Abstract
Objective:
To determine the anatomic correlate of prosopagnosia in subjects with semantic dementia.
Methods:
We identified all subjects who had been evaluated by an experienced behavioral neurologist, met criteria for semantic dementia, and had completed a volumetric head MRI scan. In all subjects, historical records were reviewed and subjects in which the presence (n = 15) or absence (n = 12) of prosopagnosia was specifically ascertained by the neurologist were identified. Voxel-based morphometry was used to assess patterns of gray matter atrophy in subjects with and without prosopagnosia compared to a group of age and gender-matched normal controls, and compared to each other.
Results:
Compared to controls, both groups showed prominent temporal lobe volume loss. Those with prosopagnosia showed bilateral loss but with greater involvement of the right temporal lobe, while those without prosopagnosia showed predominantly left anterior temporal lobe loss. On direct comparison, subjects with prosopagnosia showed greater loss predominantly in the right amygdala, hippocampus, fusiform gyrus, and anterior temporal pole than those without prosopagnosia. No regions were involved to a greater degree in those without prosopagnosia, compared to those with prosopagnosia.
Conclusions:
Prosopagnosia appears to be associated with volume loss of the right temporal lobe, particularly medial temporal lobe, fusiform gyrus, and anterior temporal pole, although in semantic dementia it is occurring in the context of bilateral temporal lobe volume loss.
GLOSSARY
- ADPR
= Alzheimer’s Disease Patient Registry;
- ADRC
= Alzheimer’s Disease Research Center;
- BNT
= Boston Naming Test;
- CDR-SB
= Clinical Dementia Rating score sum of boxes;
- DCT
= discrete cosine transformation;
- DRS
= Dementia Rating Scale;
- FTLD-U
= frontotemporal lobar degeneration with ubiquitin-only-immunoreactive changes;
- FWHM
= full-width at half-maximum;
- GM
= gray matter;
- MMSE
= Mini-Mental State Examination;
- MNI
= Montreal Neurological Institute;
- SPGR
= spoiled gradient echo;
- STMS
= Short Test of Mental Status;
- VBM
= voxel-based morphometry;
- WM
= white matter.
Semantic dementia is a multimodal disorder characterized by deficits in verbal and visual confrontation naming, poor comprehension of word meaning, semantic paraphasic errors (e.g., apple for grapefruit), and poor pronunciation of orthographically irregular words, i.e., a surface dyslexia (such as la-sag-na for lasagna).1–3 Semantic dementia, from a nosological standpoint, is one of the three well recognized variants of frontotemporal dementia, the other two being behavioral variant frontotemporal dementia and progressive nonfluent aphasia.4
In addition to aphasia, subjects with semantic dementia may also have difficulty recognizing objects by sight, smell, sound, touch, and may have difficulty recognizing familiar faces (prosopagnosia),5 such as faces of close family members or faces that tend to be seen frequently on television or in magazines. These features can occur in any combination, although one feature may be more prominent than the others. Prosopagnosia occurs in about a third of subjects with semantic dementia6 but in contrast to the visuoperceptual modality-specific prosopagnosia, it is cross-modal and hence patients are impaired in face, voice, and naming recognition.7
There have been case reports and group analyses demonstrating the association of semantic dementia with bilateral anterior temporal lobe atrophy.8 However, there are no studies that have assessed the anatomic correlate of prosopagnosia in semantic dementia using an unbiased image analysis methodology such as voxel-based morphometry (VBM). The aim of this study was therefore to determine what brain regions are associated with the presence of prosopagnosia in the context of semantic dementia.
METHODS
Subjects.
