Abstract
Background
Cognitive dysfunction is fairly common after noncardiac surgery and may be related to intraoperative blood pressure management. The authors present an analysis of risk factors for cognitive deterioration after spine surgery in older patients, with particular emphasis on intraoperative blood pressure in normotensive and hypertensive patients.
Methods
This is a post hoc cohort analysis of 45 patients enrolled before undergoing lumbar laminectomy or microdiscectomy. The patients underwent a battery of 5 neuropsychometric tests preoperatively, and 1 day and 1 month postoperatively. Computerized anesthesia records were used to obtain intraoperative mean arterial pressure (MAP) data. Simple linear regressions between intraoperative MAP and postoperative cognitive performance were performed, and multivariate linear regression models of postoperative cognitive performance were constructed to analyze potential risk factors for cognitive decline after surgery.
Results
Twenty-one normotensive patients (mean age, 62.4 yr) and 24 hypertensive patients (mean age, 67.9 yr) were included in this analysis. There was a significant positive relationship between minimum intraoperative MAP values and 1-day cognitive performance by simple linear regression in hypertensive (P = 0.003), but not normotensive, patients. In multivariate linear regression analysis of cognitive performance, there was a significant interaction between hypertension and minimum intraoperative MAP at 1 day and 1 month.
Conclusions
In hypertensive patients, there was a significant relationship between minimum intraoperative MAP and decline in cognitive function 1 day and 1 month after surgery. A prospective controlled trial of intraoperative blood pressure control, especially during induction of anesthesia when MAP values typically drop, is needed to confirm these findings.
POSTOPERATIVE cognitive dysfunction is relatively common after a variety of noncardiac surgeries.1–3 Some of the mechanisms responsible for this dysfunction probably depend on intraoperative events related to the surgery or anesthesia. For example, we believe it is likely that cognitive dysfunction after carotid endarterectomy is largely the result of cerebral ischemia occurring during carotid artery cross-clamping.1
Intraoperative decreases in systemic mean arterial pressure (MAP), which potentially lead to decreases in cerebral perfusion, are another possible mechanism of cerebral ischemia and resultant postoperative cognitive dysfunction. The relationship between intraoperative blood pressure and cognition has been examined before; notably in elderly noncardiac surgery patients,2 cardiac surgery patients on cardiopulmonary bypass,4,5 and elderly patients undergoing hypotensive epidural anesthesia for primary total hip replacement.6 Of these, only elderly patients undergoing cardiac surgery developed cognitive dysfunction associated with lower intraoperative blood pressure, and this association only pertained to certain neuropsychometric tests.4 However, in these studies the investigators did not separately analyze patients with hypertension who, as a result of altered cerebrovascular autoregulation, might be at greater risk for decreased cerebral perfusion secondary to decreased systemic blood pressure.7 This view is supported by a study of patients undergoing cardiac surgery, which demonstrated that hypertensive patients had higher postoperative increases in peripheral blood levels of S100B, a marker of cerebral injury.8 They hypothesized that this larger increase was the result of altered cerebrovascular autoregulation, and suggested that cardiopulmonary bypass management protocols may need adjustment to improve cerebral perfusion in hypertensive patients. Indeed, Gold et al.5 demonstrated less postoperative neurologic complications in patients randomized to the higher of two target blood pressure ranges while on cardiopulmonary bypass, although they did not find an association with postoperative cognition or report on the possible effect of hypertension. Thus, there is evidence that intraoperative blood pressure values can affect cerebral perfusion in significant ways, at least in cardiac surgery patients. We report a post hoc analysis of potential risk factors for poorer postoperative cognitive performance in older patients undergoing spine surgery, with special attention paid to intraoperative MAP measures of normotensive versus hypertensive patients. Computerized anesthesia records were used to eliminate the potential bias of hand-recorded blood pressure values.9
Materials and Methods
This study, approved by the Columbia University Medical Center Institutional Review Board (New York, New York), is a post hoc cohort analysis of 48 patients older than 40 yr, enrolled before scheduled lumbar laminectomy (without instrumentation) or microdiscectomy from 2003–2007. These patients were initially recruited to serve as a control group in an ongoing study of cognitive change after carotid endarterectomy.1 Before enrollment, patients provided informed written consent for study participation. Patients not proficient in English were excluded (required for neuropsychometric test validity). Upon enrollment, all patients underwent a brief medical history interview and were classified as having hypertension if they reported such a diagnosis during the interview, had a diagnosis noted in their anesthesia record, or reported taking antihypertensive medications.
Patients were assessed preoperatively (baseline), 1 day postoperatively, and 1 month postoperatively using a battery of 5 neuropsychometric tests described elsewhere (Boston Naming Test, Halstead-Reitan Neuropsychological Test Battery Trials A and B, Controlled Oral Word Association Test, and the Copy portion of the Rey Complex Figure Test).1,10 For each patient, Z scores were generated for change in neuropsychometric performance from baseline for each neuropsychometric test at 1 day and 1 month postoperatively by dividing the difference of the patient’s change in neuropsychometric test score and the mean change in neuropsychometric test score of all patients by the SD of the change in neuropsychometric test score of all patients. Composite Z scores were then calculated for each patient at 1 day and 1 month by averaging all the Z scores from the 5 neuropsychometric tests. Thus, a negative composite Z score indicates a decreased overall performance from baseline relative to the mean of all patients. Only patients who completed all five tests were included in statistical analyses for each time point. Pain was also assessed at each time point using the Wong-Baker FACES Pain Rating Scale (range from 0 to 10; 10 indicating severe pain).
