Abstract
Objective:
To investigate the association between fish consumption and subclinical brain abnormalities.
Methods:
In the population-based Cardiovascular Health Study, 3,660 participants age ≥65 underwent an MRI scan in 1992–1994. Five years later, 2,313 were scanned. Neuroradiologists assessed MRI scans in a standardized and blinded manner. Food frequency questionnaires were used to assess dietary intakes. Participants with known cerebrovascular disease were excluded from the analyses.
Results:
After adjustment for multiple risk factors, the risk of having one or more prevalent subclinical infarcts was lower among those consuming tuna/other fish ≥3 times/week, compared to <1/month (relative risk 0.74, 95% CI = 0.54–1.01, p = 0.06, p trend = 0.03). Tuna/other fish consumption was also associated with trends toward lower incidence of subclinical infarcts. Additionally, tuna/other fish intake was associated with better white matter grade, but not with sulcal and ventricular grades, markers of brain atrophy. No significant associations were found between fried fish consumption and any subclinical brain abnormalities.
Conclusions:
Among older adults, modest consumption of tuna/other fish, but not fried fish, was associated with lower prevalence of subclinical infarcts and white matter abnormalities on MRI examinations. Our results add to prior evidence that suggest that dietary intake of fish with higher eicosapentaenoic acid and docosahexaenoic acid content, and not fried fish intake, may have clinically important health benefits.
GLOSSARY
- ARR
= absolute risk reduction;
- BMI
= body mass index;
- CHD
= coronary heart disease;
- CHS
= Cardiovascular Health Study;
- DHA
= docosahexaenoic acid;
- EPA
= eicosapentaenoic acid;
- FFQ
= food frequency questionnaire;
- HDL-C
= high-density lipoprotein cholesterol;
- LDL-C
= low-density lipoprotein cholesterol;
- PUFA
= polyunsaturated fatty acid;
- RR
= relative risk.
Clinically unrecognized or “silent” brain infarcts are very common, particularly with advancing age. Among adults age ≥65 without infarcts on initial brain MRI, nearly 20% had at least one new infarct when MRI was performed about 5 years later, but without recognized TIA or clinical stroke in nearly 90% of cases.1 Persons with MRI-defined infarcts experience greater cognitive decline than persons without infarcts, and such individuals are also at higher risk of future clinical stroke.1–3
Fish consumption is associated with risk of clinically recognized stroke. Among older adults, consumption of tuna or other broiled or baked fish correlated with plasma phospholipid n-3 polyunsaturated fatty acid (PUFA) levels and was associated with lower stroke risk.4 Consumption of fried fish did not correlate with n-3 PUFA levels and was associated with higher stroke risk.4 Whether fish consumption affects the risk of subclinical infarcts or other subclinical brain abnormalities is unknown. Fish consumption is associated with decreased risk of dementia and cognitive decline,5 for which major risk factors are subclinical brain abnormalities.6 We hypothesized that, as with overt infarcts,4 consumption of tuna/other fish would be associated with lower risk of subclinical infarcts among older adults, while fried fish consumption would be associated with higher risk. We also investigated associations of fish consumption with MRI-defined white matter abnormalities and ventricular and sulcal enlargement.7,8
METHODS
Study population.
The Cardiovascular Health Study (CHS) is a prospective cohort study of 5,888 older adults. The design and recruitment experience have been described.8,9 Briefly, 5,201 men and women age ≥65 at baseline were randomly selected and enrolled in 1989–1990 from Medicare eligibility lists in four United States communities. An additional 687 black participants were enrolled in 1992–1993. Each center’s institutional review committee approved the study, and all subjects gave informed consent. All participants underwent extensive baseline evaluations including standard questionnaires, physical examination, performance measures, and laboratory testing.8–10 Parts of the baseline evaluation were repeated during annual follow-up visits. Prevalent coronary heart disease (CHD), stroke, TIA, hypertension, and diabetes were defined using patients’ reports and confirmed by centralized review of hospital and clinic records.8,9
Assessment of dietary intake.