We identified all patients with semantic dementia who had been seen by a Mayo Clinic behavioral neurologist, and who had at least one volumetric head MRI scan. All cases were then reviewed by one behavioral neurologist (K.A.J.), blinded to radiologic findings, to ensure that they met clinical criteria for semantic dementia.4 A total of 39 subjects with semantic dementia were identified. The majority of cases had been prospectively recruited into the Alzheimer’s Disease Research Center (ADRC) or Alzheimer’s Disease Patient Registry (ADPR). The medical records of all cases were reviewed to abstract clinical data, including demographic features, Mini-Mental State Examination (MMSE),9 Short Test of Mental Status (STMS),10 Mattis Dementia Rating Scale (DRS),11 Clinical Dementia Rating score sum of boxes (CDR-SB),12 and Boston Naming Test (BNT) scores.13
These subjects were then categorized based on whether there was specific ascertainment of the presence or absence of prosopagnosia throughout the clinical history. Prosopagnosia was considered present when the clinician specifically documented that subjects had problems recognizing familiar faces, such as faces of close family members, friends, or very famous personalities (such as the current US president). Subjects who had trouble naming familiar people only without the recognition component were not considered to have prosopagnosia. Prosopagnosia was present in 15 subjects. In all cases the problems were originally reported, or confirmed, by an informant. The first MRI after the first reported occurrence of prosopagnosia was used for analysis in the prosopagnosia subjects. Prosopagnosia was considered absent when the clinician specifically stated that the subject did not have problems recognizing familiar faces. Prosopagnosia was absent in 12 subjects. Once again this was confirmed by an informant in all cases. The first MRI after disease onset was used for the subjects without prosopagnosia; in all cases the MRI occurred before the date that prosopagnosia was noted as absent. Six of the subjects have since come to autopsy (five with prosopagnosia and one without prosopagnosia); all six with a pathologic diagnosis of frontotemporal lobar degeneration with ubiquitin-only-immunoreactive changes (FTLD-U).14
Each semantic dementia subject was matched by age and gender to a cognitively normal control subject. All control subjects were prospectively recruited into the ADRC or the ADPR and were identified from the ADRC/ADPR database. Control subjects were cognitively normal individuals who had been seen in internal medicine for routine physical examinations and asked to enroll in the ADRC and ADPR. All subjects were then evaluated by a neurologist to verify the normal diagnosis. Controls were identified as individuals who 1) were independently functioning community dwellers, 2) did not have active neurologic or psychiatric conditions, 3) had no cognitive complaints, 4) had a normal neurologic and neurocognitive examination, and 5) were not taking any psychoactive medications in doses that would affect cognition.
Image analysis.
T1-weighted three-dimensional volumetric spoiled gradient echo (SPGR) sequences with 124 contiguous partitions and 1.6 mm slice thickness (22 × 16.5 cm or 24 × 18.5 field of view, 25° flip angle) were performed at 1.5 T and used for analysis. An optimized method of VBM was applied, implemented using SPM2 (http://www.fil.ion.ucl.ac.uk/spm).15,16 A number of preprocessing steps were performed to ready the data for statistical analysis, including spatial normalization and segmentation. The spatial normalization step transforms all images into the same stereotactic space by registering each of the images to the same specific template, and the segmentation of the brain into gray matter (GM), white matter (WM), and CSF is performed using the voxel intensities combined with a priori knowledge of the spatial distribution of these tissues, derived from probability maps. In order to reduce any potential normalization or segmentation bias across the disease groups, customized templates and prior probability maps were created from all subjects in the study, including both controls and semantic dementia subjects. To create the customized template and prior probability maps, all images were registered to the Montreal Neurological Institute (MNI) template using a 12 degrees of freedom (df) affine transformation and segmented into GM, WM, and CSF using the MNI prior probability maps. GM images were normalized to the MNI GM template using a nonlinear discrete cosine transformation (DCT). The normalization parameters were applied to the original whole head and the images were segmented once again. Average images were created of whole head, GM, WM, and CSF, and smoothed using 8 mm full-width at half-maximum (FWHM) smoothing kernel. The average whole head image becomes the customized template, and the average GM, WM, and CSF images are then used as the customized prior probability maps for subsequent segmentations.
All images were then registered to the customized whole brain template using a 12 df affine transformation and segmented using the customized prior probability maps. The GM images were normalized to the custom GM template using a nonlinear DCT. The normalization parameters were then applied to the original whole head and the images were segmented once again. All the GM images were modulated and smoothed with 8 mm FWHM smoothing kernel. In addition, a reinitialization routine was implemented. This uses the parameters from the initial normalization to the MNI template (performed to generate the customized template) to initialize the normalization to the custom template.16
A single-subject condition and covariate model, including age, gender, and total intracranial volume as covariates, was used to compare the smoothed modulated gray matter images between the semantic dementia subjects with prosopagnosia and controls, and between the semantic dementia subjects without prosopagnosia and controls. Given that the subjects with prosopagnosia performed worse cognitively than the subjects without prosopagnosia, we also repeated the analysis accounting for differences in global gray matter volume between the groups by implementing global normalization using analysis of covariance. A direct comparison was also performed between the two semantic dementia groups. Gray matter differences were assessed at a statistical threshold of p < 0.001 corrected for multiple comparisons using the false discovery rate correction.
