Abstract
Lysophosphatidic acid (LPA) is a simple lipid with many important biological functions such as the regulation of cellular proliferation, cellular migration, differentiation, and suppression of apoptosis. Although a direct angiogenic effect of LPA has not been reported to date, there are indications that LPA promotes angiogenesis. In addition, LPA is a chemo-attractant for cultured endothelial cells and promotes barrier function in such cultures1. To test the hypothesis that LPA is angiogenic, we used the chicken chorio-allantoic membrane (CAM) assay. Sequence analysis of the cloned, full length chicken LPA receptor cDNAs revealed three receptor types that are orthologous to the mammalian LPA1, LPA2 and LPA3 receptors. We document herein that LPA is angiogenic in the CAM system and further that synthetic LPA receptor agonists and antagonists mimick or block this response, respectively. Our results predict that LPA receptor antagonists are a possible therapeutic route to interdicting angiogenesis.
Keywords: angiogenesis, chorio-allantoic membrane (CAM), lipid mediators, lysophosphatidic acid (LPA), vessels
INTRODUCTION
Lysophosphatidic acid (LPA, 1-acyl-2-hydroxy-sn-glycero-3-phosphate) is a simple lipid involved in a number of biologic processes including cellular proliferation, suppression of apoptosis, and modulation of survival and differentiation2, 3. Other important cellular effects induced by LPA are related to cytoskeletal filament reorganization, including regulation of chemotaxis in endothelial cells and smooth muscle contraction4, 5. Many of these actions of LPA are mediated by G protein-coupled receptors (GPCRs) of the Endothelial Differentiation Gene (EDG) family. Three GPCRs of the EDG family have been identified as LPA receptors, LPA1 (formerly EDG-2), LPA2 (EDG-4) and LPA3 (EDG-7). Two additional GPCRs have been suggested to be LPA receptors, GPR23/p2y9/LPA46 and GPR92/LPA57, but these are distantly related to the EDG family LPA receptors and have not yet been studied in detail. LPA receptors are developmentally regulated and differ in tissue distribution, but couple similarly to multiple types of G proteins to signal through Ras and Mitogen-Activated Protein Kinase (MAPK)8, Rho9, phospholipase C (PLC)10, and several protein tyrosine kinases11. The physiological roles of individual LPA receptors are not well understood, but studies with mutant mice have suggested that the LPA1 and LPA3 receptors are important for normal development12, 13.
LPA is found in human plasma at a concentration of 0.4 – 0.5 µM14 while in serum the concentration range is approximately 10-fold higher15. It is synthesized through a number of different pathways including de-acylation of phosphatidic acid (PA) by phospholipases A1 and A2 (PLA1 and PLA2), acylation of glycerol 3-phosphate by glycerophosphate acetyltransferase, phosphorylation of monoacylglycerol (MAG) by acylglycerol kinase (AGK), and hydrolysis of plasma lysophosphatidylcholine (LPC) by the lyso-phospholipase D (lyso-PLD), autotaxin16. Extracellular LPA is degraded by the action of integral membrane lipid phosphate phosphatases (LPPs), which hydrolyze LPA to form monoacylglycerol and inorganic phosphate. LPA can also be metabolized via acylation by LPA acyl transferases (LPAATs) to form PA17.
Several properties of LPA prompted us to test it as an angiogenic factor. For instance, LPA promotes endothelial cell migration and proliferation in vitro18, 19, it enhances matrix metalloproteinase-2 expression in endothelial cells20 and autotaxin (ATX, a plasma lyso-PLD), is angiogenic in vivo21 and is essential for vascular development in mice22. Although these results suggest that LPA is an angiogenic molecule, there has not been a demonstration that LPA itself induces angiogenesis in vivo. To test the hypothesis that LPA is angiogenic, we used the chicken chorio-allantoic membrane (CAM) assay using LPA as well as synthetic compounds that are agonists or antagonists at individual LPA receptors. We report the results of these studies herein.