Usual dietary intakes were assessed in 1989–1990 using a picture-sort version of the National Cancer Institute food frequency questionnaire (FFQ).11 Participants were asked to indicate how often, on average, they had consumed various specific foods during the past year, including tuna fish, other broiled or baked fish, and fried fish or fish sandwiches (fish burgers). Nutrient intakes were estimated from questionnaire responses and adjusted for total calories using regression analyses.12,13 Dietary eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) intakes were calculated from questionnaire responses using estimated fish and shellfish serving sizes (3–5 oz [84–140 g])14 and US commercial landings data.15 Tuna or other broiled or baked fish correlated with combined plasma phospholipid EPA+DHA concentrations (r = 0.51), a biomarker of n-3 fatty acid intake, in a subsample of participants.16 Phospholipid EPA+DHA concentrations did not correlate with fried fish consumption (r = 0.04), consistent with the lean types of fish that are typically fried (e.g., cod, pollock).16
Usual dietary intakes were assessed again in 1995–1996 using Willett’s semiquantitative FFQ.17 Participants were asked to indicate how often, on average, they had consumed given amounts of various specified foods during the past year, including canned tuna fish (3–4 oz [84–112 g]); dark-meat fish such as mackerel, salmon, sardines, bluefish, and swordfish (3–5 oz [84–140 g]); and other white fish (3–5 oz [84–140 g]). Consumption of fried fish was not specifically assessed. Nutrient intakes were calculated as frequency of intake multiplied by nutrient composition of the specified portion size. Nutrient estimates were based on US Department of Agriculture14 and Harvard University food composition database sources, and adjusted for total energy by regression analyses.13 Dietary EPA and DHA were calculated from questionnaire responses using estimates for each fish serving, as previously described,18 and validated in other cohorts by comparisons with multiple weighted 1-week dietary records and against adipose stores of n-3 fatty acids17,19; the correlation between EPA+DHA intake and proportion in adipose tissue was 0.47.19
Brain imaging.
CHS participants were invited to undergo MRI scanning between 1991 and 1994. A total of 3,660 (62%) underwent scanning and were slightly younger and healthier than those who did not undergo MRI.20 All participants were again invited to undergo MRI scanning 5 years later, between 1997 and 1999, and 2,313 were scanned. A total of 2,116 participants underwent both scans and were healthier than the 1,544 who underwent only the initial scan, including a lower prevalence of cardiovascular disease, hypertension, and diabetes, and current smoking, and higher income and education.1
The cranial MRI scanning protocol included sagittal T1-weighted localizer images and axial T1, spin density, and T2-weighted images.21 Without knowledge of participants’ clinical information, neuroradiologists at the CHS reading center identified infarcts and estimated white matter, ventricular, and sulcal grades, as detailed previously.10,20 Grades were defined using a semiquantitative 10-point scale from 0 to 9 (most abnormal), based on comparison templates.10 As previously described,22 ventricular and sulcal grades were grouped for analysis as ≤2, 3, 4, and ≥5, and white matter grade as ≤1, 2, 3, and ≥4. Brain infarct was defined as an area of abnormal signal intensity, ≥3 mm in size, in a vascular distribution that lacked mass effect.10
Statistical analysis.
We used the prospectively collected repeated measures of dietary intake and MRI scans to perform both cross-sectional and longitudinal analyses. Relative risk (RR) of subclinical infarcts was evaluated using logistic regression. RR of number of subclinical infarcts (range 0 to 4) or abnormal sulcal, ventricular, and white matter grades (four levels) was evaluated using ordinal logistic regression. Absolute risk reduction (ARR) was calculated by multiplying the absolute risk in the reference group by the multivariable-adjusted RR reduction in the comparison group.