All patients underwent lumbar laminectomy or micro-discectomy and received general anesthesia with routine monitoring. Computerized anesthesia records (CompuRecord; Philips Medical Systems, Andover, MA) were used in each case, recording all standard hemodynamic and anesthesia parameters. Blood pressure was measured by pneumatic cuff approximately every 2–3 min (4 patients had arterial catheters that recorded blood pressures every 15 s). All blood pressure values were retained, except when blood pressure values from the arterial catheters at one time point exceeded the values before and after it by more than 50%.
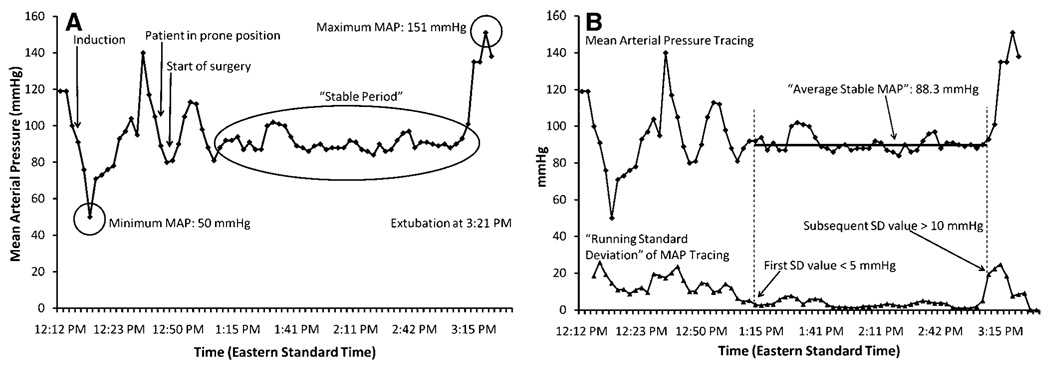
Preoperative mean blood pressures for each patient were recorded by the caring anesthesiologist in the anesthesia record. These pressures were obtained in the preoperative waiting area or during the preoperative medical examination. Intraoperative blood pressure management was left to the discretion of the caring anesthesiologist (there were no study protocols or guide-lines for blood pressure management). Patients’ computerized anesthesia records of intraoperative MAP (calculated by computerized record from systolic and diastolic blood pressure recordings) were analyzed as follows: Minimum intraoperative MAP, maximum intraoperative MAP, and “average stable” intraoperative MAP (after induction and intubation and before extubation, when blood pressures are generally relatively stable) were recorded (fig. 1A). “Average stable” intraoperative MAP was defined and determined in the following way: A “running MAP SD” curve was generated from the MAP tracings by calculating the SD of each group of five consecutive MAP recordings (each five consecutive MAP recordings group began one recording later in time than the previous, so that the groups were overlapping) (fig. 1B). At the first point in time when this SD curve decreased below 5 mmHg, subsequent blood pressures were averaged until the SD curve rose above 10 mmHg (if at least 30 min of recordings could be averaged over this period). The 5- and 10-mmHg cutoff values were determined by trial and error analysis of intraoperative MAP data from the first seven patients, and were then prospectively applied to the remaining patients. Each patient had at least one period meeting these criteria. The duration of the “average stable MAP” period for each patient was noted. All intraoperative MAP data were presented as absolute values (in mmHg) and as unitless fractions of preoperative MAP (denoted as “fractional minimum/maximum/‘average stable’ MAP”).
Fig. 1.
(A), mean arterial pressure (MAP) plotted as a function of time, shown as black diamonds. Induction, patient in prone position, start of surgery, and extubation were noted. Three other key events are denoted by circled areas: Minimum MAP, maximum MAP, and the period of stable MAP (“stable period”). (B) “Average Stable MAP,” defined and determined in the following way: A “running MAP SD” curve is generated from the MAP tracings by calculating the SD of each group of five consecutive MAP recordings (each five consecutive MAP recordings group began one recording later in time than the previous, so that the groups were overlapping) and is shown as black triangles in the lower part of the graph and labeled “‘Running Standard Deviation’ of MAP Tracing.” When this SD curve decreases below 5 mmHg (shown at the arrow labeled “First SD value < 5 mmHg”), subsequent blood pressures are averaged (shown as the horizontal line labeled “Average Stable MAP”) until the SD curve rises above 10 mmHg (if at least 30 min of recordings could be averaged over this period) (shown as the arrow labeled “Subsequent SD value > 10 mmHg”). The 5- and 10-mmHg cutoff values were determined by trial and error analysis of intraoperative MAP data from the first seven subjects.