Statistics.
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 6.0.0; SAS Institute Inc., Cary, NC) with α set at 0.05. Gender ratios were compared across groups with χ2 test. Kruskal-Wallis test was used to compare continuous data across the three groups. For any significant comparison, Wilcoxon rank sum test was used to determine if there was a significant difference between those with and without prosopagnosia.
RESULTS
The table shows the subject demographics for the 15 subjects with and 12 subjects without prosopagnosia, as well as controls. There was no significant difference between the semantic dementia subjects with and without prosopagnosia in age at onset, age at scan, time from onset to scan, education, MMSE, DRS, or BNT. The subjects with prosopagnosia, however, were more likely to be female and had worse CDR-SB scores.
Table Subject demographics
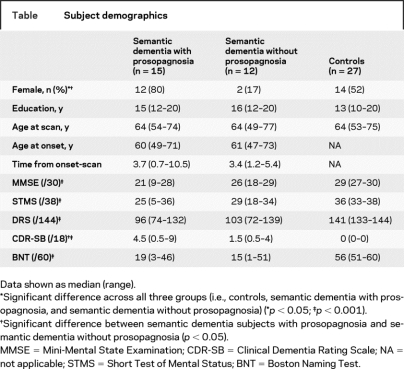
Compared with controls, the semantic dementia subjects with prosopagnosia showed gray matter loss bilaterally in the temporal lobes, with a greater degree of loss observed in the right temporal lobe (figure 1A). On the right, gray matter loss was observed throughout the temporal lobe with relative sparing of the posterior superior temporal gyrus. Gray matter loss was present from the anterior temporal pole, through the medial temporal lobe, inferior and middle temporal gyri, parahippocampal gyrus, and fusiform gyrus back to the most posterior extent of the fusiform and inferior temporal gyrus. The same regions were involved in the left temporal lobe, although to a lesser extent and severity. Gray matter loss was also observed in the right head of the caudate nucleus, bilateral insula, frontal lobes, and cerebellum. Compared with controls, the semantic dementia subjects without prosopagnosia showed gray matter loss restricted to the left temporal lobe, predominantly involving medial and inferior temporal regions and the anterior temporal pole (figure 1B). The left insula was also involved.
Figure 1 Patterns of gray matter loss observed in subjects with semantic dementia and prosopagnosia compared to controls (A), and subjects with semantic dementia without prosopagnosia compared to controls (B)
Results are shown on three-dimensional renders of the brain and representative coronal slices.
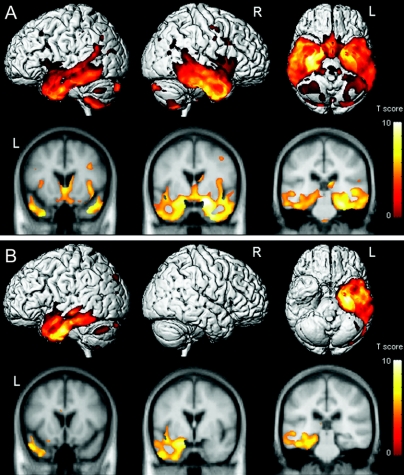
After normalization for global gray matter volume, the semantic dementia subjects with prosopagnosia showed a more asymmetric pattern of loss predominantly involving the right temporal lobe, with only minor involvement of the left temporal lobe, compared to controls (figure 2A). The semantic dementia subjects without prosopagnosia once again show a predominately left anterior and inferior temporal pattern of loss compared to controls (figure 2B).
Figure 2 Three-dimensional renders showing patterns of gray matter loss in subjects with semantic dementia and prosopagnosia compared to controls (A), and subjects with semantic dementia without prosopagnosia compared to controls (B), after global normalization
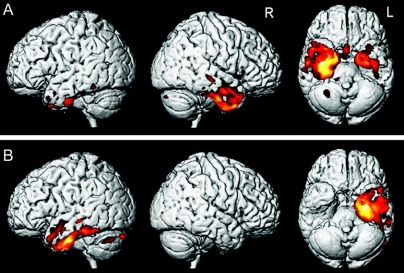
Direct comparisons were also performed between the two semantic dementia groups. The first comparison looked for regions that showed greater gray matter loss in the semantic dementia subjects with prosopagnosia than those without. Regions were identified in the right medial temporal lobe, including the amygdala, hippocampus and parahippocampal gyrus, right fusiform gyrus, and right temporal pole (figure 3). In the reverse comparison between the two groups, there were no regions that showed greater gray matter loss in the semantic dementia subjects without prosopagnosia than those with prosopagnosia.