For cross-sectional analyses of prevalent subclinical neurologic abnormalities, dietary intake in 1989–1990 was related to MRI findings in 1991–1994, and dietary intake in 1995–1996 to MRI findings in 1997–1999. After confirming in preliminary analyses that the observed relationships were similar in the two periods evaluated separately, data were combined to assess the repeated measures. All analyses were clustered within-individual with robust variance estimates to assess both between- and within-individual covariance (specifying that observations were independent across individuals but not within individuals).
For each diet-MRI pairing, we excluded participants with history of prevalent TIA or stroke, incomplete data on fish consumption, or incomplete data on sulcal, ventricular, or white matter grades (figure 1). For evaluation of subclinical infarcts, we also excluded possible (<3 mm) infarcts or evidence of prior hemorrhage (figure 1). For longitudinal analyses of incident subclinical neurologic abnormalities, we excluded participants with any MRI-defined (clinical or subclinical) infarcts or prior hemorrhage at the first MRI (figure 1). We then related dietary intake from the first questionnaire to subclinical MRI findings on the second MRI, excluding participants with clinically diagnosed TIA or stroke before the first MRI or between the two MRIs (figure 2).
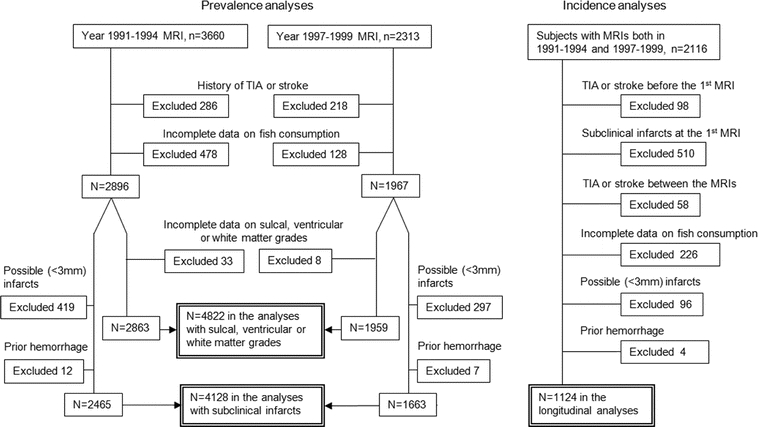
Figure 1 Exclusion criteria for the cross-sectional and longitudinal analyses
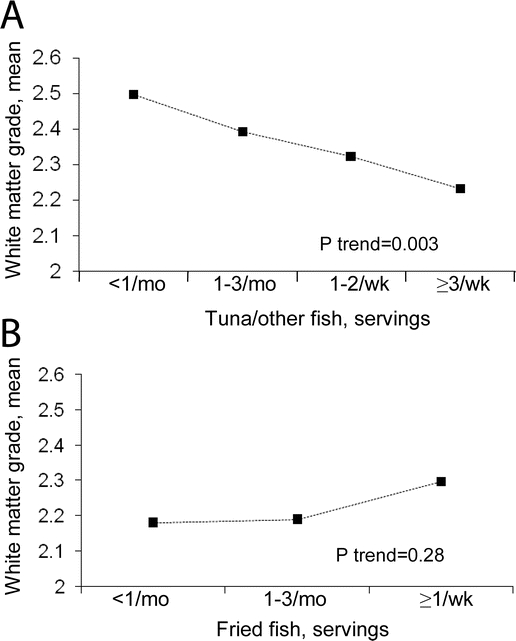
Figure 2 White matter grades according to fish consumption
Adjustments were made for fried fish or tuna/other fish consumption and age, sex, race, enrollment center, diabetes, education, smoking status, pack-years of smoking, body mass index, coronary heart disease at the time of MRI, alcohol use, physical activity, energy intake, meat consumption, and vegetable consumption.