Angiogenesis – the sprouting of capillaries from existing blood vessels – is required in normal embryogenesis as well as wound healing. However, this process is also critical for solid tumor growth. Tumors attract new blood vessels to receive nutrients and oxygen necessary for expansion. Angiogenesis is a tightly regulated process (by the strict balance of angiostatic/inhibitors vs. angiogenic/stimulators factors), thus it is important to understand how it is regulated and to identify molecules that are involved in the process. If LPA induces angiogenesis in vivo, it would be helpful to determine the function of individual LPA receptors in the regulation of this process so as to direct the development of LPA receptor-directed anti-angiogenic compounds.
METHODS
Materials and Reagents
Lipids (1-oleoyl LPA, S-OMPT, sphingosine 1-phosphate) and the LPA receptor antagonist VPC32183 were purchased from Avanti Polar Lipids, Alabaster, AL. Fatty acid free-bovine serum albumin (FAF-BSA), water-soluble hydrocortisone and Vascular Endothelial Growth Factor (VEGF) were purchased from Sigma-Aldrich, St. Louis, MO. Recombinant spider sphingomyelinase D (SMaseD) and the two catalytically inactive mutants were prepared and purified by Sangderk Lee, Ph.D. in our laboratory.
RNA extraction from chicken embryos
Total RNA was extracted from the chicken embryos using TRI reagent (Sigma-Aldrich, St. Louis, MO). Briefly, tissue from the embryo was cut and immediately transferred to a tube containing RNAlater® solution to inhibit degradation of RNA. The tissue was homogenized in TRI reagent and chloroform was added. The mixture was centrifuged (12,000 × g for 15 minutes at 4°C), the phase containing the RNA was removed, transferred to a new RNase-free tube and isopropanol was added. After centrifugation, the supernatant fluid was removed, the RNA pellet was washed with 75% ethanol and collected by centrifugation (7,500 × g for 5 minutes). The RNA pellet was then air-dried and dissolved in DEPC-treated (RNase-free) water (Ambion, Austin, TX).
Cloning of LPA receptors from chicken
The three EDG chicken LPA receptors (LPA1, 2, 3) were cloned using total RNA extracted from a chicken embryo. In the case of cLPA1, the full translational open reading frame (ORF) was amplified using the following primer sets designed from the nucleotide sequence present in the chicken genome record (sense: 5′- ATG GAT ATC CCC ACT GAT TTG GTG CCA and anti-sense: 5′- TCC ACA GCA ACG ACC ACT CGG TGG TGT AA (for the construct with stop codon (N-terminal tagged)) or anti-sense: 5′- TCC ACA GCA ACG ACC ACT CGG TGG TG (for the construct without the stop codon (C-terminal tagged)). In the case of cLPA2, we designed the following sense primer (5′- ATG GTA GAG GTG CGG TGT GGA T) from the known nucleotide sequence encoding a fragment (~900 bp) of this receptor. We amplified the full ORF sequence using 3′-rapid amplification of cDNA ends (RACE) using this sense primer and an oligo-dT anti-sense primer using the Generacer kit (Invitrogen, Carlsbad, CA).
Similarly, for cLPA3, we designed a sense primer from the known nucleotide sequence (5′- ATG AAT GAA TGC TAC TAT GAT AAG CAC AT) and we used 3′-RACE (with the oligo-dT anti-sense primer) to amplify the sequence encoding the entire translational open reading frame (ORF). For all the receptors, the DNA encoding the full translational ORF was sub-cloned into pcDNA3.1/V5-His -TOPO (for C-terminal epitope tag) or pcDNA4/HisMax -TOPO (for N-terminal tag) plasmids for eventual expression in mammalian cells. The fidelity of the plasmid constructs were verified by automated DNA sequencing (UVA Biomedical Research Core Facility).