Total intraoperative vasopressor dosages and American Society of Anesthesiologists (ASA) status classification, as determined by the caring anesthesiologist, were also obtained from the computerized anesthesia records.
Statistical Analysis
Statistical analyses were performed using Excel (Microsoft Corp., Redmond, WA) and JMP 7 software (SAS Institute Inc., Cary, NC). All statistical tests were considered significant if they achieved P < = 0.05. Mean 1-day and 1-month composite Z scores and 1-month follow-up rates were also compared using Mann–Whitney U tests.
Simple linear regressions between all fractional MAP measures (minimum, maximum, and “average stable”) and 1-day and 1-month composite Z scores were performed separately for normotensive and hypertensive patients. In addition, Pearson’s correlation analyses were performed to screen for potential relationships between demographic/perioperative variables and 1-day and 1-month composite Z scores. Multivariate linear regression models of 1-day and 1-month composite Z scores were created using a reverse elimination technique. Initially, all demographic and anesthetic variables were included that had a correlation to 1-day or 1-month composite Z scores achieving P < = 0.10 by Pearson’s analysis, and/or are known to affect neuropsychometric performance in surgical populations (years of education,2,3 age,2,3,11 pain at time of testing,12 diabetes,10 and previous cerebrovascular accident3). In addition, intra-operative MAP measure(s) that were found to be significantly associated with 1-day or 1-month composite Z scores by simple linear regression were also included as individual independent variables, and as variables crossed with hypertension to analyze for potential interactions. Variables were then eliminated from the model in a stepwise fashion until only variables achieving P < = 0.05 remained (or variables that were part of an interaction term with P < = 0.05).
To confirm assumptions made in the multivariate and simple linear regression analyses, the residual distributions for all regressions were assessed for normality using Shapiro-Wilk W testing.
Results
Forty-five patients were included in this analysis (all completed the 5 neuropsychometric tests at the preoperative and 1-day postoperative time points), 24 (53.3%) of whom reported a diagnosis of hypertension or had it noted in their anesthesia record (no patient reported taking antihypertensive medications who did not also report a diagnosis of hypertension or had it noted in his or her record). The prevalence of other demographic and anesthetic variables and pain measures of normotensive and hypertensive patients are shown in table 1. As compared with normotensive patients, hypertensive patients reported less 1-day postoperative pain according to the Wong-Baker FACES Pain Rating Scale. There was no significant difference in ASA status between normotensive and hypertensive patients; 2 hypertensive patients and 1 normotensive patient were classified as ASA 3, and all other patients were classified as ASA 2. No patient suffered an intraoperative or postoperative (within 24 h of surgery) cerebrovascular accident or myocardial infarction. Follow-up rates at 1 month were not significantly different between normotensive and hypertensive patients (15/21 for normotensive, 16/24 for hypertensive patients; P = 0.76).
Table 1.
Demographic/Anesthetic Variables and Pain Scores for Normotensive and Hypertensive Patients
| Normotensive Patients |
Hypertensive Patients |
|
|---|---|---|
| N | 21 | 24 |
| Age | 62.4 ± 10.5 | 67.9 ± 7.7 |
| Male sex (%) | 10/21 (47.6%) | 15/24 (62.5%) |
| Diabetes mellitus (%) | 0/21 (0%) | 4/24 (16.7%) |
| Hypercholesterolemia (%) | 7/21 (33.3%) | 11/24 (45.8%) |
| Body mass index (kg/m2) | 25.9 ± 4.0 | 27.8 ± 4.9 |
| History of smoking (%) | 10/21 (47.6%) | 8/24 (33.3%) |
| Previous CVA (%) | 1/21 (4.8%) | 2/24 (8.3%) |
| Previous MI (%) | 1/21 (4.8%) | 1/24 (4.2%) |
| ASA status ± 3 | 1/21 (4.8%) | 2/24 (8.3%) |
| Years of education | 17.0 ± 4.0 | 15.6 ± 3.2 |
| Duration of surgery (min) | 148.0 ± 36.3 | 160.5 ± 41.6 |
| Induction agent administered (%) |
||
| Propofol | 20/21 (95.2%) | 22/24 (91.6%) |
| Etomidate | 0/21 (0.0%) | 2/24 (8.3%) |
| Thiopental | 1/21 (4.8%) | 0/24 (0.0%) |
| Wong/Baker FACES | 4.2 ± 1.9 | 3.6 ± 2.0 |
| Rating Scale | ||
| Baseline | 4.2 ± 1.9 | 3.6 ± 2.0 |
| 1 day | 3.9 ± 2.5 | 1.9 ± 2.0 |
| 1 Month | 1.6 ± 1.1 | 1.0 ± 1.5 |
Data presented as mean ± SD, or as proportion of patients (percentage of patients).
ASA = American Society of Anesthesiologists; CVA = cerebrovascular accident; MI = myocardial infarction.