Multivariate models included possible confounders based upon clinical interest, previously published associations with MRI findings,1,4,23 or associations with exposures or outcomes in the present analysis. Covariates were those from the examination closest in time to the brain imaging. The final models included tuna/other fish intake, fried fish intake, age, sex, race, enrollment center, diabetes, education, smoking, pack-years of smoking, body mass index, prevalent CHD, alcohol use, physical activity, energy intake, meat consumption, and vegetable consumption. Because the second dietary questionnaire did not include information on fried fish consumption, only data from the first MRI were used for analyses of relationships of fried fish intake with MRI abnormalities. For covariate adjustment only, fried fish values for the second questionnaire were imputed using responses on the 1989–1990 FFQ and age, sex, race, enrollment center, diabetes, education, smoking, pack-years of smoking, body mass index, prevalent CHD, alcohol use, physical activity, energy intake, meat consumption, and vegetable consumption. Further adjustments for systolic blood pressure, atrial fibrillation, exercise intensity, use of estrogen, aspirin, lipid-lowering or hypertension medication, LDL or HDL cholesterol, triglycerides, time between MRI scans, carotid intima-media thickness, forced expiratory volume, history of frequent falls, serum creatinine, serum c-reactive protein, fasting glucose, insulin, fibrinogen, factor VII, use of fish oil (<5% of participants), or consumption of saturated fat, fiber, or fruit did not appreciably alter (<5%) the risk estimates between fish or EPA+DHA intake and the MRI findings.
Likelihood ratio tests using multiplicative interaction terms were used to explore potential effect modification by age, sex, race, education, diabetes, prevalent CHD, hypertension, systolic blood pressure, alcohol intake, smoking, and use of aspirin, lipid lowering, or antihypertensive medications. All p values were two-tailed (α = 0.05). Analyses were performed using Stata 8.0 (Stata Corp, College Station, TX).
RESULTS
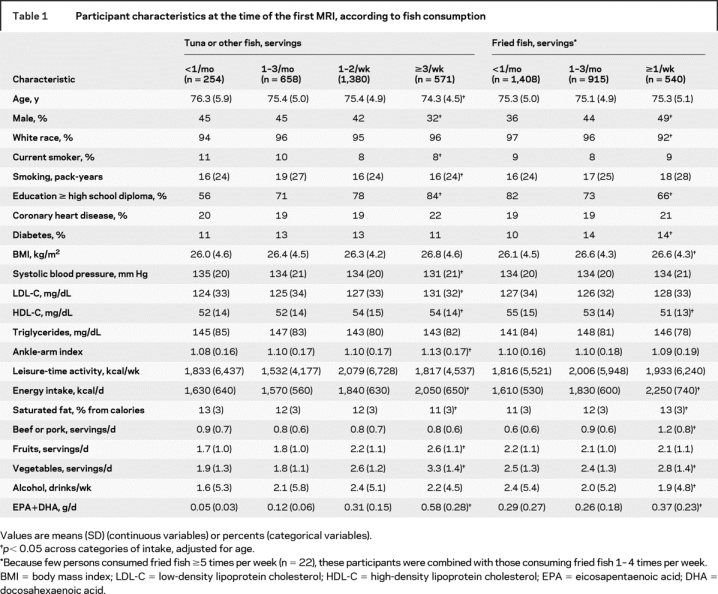
At baseline, higher tuna/other fish consumption was associated with younger age, female sex, higher education, lower systolic blood pressure, higher LDL and HDL cholesterol, and higher ankle-arm index, whereas higher fried fish consumption was associated with male sex, nonwhite race, lower education, higher prevalence of diabetes, higher BMI, and lower HDL cholesterol (table 1). Tuna/other fish consumption was also associated with higher energy intake, higher fruit and vegetable consumption, and lower saturated fat intake. Fried fish consumption was associated with higher energy intake, saturated fat intake, vegetable consumption, and beef or pork consumption, and lower alcohol intake.