Figure 3 Regions of greater gray matter loss in the semantic dementia subjects with prosopagnosia compared to the semantic dementia subjects without prosopagnosia
Results are shown on representative coronal slices through the temporal lobe.
DISCUSSION
In this study, we found that subjects with semantic dementia and prosopagnosia had gray matter loss bilaterally in the temporal lobes, with greatest loss in the right temporal lobe. Regions in the right temporal lobe, particularly the amygdala, hippocampus, parahippocampal gyrus, fusiform gyrus, and anterior temporal pole, were found to have significantly greater volume loss in those with prosopagnosia compared to those without prosopagnosia.
Relatively few studies have been performed on subjects with prosopagnosia and even fewer have investigated prosopagnosia in the context of semantic dementia. However, a number of case reports investigating subjects with acquired prosopagnosia have similarly shown atrophy in the right anterior inferior temporal lobe in these subjects.7,17–20 A longitudinal case report demonstrated that the onset of problems recognizing faces coincided with the development of atrophy in the right anterior temporal lobe19 and it has been suggested that the anterior temporal region is involved with retrieval of biographical information.21,22 The fusiform gyrus plays a central role in face processing, particularly in the perceptual analysis of faces,21 and has been shown to be atrophic in subjects with prosopagnosia.19,23 In our study the fusiform gyrus did show greater gray matter loss in the prosopagnosia subjects compared to those without prosopagnosia. A small group study has also demonstrated that the fusiform and middle temporal gyri are atrophic in subjects with congenital prosopagnosia, and that the volume of the fusiform gyrus correlates to performance on a famous face recognition test.24 A number of different studies have also implicated the hippocampus in prosopagnosia. A small study looking at three subjects who presented with problems recognizing faces demonstrated atrophy in the right hippocampus, as well as the right inferior and middle temporal gyri.25 Medial temporal lobe atrophy has similarly been reported in a subject with frontotemporal dementia and deficits in the recognition of familiar faces.20 Functional MRI studies have also shown that the hippocampus and parahippocampal gyrus are activated during the recognition of famous faces, although the laterality of this activation varies.22,26–28 It has been suggested that the hippocampus (specifically the right hippocampus) plays a role with retrieving relevant information from semantic memory.28,29 Whether, and how, the amygdala plays a role in the recognition of familiar faces is less clear since it is not a structure that has typically been implicated in prosopagnosia. However, the amygdala is important in the recognition of emotion and expression from faces,30 and it has been suggested that the emotional response we experience when seeing a familiar face plays an important role in successful recognition.31 The presence, and recognition, of a facial expression improves the ability to recognize familiar faces.32
It is therefore possible that these regions are specifically associated with the occurrence of prosopagnosia in these subjects. Previous authors have suggested that right-sided atrophy is responsible for prosopagnosia.33 However, it has also been suggested that prosopagnosia occurs in the context of bilateral damage,34 and bilateral structures have been associated with recognizing familiar faces.35,36 Our subjects with prosopagnosia did indeed show bilateral loss of the temporal lobes supporting this hypothesis, but it is important to remember that our results reflect the fact that these subjects all fulfill criteria for semantic dementia. Therefore, the left sided atrophy may simply be reflecting other nonprosopagnosia semantic deficits.
The main analysis shows a bilateral pattern of loss and is therefore not very helpful in determining whether the disease in the prosopagnosia subjects first develops in the left temporal lobe and then progresses to the right temporal lobe, whether it develops first in the right temporal lobe and then spreads to the left, or whether it progresses bilaterally. Furthermore, it is notable that the degree of left temporal lobe atrophy was comparable between the subjects with and without prosopagnosia (as seen in figure 1) and both groups also performed comparably on the BNT in which poor performance is associated with deficits in the left temporal lobe. If indeed the disease starts in the right temporal lobe and then spreads to the left one would expect the subjects with prosopagnosia to have less left temporal lobe atrophy than the subjects without prosopagnosia, given that both groups have the same time from disease onset to scan. Interpreting our findings is difficult since the subjects with prosopagnosia appear to be further along in their disease course than the subjects without prosopagnosia, evidenced by poorer performance on cognitive testing. Most likely, the subjects with prosopagnosia are not coming to medical attention until naming problems are evident, at which point the left temporal lobe would be expected to be affected; in essence, the prosopagnosia and disease goes undetected for many years.