By univariate analysis, hypertensive patients’ mean 1-day composite Z score (± SD, −0.11 ± 0.48) was not significantly lower than normotensive patients’ (± SD, 0.03 ± 0.40), indicating that as a group and without regard to intraoperative MAP measures, hypertensive patients did not perform worse (P = 0.33). Although the number of patients decreased to 31, similar results were seen with 1-month composite Z scores (± SD, −0.05 ± 0.42 and 0.06 ± 0.54 in hypertensive and normotensive patients, respectively; P = 0.35).
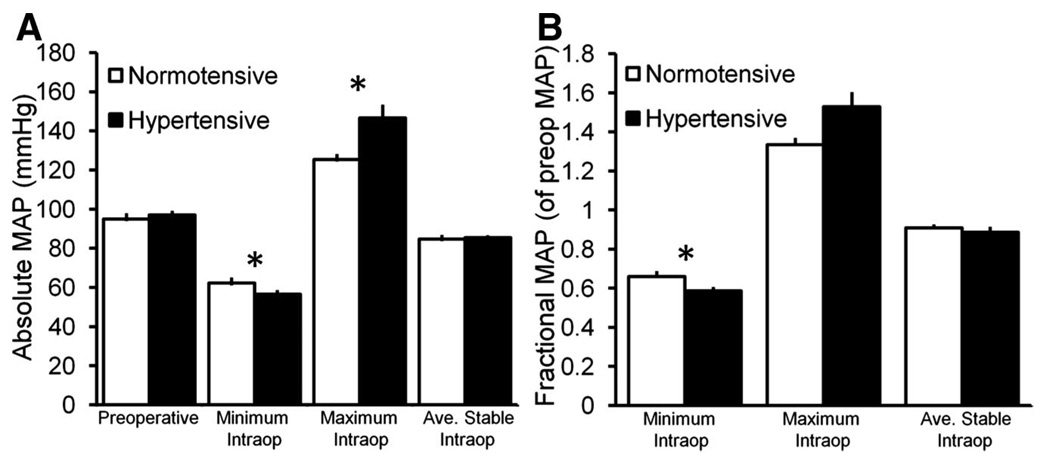
Figure 2A, in which absolute MAP data are plotted, demonstrates that hypertensive patients achieved significantly higher absolute maximum and lower absolute minimum intraoperative MAP. Fractional MAP data (intraoperative MAP measures as a fraction of preoperative MAP) are shown in figure 2B, and fractional minimum MAP was significantly lower in hypertensive patients as well. Absolute and fractional “average stable” MAP was nearly identical between hypertensive and normotensive patients, and was calculated over similar mean durations of slightly less than 2 h.
Fig. 2.
Pre- and intraoperative mean arterial pressure (MAP) measures for normotensive and hypertensive patients. (A) Mean absolute MAP measures. (B) Mean MAP measures as a fraction of preoperative MAP. Hypertensive patients had a significantly lower absolute minimum, higher absolute maximum (A) and lower fractional minimum (B) intraoperative MAP than normotensive patients. (*denotes statistically significant difference between normo- and hypertensive patients by Mann–Whitney U test. Error bars = SE. Ave. = average, intraop =in-traoperative.)
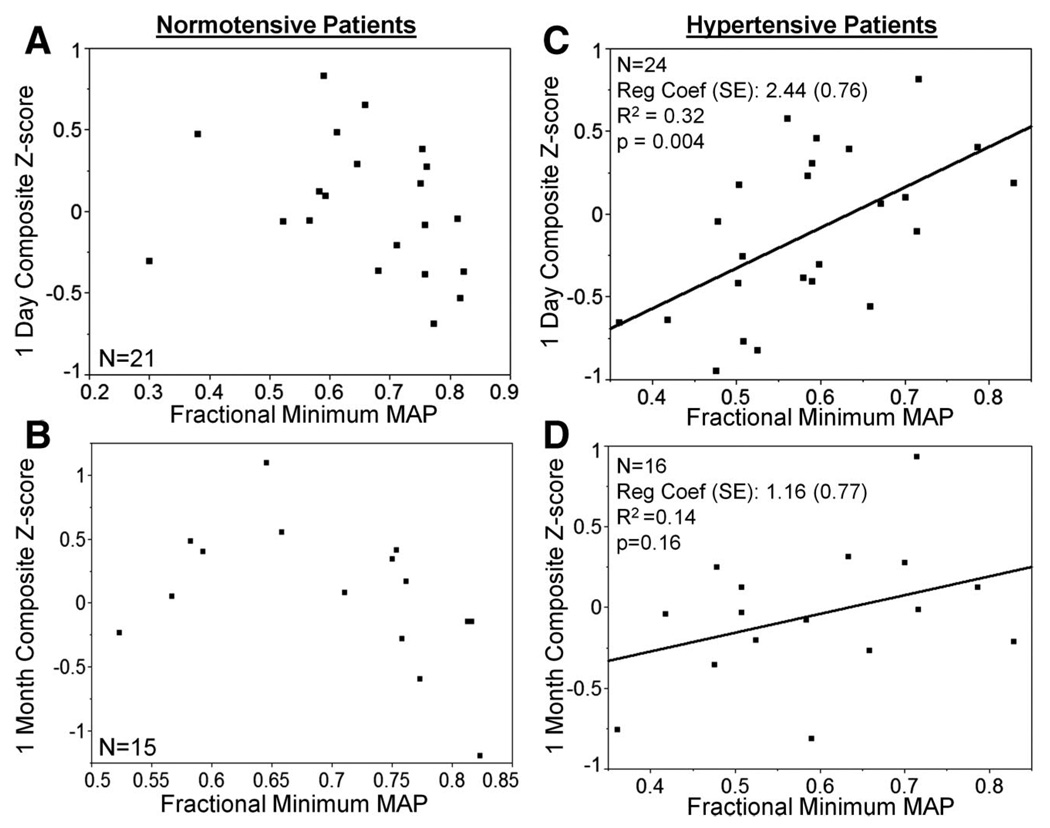
Simple linear regressions between intraoperative blood pressure measures (fractional minimum MAP, fractional maximum MAP, and fractional “average stable” MAP) and composite Z scores at 1 day and 1 month were performed separately for normotensive and hypertensive patients. In normotensive patients, no significant linear relationship was found between fractional minimum MAP and 1-day or 1-month composite Z scores (fig. 3, A and B). However, in hypertensive patients, there was a significant linear relationship between fractional minimum intraoperative MAP and 1-day composite Z scores (P = 0.003), with lower minimum MAP associated with lower Z scores (worse cognitive performance) (fig. 3C). A trend in this direction remained in hypertensive patients at 1 month (lower fractional minimum intraoperative MAP was associated with lower 1-month composite Z score; fig. 3D). There was no significant linear relationship between factional maximum or “average stable” MAP and composite Z score in either group at 1 day or 1 month (data not shown).
Fig. 3.
Simple linear regression between fractional minimum mean arterial pressure (MAP) and 1-day ([A] and [C]) and 1-month ([B] and [D]) composite Z score for normotensive ([A] and [B]) and hypertensive ([C] and [D]) patients. (C) There is a significant positive linear relationship between factional minimum intraoperative MAP and 1-day composite Z score for hypertensive patients. Although this trend continues at 1 month, there is no significant relationship in any of the other simple linear regressions. (Reg Coef (SE) = regression coefficient [SE of regression coefficient]).
