller BL. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 2003;60:1094–1097. [DOI] [PubMed] [Google Scholar]
- 9.Robinson KM, Lacey SC, Grugan P, Glosser G, Grossman M, McCluskey LF. Cognitive functioning in sporadic amyotrophic lateral sclerosis: a six month longitudinal study. J Neurol Neurosurg Psychiatry 2006;77:668–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caselli RJ, Windebank AJ, Petersen RC. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol 1993;33:200–207. [DOI] [PubMed] [Google Scholar]
- 11.Strong MJ, Grace GM, Orange JB, Leeper HA. Cognition, language, and speech in amyotrophic lateral sclerosis: a review. J Clin Exp Neuropsychol 1996;18:291–303. [DOI] [PubMed] [Google Scholar]
- 12.Bak T, O’Donovan DG, Xuereb J, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain 2001;124:103–120. [DOI] [PubMed] [Google Scholar]
- 13.Ellis CM, Suckling J, Amaro E. Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology 2001;57:1571–1578. [DOI] [PubMed] [Google Scholar]
- 14.Chang JL, Lomen-Hoerth C, Murphy J, Henry RG, Kramer JH, Miller BL. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 2005;65:75–80. [DOI] [PubMed] [Google Scholar]
- 15.Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron 2004;41:301–307. [DOI] [PubMed] [Google Scholar]
- 16.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 1994;124:96–107. [DOI] [PubMed] [Google Scholar]
- 17.Cedarbaum J, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169: 13–21. [DOI] [PubMed] [Google Scholar]
- 18.Ashworth MB. Preliminary trial of Carisoprodol in multiple sclerosis. Practitioner 1964. [PubMed] [Google Scholar]
- 19.Howard D, Patterson KE. Pyramids and Palm Trees. Bury St. Edmonds: Thames Valley Test Company; 1992. [Google Scholar]
- 20.Francis WN, Kucera H. The Frequency Analysis of English Usage. Boston: Houghton-Mifflin Co.; 1982. [Google Scholar]
- 21.Yi HA, Moore P, Grossman M. Reversal of the concreteness effect for verbs in semantic dementia. Neuropsychology 2007;21:9–19. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale–Revised. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 23.Mickanin J, Grossman M, Onishi K, Auriacombe S, Clark C. Verbal and non-verbal fluency in patients with probable Alzheimer’s disease. Neuropsychology 1994;8:385–394. [Google Scholar]
- 24.Bishop R Test for the Reception of Grammar. Bury St. Edmonds, UK: Thames Testing Company; 1989. [Google Scholar]
- 25.Grossman M, Libon DJ, Forman MS, et al. Distinct antemortem profiles in pathologically defined patients with frontotemporal dementia. Arch Neurol 2007;64:1601–1609. [DOI] [PubMed] [Google Scholar]
- 26.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006;66:41–48. [DOI] [PubMed] [Google Scholar]
- 27.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006;59:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knopman DS, Boeve BF, Parisi JE, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol 2004;57:480–488. [DOI] [PubMed] [Google Scholar]
- 29.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–133. [DOI] [PubMed] [Google Scholar]
- 30.Davidson Y, Kelley T, Mackenzie IR, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 2007;113. [DOI] [PubMed] [Google Scholar]
- 31.Grossman M, Wood EM, Moore P, et al. TDP-43 pathology and clinical phenotype in frontotemporal lobar degeneration with ubiquitin. Arch Neurol 2007;64. [DOI] [PubMed] [Google Scholar]
- 32.Rhee J, Moore P, Grossman M. Verb comprehension in frontotemporal degeneration: The role of grammatical, semantic and executive components. Neurocase 2001;7:173–184. [DOI] [PubMed] [Google Scholar]
- 33.Thompson-Schill SL, D’Esposito M, Aguirre G, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA 1997;94:14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron 2001;31:329–336. [DOI] [PubMed] [Google Scholar]
- 35.Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Gee JC. Age-related changes in working memory during sentence comprehension: an fMRI study. NeuroImage 2002;15:302–317. [DOI] [PubMed] [Google Scholar]
- 36.Koenig P, Smith EE, Glosser G, et al. The neural basis for novel semantic categorization. NeuroImage 2005;24:369–383. [DOI] [PubMed] [Google Scholar]
- 37.Friederici A, Opitz B, von Cramon DY. Segregating semantic and syntactic aspects of processing in the human brain: an fMRI investigation of different word types. Cereb Cortex 2000;10:698–705. [DOI] [PubMed] [Google Scholar]
- 38.Friederici A, Meyer M, von Cramon DY. Auditory language comprehension: an event-related fMRI study on the processing of syntactic and lexical information. Brain Lang 2000;74:289–300. [DOI] [PubMed] [Google Scholar]
- 39.Cooke A, DeVita C, Gonzalez-Atavales J, et al. Large-scale neural network for sentence processing. Brain Lang 2005;96:14–36. [DOI] [PubMed] [Google Scholar]
- 40.Novais-Santos S, Gee J, Shah M, Troiani V, Work M, Grossman M. Resolving sentence ambiguity with planning and working memory resources: evidence from fMRI. NeuroImage 2007;37:361–378. [DOI] [PubMed] [Google Scholar]