To address this problem we corrected for the confounder of disease stage by including global gray matter volume as a covariate in the statistical model. We then demonstrated that the subjects with prosopagnosia showed much less loss of the left temporal lobe than the subjects without prosopagnosia (as seen in figure 2) and much less left temporal lobe than right temporal lobe atrophy. This finding would suggest that in those with prosopagnosia the left temporal lobe atrophy is a component of the more widespread or global atrophy and related to disease severity but that there is right temporal lobe atrophy beyond the degree of global atrophy. This is in keeping with the hypothesis that atrophy is beginning on the right and then progressing to the left and would not support a hypothesis that prosopagnosia is just a marker of disease severity in semantic dementia.
The semantic dementia subjects without prosopagnosia showed loss restricted to the left temporal lobe, particularly involving anterior medial and inferior temporal regions as has been previously emphasized in subjects with semantic dementia,8,37,38 but also involving more posterior aspects of the temporal lobe. It is very likely, however, that atrophy in these subjects will eventually spread to involve the right temporal lobe, as has been suggested by a couple of longitudinal studies in semantic dementia.39,40
An unexpected finding was the fact that the prosopagnosia subjects had a higher female to male ratio than the subjects without prosopagnosia. Given that we have a relatively small sample size this could have occurred by chance. Further studies are needed to investigate this association. In addition, future studies could correlate the degree of gray matter atrophy with the degree of prosopagnosia. This would require formal testing for prosopagnosia, which was not performed in our study. Formal testing could also allow us to determine whether the prosopagnosia was purely an associative phenomenon or whether perceptual deficits play a role.
Address correspondence and reprint requests to Dr. Keith A. Josephs, Department of Neurology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905 josephs.keith@mayo.edu
K.A.J. is supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078. Co-authors on this study are also supported by NIH grants P50-AG16574, U01-AG06786, R01-AG11378, as well as the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation, and the NIH Construction Grant (NIH C06 RR018898).
Disclosure: D.S.K. has been a consultant to GE HealthCare, GlaxoSmithKline, and Myriad Pharmaceuticals, has served on a Data Safety Monitoring Board for Neurochem Pharmaceuticals, and is an investigator in a clinical trial sponsored by Elan Pharmaceuticals. B.F.B. is an investigator in a clinical trial sponsored by Myriad Pharmaceuticals. R.C.P. has been a consultant to GE Healthcare and has served on a data safety monitoring board in a clinical trial sponsored by Elan Pharmaceuticals. C.R.J. receives research support from Pfizer in the form of research grants.
Received May 23, 2008. Accepted in final form August 8, 2008.
REFERENCES
- 1.Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol 1975;27:635–657. [DOI] [PubMed] [Google Scholar]
- 2.Snowden JS, Goulding P, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol 1989;2:167–182. [Google Scholar]
- 3.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain 1992;115:1783–1806. [DOI] [PubMed] [Google Scholar]
- 4.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 5.Bozeat S, Lambon Ralph MA, Patterson K, et al. Non-verbal semantic impairment in semantic dementia. Neuropsychologia 2000;38:1207–1215. [DOI] [PubMed] [Google Scholar]
- 6.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 2003;61:1196–1203. [DOI] [PubMed] [Google Scholar]
- 7.Evans JJ, Heggs AJ, Antoun N, Hodges JR. Progressive prosopagnosia associated with selective right temporal lobe atrophy: a new syndrome? Brain 1995;118:1–13. [DOI] [PubMed] [Google Scholar]
- 8.Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol 2001;49:433–442. [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 10.Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc 1987;62:281–288. [DOI] [PubMed] [Google Scholar]
- 11.Mattis S Mental status examination for organic mental syndrome in the elderly patients. In: Bellack LT, ed. Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. New York: Grune and Stratton; 1976:77–121. [Google Scholar]