Pearson’s correlation analysis between key demographic and perioperative variables and 1-day and 1-month composite Z scores are presented in table 2 to define those variables for inclusion in the multivariate linear regression model. As the correlation between 1-day composite Z score and age and 1-day Wong-Baker FACES pain rating both achieved a P < 0.10, and the correlation between 1-month composite Z score and duration of surgery achieved a P < 0.10; these variables were included in their respective multivariate linear regression models of composite Z scores (table 3). At 1 day, the multivariate model also initially included hypertension, diabetes mellitus, history of cerebrovascular accident, years of education, fractional minimum MAP, and hypertension crossed with fractional minimum MAP (hypertension*fractional minimum MAP). Following stepwise reverse elimination, the final model included 4 variables (hypertension, 1 day Wong/Baker FACES Rating Scale, fractional minimum MAP, and hypertension*fractional minimum MAP), of which only day 1 Wong/Baker FACES Rating Scale and hypertension*fractional minimum MAP were significant (table 2). At 1 month, age, hypertension, diabetes mellitus, history of cerebrovascular accident, years of education, duration of surgery, fractional minimum MAP, and hypertension*fractional minimum MAP were initially included; with hypertension, duration of surgery, fractional minimum MAP, and hypertension *fractional minimum MAP remaining after reverse elimination. Only hypertension *fractional minimum MAP (P = 0.02) and duration of surgery (P = 0.02) were statistically significant in the final model. Hypertension*fractional minimum MAP interaction term denotes the interaction of hypertension and minimum mean arterial pressure in a multivariate regression model. The presence of a significant interaction (also called moderator effect) indicates that the effect of minimum mean arterial pressure on cognitive performance is different in patients with and with-out hypertension.
Table 2.
Pearson’s Univariate Correlation between Demographic and Perioperative Variables and Composite Z scores at 1 Day and 1 Month
| 1 Day | 1 Month | |||
|---|---|---|---|---|
| Test | Coefficient | P | Coefficient | P |
| Age | −0.22 | 0.15 | 0.23 | 0.20 |
| Hypertension | −0.15 | 0.33 | −0.17 | 0.35 |
| Diabetes mellitus | 0.03 | 0.85 | 0.04 | 0.81 |
| Hypercholesterolemia | 0.06 | 0.68 | 0.10 | 0.61 |
| Obesity | 0.02 | 0.91 | −0.06 | 0.75 |
| History of smoking | 0.03 | 0.86 | −0.26 | 0.15 |
| Previous CVA | 0.21 | 0.18 | 0.09 | 0.63 |
| Years of education | 0.31 | 0.04* | 0.02 | 0.91 |
| Duration of surgery | −0.12 | 0.41 | −0.41 | 0.02* |
| Wong/Baker FACES rating | ||||
| Baseline | 0.16 | 0.28 | 0.24 | 0.19 |
| 1 day | −0.26 | 0.08* | — | — |
| 1 month | — | — | 0.17 | 0.35 |
P < 0.10, thus variable is to be included in multivariate linear regression models of composite Z score.