Table 1 Participant characteristics at the time of the first MRI, according to fish consumption
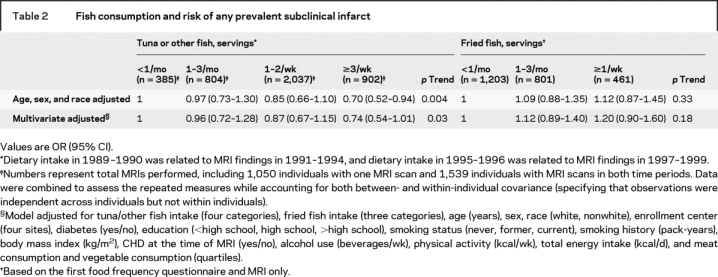
A total of 567 of 2,465 participants (23.0%) had one or more subclinical infarcts on the first MRI. On the second MRI, 382 of 1,663 participants (23.1%) had one or more subclinical infarcts. In multivariable-adjusted analyses, the RR for having a subclinical infarct was 0.74 (95% CI = 0.54–1.01, p = 0.06; p trend = 0.03) in individuals consuming tuna/other fish ≥3 times/week, compared to consumption <1/month (table 2) (reference group absolute risk = 26.5%; ARR = 6.9%). In contrast, fried fish consumption was associated with nonsignificant trends toward higher prevalence of subclinical infarct (table 2). Evaluated continuously, each one serving/week of tuna/other fish was associated with 7% lower RR of having any prevalent subclinical infarct (95% CI = 0.6–12%, p = 0.03). Each one serving/week of tuna/other fish was also associated with 7% lower RR of each additional multiple infarct (95% CI = 0.8–12%, p = 0.03).
Table 2 Fish consumption and risk of any prevalent subclinical infarct
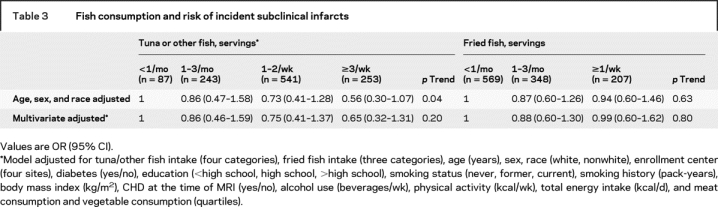
In the longitudinal analyses, 187 of 1,124 participants (16.6%) had one or more new subclinical infarcts between the two MRIs. Tuna/other fish consumption tended to be associated with lower incidence of subclinical infarct after adjustment for age, sex, race, and fried fish consumption (table 3). The RR in those consuming tuna/other fish ≥3 times/week was 0.56 (95% CI = 0.30–1.07, p = 0.08; p trend = 0.04; ARR = 9.6%), compared to <1/month. Each one serving/week of tuna/other fish was associated with trends toward 11% lower RR of any incident subclinical infarct (95% CI = −0.7–22%, p = 0.07) and 12% lower RR of each additional multiple infarct (95% CI = −0.1–22%, p = 0.05). Further adjustment for other risk factors attenuated these trends, although the directions of effect remained similar to findings seen for prevalent infarct. No associations were seen between fried fish consumption and incident subclinical infarcts (table 3).
Table 3 Fish consumption and risk of incident subclinical infarcts
Consumption of tuna/other fish was associated with better white matter grade (figure 1). Individuals consuming tuna/other fish ≥3 times/week had 10.6% better white matter grade scores, compared to <1/month (p trend = 0.003), after adjustment for other risk factors. Evaluated continuously, each one serving/week of tuna/other fish was associated with a 3.8% better white matter grade score (p = 0.06). Tuna/other fish consumption was not significantly associated with sulcal or ventricular grades (p > 0.10 for each). Conversely, in these models, strong associations with sulcal and ventricular grades were seen for male gender, white race, and higher age, consistent with previously reported findings.24 Fried fish consumption was not associated with white matter grade (figure 1) or sulcal or ventricular grades (p > 0.10 for each).