- 12.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan E. The Boston Naming Test. Boston: Veterans Administration Medical Center; 1978. [Google Scholar]
- 14.Josephs KA, Holton JL, Rossor MN, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 2004;30:369–373. [DOI] [PubMed] [Google Scholar]
- 15.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–821. [DOI] [PubMed] [Google Scholar]
- 16.Senjem ML, Gunter JL, Shiung MM, et al. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage 2005;26:600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyrrell PJ, Warrington EK, Frackowiak RS, Rossor MN. Progressive degeneration of the right temporal lobe studied with positron emission tomography. J Neurol Neurosurg Psychiatry 1990;53:1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gainotti G, Barbier A, Marra C. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain 2003;126:792–803. [DOI] [PubMed] [Google Scholar]
- 19.Joubert S, Felician O, Barbeau E, et al. Progressive prosopagnosia: clinical and neuroimaging results. Neurology 2004;63:1962–1965. [DOI] [PubMed] [Google Scholar]
- 20.Gainotti G, Ferraccioli M, Quaranta D, Marra C. Cross-modal recognition disorders for persons and other unique entities in a patient with right fronto-temporal degeneration. Cortex 2008;44:238–248. [DOI] [PubMed] [Google Scholar]
- 21.Gorno-Tempini ML, Price CJ, Josephs O, et al. The neural systems sustaining face and proper-name processing. Brain 1998;121:2103–2118. [DOI] [PubMed] [Google Scholar]
- 22.Leveroni CL, Seidenberg M, Mayer AR, et al. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci 2000;20:878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton JJ, Press DZ, Keenan JP, O’Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology 2002;58:71–78. [DOI] [PubMed] [Google Scholar]
- 24.Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cereb Cortex 2007;17:2354–2363. [DOI] [PubMed] [Google Scholar]
- 25.Joubert S, Felician O, Barbeau E, et al. The right temporal lobe variant of frontotemporal dementia: cognitive and neuroanatomical profile of three patients. J Neurol 2006;253:1447–1458. [DOI] [PubMed] [Google Scholar]
- 26.Kapur N, Friston KJ, Young A, et al. Activation of human hippocampal formation during memory for faces: a PET study. Cortex 1995;31:99–108. [DOI] [PubMed] [Google Scholar]
- 27.Bernard FA, Bullmore ET, Graham KS, et al. The hippocampal region is involved in successful recognition of both remote and recent famous faces. Neuroimage 2004;22:1704–1714. [DOI] [PubMed] [Google Scholar]
- 28.Elfgren C, van Westen D, Passant U, et al. fMRI activity in the medial temporal lobe during famous face processing. Neuroimage 2006;30:609–616. [DOI] [PubMed] [Google Scholar]
- 29.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol 1995;5:169–177. [DOI] [PubMed] [Google Scholar]
- 30.Rosen HJ, Perry RJ, Murphy J, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain 2002;125:2286–2295. [DOI] [PubMed] [Google Scholar]
- 31.Gobbini MI, Haxby JV. Neural response to the visual familiarity of faces. Brain Res Bull 2006;71:76–82. [DOI] [PubMed] [Google Scholar]
- 32.de Gelder B, Frissen I, Barton J, Hadjikhani N. A modulatory role for facial expressions in prosopagnosia. Proc Natl Acad Sci USA 2003;100:13105–13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Renzi E, Perani D, Carlesimo GA, et al. Prosopagnosia can be associated with damage confined to the right hemisphere: an MRI and PET study and a review of the literature. Neuropsychologia 1994;32:893–902. [DOI] [PubMed] [Google Scholar]
- 34.Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology 1982;32:331–341. [DOI] [PubMed] [Google Scholar]
- 35.Tsukiura T, Namiki M, Fujii T, Iijima T. Time-dependent neural activations related to recognition of people’s names in emotional and neutral face-name associative learning: an fMRI study. Neuroimage 2003;20:784–794. [DOI] [PubMed] [Google Scholar]
- 36.Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull 2005;67:87–93. [DOI] [PubMed] [Google Scholar]
- 37.Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology 2001;57:216–225. [DOI] [PubMed] [Google Scholar]
- 38.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002;58:198–208. [DOI] [PubMed] [Google Scholar]
- 39.Whitwell JL, Anderson VM, Scahill RI, et al. Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 2004;17:307–310. [DOI] [PubMed] [Google Scholar]
- 40.Brambati SM, Rankin KP, Narvid J, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging Epub 2007 Jun 29. [DOI] [PMC free article] [PubMed]