CVA = cerebrovascular accident.
Table 3.
Multivariate Linear Regression Analyses of Composite Z scores at 1 Day and 1 Month after Reverse Elimination
| 1 Day | 1 Month | |||
|---|---|---|---|---|
| Test | Coefficient (SE) |
P | Coefficient (SE) |
P |
| Age | — | — | — | — |
| Hypertension | −0.13 (0.07) | 0.06 | −0.07 (0.08) | 0.42 |
| Previous CVA | — | — | — | — |
| Years of education | — | — | — | — |
| Duration of surgery | — | — | −0.005 (0.002) | 0.02* |
| Minimum MAP (% baseline) |
0.24 (0.48) | 0.61 | −0.30 (0.69) | 0.67 |
| Wong/Baker FACES rating |
||||
| —Baseline | — | — | — | — |
| —1 Day | −0.08 (0.03) | 0.01* | — | — |
| Hypertension*Minimum MAP |
1.56 (0.45) | 0.001* | 1.75 (0.69) | 0.02* |
Data presented only for variables included in the final models. – indicates variable was initially included in the model but was removed during reverse elimination.
Statistically significant with P values as indicated.
CVA = cerebrovascular accident; MAP = mean arterial pressure; SE = standard error of independent variable in final multivariate model.
Intraoperative vasopressor usage data are presented separately for normotensive and hypertensive patients in table 4 (ephedrine and phenylephrine were the only vasopressors administered to any of the patients).
Table 4.
Intraoperative Vasopressor Medication Use
| Normotensive | Hypertensive | |
|---|---|---|
| Ephedrine | ||
| Patients administered (%) | 8/21 (38.1%) | 11/24 (45.8%) |
| Mean total dose (mg) | 8.13 ± 2.59 | 18.64 ± 12.87 |
| Phenylephrine | ||
| Patients administered (%) | 5/21 (23.8%) | 6/24 (25.0%) |
| Mean total dose (mcg) | 256.0 ± 156.5 | 120.0 ± 121.3 |
Doses are presented as means ± SD of only those patients who received the respective medication.
Discussion
The goal of this analysis was to determine the risk factors for poorer postoperative cognitive function in older normotensive and hypertensive patients undergoing lumbar laminectomy and microdiscectomy. Using computerized anesthesia record data and multivariate linear regression models of cognitive performance, we demonstrate a significant interaction between presence of hypertension and intraoperative minimum MAP (as a fraction of baseline) at 1 day and 1 month. This suggests that in hypertensive patients, lower minimum intraoperative MAPs may be associated with poorer postoperative cognitive performance.
We hypothesize that a portion of the hypertensive patients presented here experienced intraoperative blood pressures that were too low to maintain normal cerebral perfusion, resulting in ischemia manifested as poorer postoperative cognitive performances.
The mechanism of these findings likely involves cerebrovascular autoregulation, the intrinsic ability of the cerebral arterial system to contract and dilate to maintain constant cerebral perfusion across a range of systemic blood pressures.7 In hypertensive patients, arteriosclerosis and other processes cause this range to shift to higher blood pressures. As a result of this shift, hypertensive patients experience decreased cerebral perfusion at pressures that fall safely within the acceptable range for normotensive patients.7 This may explain why we demonstrate an association between intraoperative MAP minimums and poorer cognitive performance in hypertensive, but not normotensive, patients.
Possible associations between intra- and perioperative blood pressure and postoperative cognitive change in elderly patients have been studied before, most notably in the International Study on Post-Operative Cognitive Dysfunction 1 trial.2 This study showed no association between intra- or perioperative (24 h after surgery) hypotension (binomially defined as presence of at least 1 period of mean blood pressure below 60% of baseline for 30 min) and postoperative cognitive dysfunction (a binomial categorical variable based on comparison to non-surgical controls) at 1 week or 3 months. The disparities between their conclusions and the data presented here may be the result of methodological differences. We analyzed both intraoperative MAP measures (as a fraction of preoperative levels) and neuropsychometric performance as continuous variables. Presenting MAP measures as a relative, continuous variable avoids potential misrepresentations in defining patients as being “hypotensive,” as there is no widely accepted or universally applicable definition for this term.13 Neuropsychometric performance is presented in this analysis as a continuous variable to avoid categorizing patients as having or not having “cognitive dysfunction,” a distinction that is best not made without an appropriate control group. Further, the authors of the International Study on Post-Operative Cognitive Dysfunction 1 trial state that “hypotension” may be important in certain patients; however, they report no analysis of a potential interaction between hypertension and intraoperative hypotension. Also, postoperative neuropsychometric testing was performed in the study presented here 1 day and 1 month postoperatively, as compared with 1 week and 3 months in the International Study on Post-Operative Cognitive Dysfunction 1 trial.