Findings for estimated consumption of dietary EPA+DHA were generally similar to those for consumption of tuna/other fish. For example, compared to the lowest quintile of EPA+DHA intake, individuals in the highest quintile had 23% lower risk of prevalent subclinical infarct (RR = 0.77, 95% CI = 0.59–1.00, p = 0.048; p trend = 0.03; corresponding ARR = 6.0%), and 8.7% better white matter grade score (p trend = 0.01).
Little evidence was present for significant effect modification (statistical interaction) by age, sex, race, education, diabetes, CHD at the time of MRI, hypertension, systolic blood pressure, alcohol intake, smoking, or use of aspirin, lipid lowering, or hypertension medications (p > 0.05).
DISCUSSION
In this large cohort of older adults, higher consumption of tuna/other fish or dietary EPA+DHA was associated with lower prevalence of clinically unrecognized (MRI-defined) infarcts and better white matter grade but not with measures of brain atrophy (ventricular or sulcal grades) among individuals without known TIA or stroke. Tuna/other fish and EPA+DHA consumption were also associated with trends toward lower incidence of subclinical infarcts, although the associations were attenuated and no longer significant after multivariate adjustment, possibly related to limited power (only 187 incident subclinical infarcts). No associations were found between fried fish consumption and any subclinical neurologic abnormalities.
Both subclinical infarcts and white matter abnormalities are commonly observed on MRI scans in the elderly,1,10,23,24 but most often do not cause acute symptoms or signs and are clinically unrecognized. However, these abnormalities are not benign. Both are associated with cognitive and neurobehavioral impairments and with higher risk of future overt stroke.2,3,6,25 Recent articles from the Zutphen Elderly Study26 and the Atherosclerosis Risk in Communities Study27 demonstrated inverse associations between dietary or plasma EPA+DHA and cognitive decline among elderly participants. Similar results have been found in some other,28 but not all,29 studies. Fish or EPA+DHA consumption has also been associated with lower risk of dementia and Alzheimer disease,5,30 which are characterized by progressive cognitive decline. Our findings suggest that prevention of subclinical infarcts and white matter abnormalities may be one mechanism whereby fish or EPA+DHA consumption may decrease the development of these debilitating conditions.
Subclinical infarcts and white matter abnormalities are considered to be of vascular origin, presumably resulting from occlusion of small arteries in the brain and subsequent ischemia.31 Fish consumption has been associated with lower risk of clinical ischemic strokes.4,32 This lower risk may be related to favorable effects of EPA+DHA on blood pressure, lipids, red blood cell deformability, inflammation, endothelial cell function, cerebral arteriolar reactivity, and platelet function33–36 (although impact on platelets is very minor at dietary doses of EPA+DHA37). In contrast to large clinical strokes, small subclinical infarcts are not thought to be due to arterial stenosis or emboli from the heart or large arteries. Rather, disease of small cerebral vessels is regarded as the most important cause of lacunar infarcts and white matter abnormalities, although exact mechanisms are poorly understood.38
Tuna/other fish and EPA+DHA consumption were not associated with sulcal or ventricular grades, indicators of brain atrophy. Thus, the observed associations with subclinical infarcts and white matter abnormalities were relatively specific, rather than reflecting general associations of tuna/other fish or EPA+DHA intake with fewer brain abnormalities. The specificity of the findings suggests that fish consumption, and EPA+DHA, may affect mechanisms related to ischemic injury, particularly related to small vessel disease.
Few prior studies have investigated the relationships between consumption of different types of fish meals and risk of stroke.4,39 We did not find any beneficial associations between fried fish consumption and subclinical brain abnormalities, in accordance with previous studies of fried fish intake and risk of ischemic stroke and CHD.4,16 In contrast to tuna or other broiled or baked fish meals, the types of fish used in fried fish meals, such as fish burgers or fish sticks, are typically low in EPA+DHA, as supported by the lack of association of fried fish intake with blood levels of these fatty acids.16 Commercially prepared fried fish meals may also contain trans fatty acids or lipid oxidation products formed when frying oils are used repeatedly.40 Although not all studies have found differences between types of fish meals consumed,39 the results of the present article support the growing evidence that the type of fish meal consumed is important for obtaining the health benefits of fish consumption. The lower risk of subclinical brain abnormalities with estimated dietary EPA+DHA consumption suggests that the n-3 fatty acid content of the fish meal may be particularly important.
Our analysis has strengths, including the use of prospectively collected data on dietary intake and MRI findings, the population-based recruitment, the large numbers of participants enrolled, and the extensive standardized examinations of other risk factors. Potential limitations are also present. Participants who underwent MRI scans were somewhat healthier than those who did not, so results may not be fully applicable to the general elderly population. Although interreader reliabilities of white matter and ventricular grades are good, estimates of sulcal grade have greater interreader variability.24 Information on fish preparation methods (e.g., frying) was unavailable from the second FFQ, limiting our ability to separately evaluate fried fish at the second MRI and possibly biasing results for overall fish consumption toward the null. The estimation of dietary fish and EPA+DHA consumption by FFQ is imperfect and would result in some exposure misclassification. Because this would largely be random with respect to the outcomes, such errors would diminish ability to detect relationships between dietary habits and disease risk; thus, our results are likely to underestimate the true association between fish or EPA+DHA consumption and risk of subclinical brain abnormalities. The FFQs and MRI scans were not administered simultaneously; however, FFQs assess long-term habitual dietary intakes. Observed associations could be related to other differences related to fish consumption, such as a healthier lifestyle in general. However, we adjusted for a variety of other risk factors and lifestyle habits.
Our results support the need for randomized trials of fish or fish oil intake to reduce subclinical ischemic events, which would be feasible and important given the high incidence of such events in older adults.
ACKNOWLEDGMENT
The authors thank Eric B. Rimm from the Harvard School of Public Health for his comments. The authors also thank the CHS participants.
Address correspondence and reprint requests to Dr. Jyrki K. Virtanen, University of Kuopio, School of Public Health and Clinical Nutrition, Research Institute of Public Health, P.O. Box 1627, 70211 Kuopio, Finland jyrki.virtanen@uku.fi
The research reported in this article was supported by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01- HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org. Additional support was provided by grants from the Finnish Cultural Foundation, Helsingin Sanomat Centennial Foundation, Finnish Foundation for Cardiovascular Research, Yrjö Jahnsson Foundation, and University of Kuopio, for J.K. Virtanen.
Disclosure: The authors report no disclosures.
Received November 2, 2007. Accepted in final form May 1, 2008.
REFERENCES
- 1.Longstreth WT Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study. Stroke 2002;33:2376–2382. [DOI] [PubMed] [Google Scholar]
- 2.Bernick C, Kuller L, Dulberg C, et al. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology 2001;57:1222–1229. [DOI] [PubMed] [Google Scholar]
- 3.Price TR, Manolio TA, Kronmal RA, et al. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. CHS Collaborative Research Group Stroke 1997;28:1158–1164. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Longstreth WT Jr, Lemaitre RN, et al. Fish consumption and stroke risk in elderly individuals: the Cardiovascular Health Study. Arch Intern Med 2005;165:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim WS, Gammack JK, Van Niekerk J, Dangour AD. Omega 3 fatty acid for the prevention of dementia. Cochrane Database Syst Rev 2006;1:CD005379. [DOI] [PubMed] [Google Scholar]
- 6.Kuller LH, Lopez OL, Jagust WJ, et al. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 2005;64:1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosley TH Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: The Atherosclerosis Risk in Communities Study. Neurology 2005;64:2056–2062. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 9.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993;3:358–366. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth WT Jr, Bernick C, Manolio TA, et al. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: The Cardiovascular Health Study. Arch Neurol 1998;55:1217–1225. [DOI] [PubMed] [Google Scholar]
- 11.Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture-sort approach. Am J Clin Nutr 1997;65(4 suppl):1123S–1129S. [DOI] [PubMed] [Google Scholar]
- 12.Smucker R, Block G, Coyle L, Harvin A, Kessler L. A dietary and risk factor questionnaire and analysis system for personal computers. Am J Epidemiol 1989;129:445–449. [DOI] [PubMed] [Google Scholar]
- 13.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, release 17. Nutrient Data Laboratory Home Page. Available at: http://www.ars.usda.gov/Main/site_main.htm?modecode=12-35-45-00. Accessed August 9, 2007.