Williams-Russo et al. performed a prospective study with 235 older adults (> 50 yr) having elective total hip replacement with epidural anesthesia.6 The patients were randomly assigned to 1 of 2 levels of intraoperative MAP management, either 45–55 mmHg or 55–70 mmHg. Cognitive outcome was assessed by within-patient-change on 10 neuropsychometric tests before, and 1 and 4 months after surgery. They analyzed performance by group-rate analysis, evaluating change relative to baseline for each of their tests individually. They found no differences in long-term cognitive deterioration between the two groups. While they used a multivariate analysis to see if age, blood pressure group, and the interaction term (age-MAP) had any effect on cognitive outcome, they did not report whether neuropsychometric test performance depended on an interaction between a history of hypertension and intraoperative MAP.
In this study, there was no significant linear relationship demonstrated between fractional “average stable MAP” and postoperative cognitive performance in nor-motensive or hypertensive patients. This blood pressure measure was of interest, however, because it represents an average blood pressure for a large portion of the cases in both patient groups (a mean of approximately 2 h) and, presumably, is the blood pressure at which the anesthesiologist was comfortable maintaining the patient. Interestingly, the mean of this measure was almost identical between normotensive and hypertensive patients, and was insignificantly lower in hypertensive patients when reported as a percentage of preoperative MAP. This may be secondary to concerns regarding intraoperative cardiac stress, as hypertensive patients are at increased risk for coronary artery disease.14 However, more hypertensive patients received ephedrine, which has nonspecific sympathomimetic effects including increased heart rate and myocardial oxygen demand, and at significantly higher total doses than normotensive patients. Further, mild to moderate hypertension has never been independently associated with increased perioperative cardiac risk,15 and there was no significant difference in ASA status between the groups. Regardless, the difference in “average stable MAP” was apparently not significant enough to produce changes in cerebral perfusion that resulted in poorer cognitive performance.
The “average stable MAP” measure, given that it is a mean MAP calculated over a significant time period, is similar to the blood pressure measure used in the International Study on Post-Operative Cognitive Dysfunction 1 trial. This study categorized patients either as experiencing “hypotension” (MAP below 60% of baseline for 30 min) versus not experiencing hypotension, and showed no association between hypotension and post-operative cognitive dysfunction.2 Thus, the lack of association between “average stable MAP” and 1-day or 1-month composite Z score is consistent with this previous report.
Using a multivariate linear regression model, we also demonstrate that increased duration of surgery was also independently associated with poorer cognitive outcome at 1 month, which is consistent with previous reports.2 Although the cause of this is unclear, poorer cognition with longer surgical times may be the result of increased surgical trauma and/or duration of anesthetic exposure. At 1 day, a high Wong-Baker FACES Pain Rating Scale (indicating increased pain) was associated with poorer 1-day postoperative cognitive performance, confirming results published previously by our group (using a previous cohort of spine surgery patients) that severe pain confounds neuropsychometric testing.12
There are a number of limitations to our study. First, cerebral ischemia is believed to result from decreased cerebral perfusion over some period of time, and as our minimum MAP measure does not incorporate a time component, our study is limited in this regard. Blood pressures were recorded every 2–3 min, so, at a maximum, this was the duration of the period of minimum MAP. However, there is evidence suggesting that even brief periods of decreased systemic blood pressure can lead to cognitive impairment. Murkin et al.16 demonstrated cognitive dysfunction in 10 of 14 patients after implantable cardioverter-defibrillator placement, during which an average of 12 periods of ventricular fibrillation were induced for an average of 17 s (leading to an average of 11 s of mean blood pressure below 30 mmHg). Their unusual definition “… for impaired functioning in the patient group … [was] … a change in scores that reflected a drop in performance of more than 1 SD from baseline performance. Cognitive dysfunction was defined as impaired performance in one of four domains.”16 They point out that multiple animal brain tissue studies have shown metabolic and functional derangements after seconds of ischemia,17,18 and that similar changes have been observed in human cerebral cortex samples obtained during craniotomy procedures.19 Thus, neuropsychometric testing, which we believe to be a sensitive marker for cerebral ischemic damage, may reflect such changes in this study cohort.
Another limitation of this study is that it is a post hoc analysis with a limited number of patients. The latter is especially important regarding the multivariate analyses. A further limitation of this study is that there is no external control group, and thus we are neither able to make categorical distinctions regarding postoperative cognitive dysfunction (POCD), nor are we able draw conclusions regarding the absolute magnitude or significance of the change. Further, there is no data regarding duration of treatment with antihypertensive medications, medication dosages, and the time of the last dose before surgery, which may have allowed for a better understanding of the intraoperative blood pressure data presented. Our measure of baseline blood pressure, usually obtained in the preoperative waiting area, is also not ideal, as patients may have elevated blood pressure because of anxiety regarding their surgery, and the extent of this elevation may be influenced by standing use of antihypertensive medications. Interestingly, this may have contributed to the fact that baseline MAP values did not significantly differ between the normotensive and hypertensive group. Further, this finding suggests that blood pressures in the hypertensive group may have been reasonably well controlled with home medications.
This post hoc analysis demonstrates that in older hypertensive patients undergoing lumbar laminectomy or microdiscectomy, lower intraoperative minimum MAP (as a fraction of baseline MAP) is associated with poorer cognitive performance at 1 day and 1 month postoperatively. Alterations in cerebrovascular autoregulation may explain why this effect is only seen in hypertensive patients. This relationship warrants further investigation, including a prospective controlled trial of the effect of intraoperative blood pressure control, especially during induction when pressure tends to reach a minimum, on postoperative cognitive change, to confirm these findings.
Acknowledgments
The authors thank Brian Thumm, B.A., Senior System Analyst, Imaginous Technology, Brooklyn, New York, for his kind assistance with computerized anesthesia records.
Eric J. Heyer was supported in part by a grant from the National Institute on Aging (RO1AG17604); Bethesda, Maryland. Gene T. Yocum was supported in part by a grant from Doris Duke Charitable Foundation, New York, New York.
References
- 1.Heyer EJ, Sharma R, Rampersad A, Winfree CJ, Mack WJ, Solomon RA, Todd GJ, McCormick PC, McMurtry JG, Quest DO, Stern Y, Lazar RM, Connolly ES. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 4.Newman MF, Kramer D, Croughwell ND, Sanderson I, Blumenthal JA, White WD, Smith LR, Towner EA, Reves JG. Differential age effects of mean arterial pressure and rewarming on cognitive dysfunction after cardiac surgery. Anesth Analg. 1995;81:236–242. doi: 10.1097/00000539-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Gold JP, Charlson ME, Williams-Russo P, Szatrowski TP, Peterson JC, Pirraglia PA, Hartman GS, Yao FS, Hollenberg JP, Barbut D, Hayes JG, Thomas SJ, Purcell MH, Mattis S, Gorkin L, Post M, Krieger KH, Isom OW. Improvement of outcomes after coronary artery bypass. A randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1302–1314. doi: 10.1016/S0022-5223(95)70053-6. [DOI] [PubMed] [Google Scholar]
- 6.Williams-Russo P, Sharrock NE, Mattis S, Liguori GA, Mancuso C, Peterson MG, Hollenberg J, Ranawat C, Salvati E, Sculco T. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91:926–935. doi: 10.1097/00000542-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Paulson OB, Waldemar G, Schmidt JF, Strandgaard S. Cerebral circulation under normal and pathologic conditions. Am J Cardiol. 1989;63:2C–5C. doi: 10.1016/0002-9149(89)90396-2. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Scheunert T, Steinbach G, Schirmer U, Marx T, Freitag N, Reinelt H. Hypertension as a risk factor for cerebral injury during cardiopulmonary bypass. Protein S100B and transcranial Doppler findings. Anaesthesia. 2001;56:733–738. doi: 10.1046/j.1365-2044.2001.02105.x. [DOI] [PubMed] [Google Scholar]
- 9.Reich DL, Wood RK, Jr, Mattar R, Krol M, Adams DC, Hossain S, Bodian CA. Arterial blood pressure and heart rate discrepancies between handwritten and computerized anesthesia records. Anesth Analg. 2000;91:612–616. doi: 10.1097/00000539-200009000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Mocco J, Wilson DA, Komotar RJ, Zurica J, Mack WJ, Halazun HJ, Hatami R, Sciacca RR, Connolly ES, Heyer EJ. Predictors of neurocognitive decline after carotid endarterectomy. Neurosurgery. 2006;58:844–850. doi: 10.1227/01.NEU.0000209638.62401.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: A systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Heyer EJ, Sharma R, Winfree CJ, Mocco J, McMahon DJ, McCormick PA, Quest DO, McMurtry JG, Riedel CJ, Lazar RM, Stern Y, Connolly ES. Severe pain confounds neuropsychological test performance. J Clin Exp Neuropsychol. 2000;22:633–639. doi: 10.1076/1380-3395(200010)22:5;1-9;FT633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: Literature definitions applied to a retrospective cohort using auto-mated data collection. Anesthesiology. 2007;107:213–220. doi: 10.1097/01.anes.0000270724.40897.8e. [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Tarkington LG, Yancy CW. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–e499. doi: 10.1161/CIRCULATIONAHA.107.185699. [DOI] [PubMed] [Google Scholar]
- 16.Murkin JM, Baird DL, Martzke JS, Yee R. Cognitive dysfunction after ventricular fibrillation during implantable cardiovertor/defibrillator procedures is related to duration of the reperfusion interval. Anesth Analg. 1997;84:1186–1192. doi: 10.1097/00000539-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg ND, Passonneau JV, Lowry OH. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem. 1966;241:3997–4003. [PubMed] [Google Scholar]
- 18.Lowry OH, Passonneau JV. The relationships between substrates and enzymes of glycolysis in brain. J Biol Chem. 1964;239:31–42. [PubMed] [Google Scholar]
- 19.Kirsch WM, Leitner JW. Glycolytic metabolites and co-factors in hum an cerebral cortex and white matter during complete ischemia. Brain Res. 1967;4:358–368. doi: 10.1016/0006-8993(67)90165-5. [DOI] [PubMed] [Google Scholar]