- 15.National Marine Fisheries Service, Fisheries of the United States–2000. Silver Spring, MD: US Department of Commerce; 2001. [Google Scholar]
- 16.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: The Cardiovascular Health Study. Circulation 2003;107:1372–1377. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126. [DOI] [PubMed] [Google Scholar]
- 18.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and n-3 fatty acids and risk of stroke in women. JAMA 2001;285:304–312. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 1992;135:418–427. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: The Cardiovascular Health Study. Stroke 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 21.Bryan R, Manolio T, Schertz L, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The Cardiovascular Health Study. AJNR Am J Neuroradiol 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 22.Mukamal KJ, Longstreth WT Jr, Mittleman MA, Crum RM, Siscovick DS. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: the cardiovascular health study. Stroke 2001;32:1939–1946. [DOI] [PubMed] [Google Scholar]
- 23.Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study. Stroke 2005;36:56–61. [DOI] [PubMed] [Google Scholar]
- 24.Yue NC, Arnold AM, Longstreth WT Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology 1997;202:33–39. [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH, Longstreth WT, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ Jr . White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 26.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study. Am J Clin Nutr 2007;85:1142–1147. [DOI] [PubMed] [Google Scholar]
- 27.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr 2007;85:1103–1111. [DOI] [PubMed] [Google Scholar]
- 28.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004;62:275–280. [DOI] [PubMed] [Google Scholar]
- 29.Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ. n-3 Fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis 2003;5:315–322. [DOI] [PubMed] [Google Scholar]
- 30.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60:940–946. [DOI] [PubMed] [Google Scholar]
- 31.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–659. [DOI] [PubMed] [Google Scholar]
- 32.He K, Rimm EB, Merchant A, et al. Fish consumption and risk of stroke in men. JAMA 2002;288:3130–3136. [DOI] [PubMed] [Google Scholar]
- 33.Nestel PJ. Fish oil and cardiovascular disease: lipids and arterial function. Am J Clin Nutr 2000;71(1 Suppl):228S–231S. [DOI] [PubMed] [Google Scholar]
- 34.Agren JJ, Hanninen O, Hanninen A, Seppanen K. Dose responses in platelet fatty acid composition, aggregation and prostanoid metabolism during moderate freshwater fish diet. Thromb Res 1990;57:565–575. [DOI] [PubMed] [Google Scholar]
- 35.Ellis EF, Police RJ, Dodson LY, McKinney JS, Holt SA. Effect of dietary n-3 fatty acids on cerebral microcirculation. Am J Physiol 1992;262(5 Pt 2):H1379–1386. [DOI] [PubMed] [Google Scholar]
- 36.Knapp HR. n-3 Fatty acids and human hypertension. Curr Opin Lipidol 1996;7:30–33. [DOI] [PubMed] [Google Scholar]
- 37.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296:1885–1899. [DOI] [PubMed] [Google Scholar]
- 38.Fisher CM. Lacunar strokes and infarcts: a review. Neurology 1982;32:871–876. [DOI] [PubMed] [Google Scholar]
- 39.Wennberg M, Bergdahl IA, Stegmayr B, et al. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br J Nutr 2007;98:1038–1045. [DOI] [PubMed] [Google Scholar]
- 40.Candela M, Astiasaran I, Bello J. Deep-fat frying modifies high-fat fish lipid fraction. J Agric Food Chem 1998;46:2793–2796. [Google Scholar]