Chicken Chorio-Allantoic Membrane (CAM) assay for angiogenesis
White Leghorn chicken eggs were purchased from CBT Farms (Chestertown, MD). After shipment, eggs were incubated in a rocking incubator at 37°C for three days (the first day of incubation is considered the first day of embryonic development, or E1). On day E4, a 1 cm2 window was cut in the shell of the eggs and the underlying membrane was removed. The window was covered with a plastic cover slip and the eggs were returned to the incubator. At day E8 the CAMs (~8–10 per group) were treated with the appropriate compounds (20 µl at the desired concentrations) or controls. Compounds were delivered by applying to 0.5 cm diameter Whatman GF/C filter paper placed on the surface of the CAM. Eggs were treated daily for three consecutive days (E8, E9 and E10) by adding fresh drug to the same filter paper disc. On day E11, CAMs were harvested by injecting a 37% formaldehyde solution under and around the filter disc, the disc was removed and the number of new vessels (all vessels that intersect the filter paper disc at an angle greater than 45°) was determined using a light microscope. Images of CAM membranes were captured using a dissecting microscope equipped with a digital camera.
Statistical Analysis
One-Way ANOVA or Student t test were performed between groups.
RESULTS
Chicken LPA Receptors
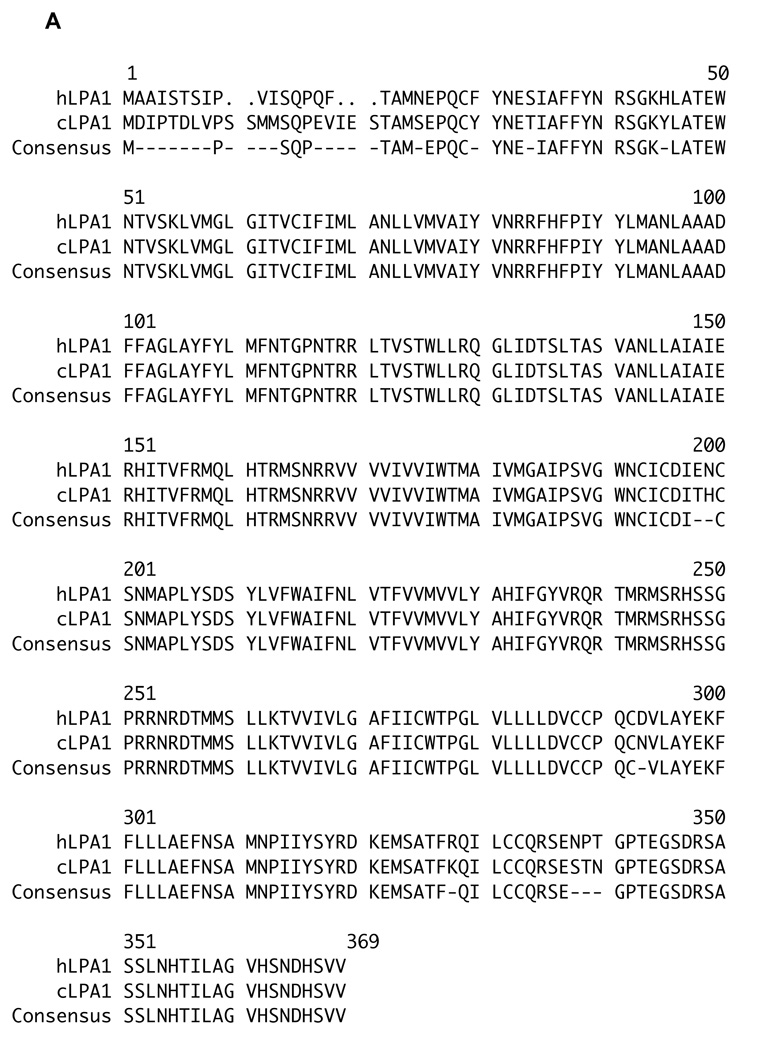
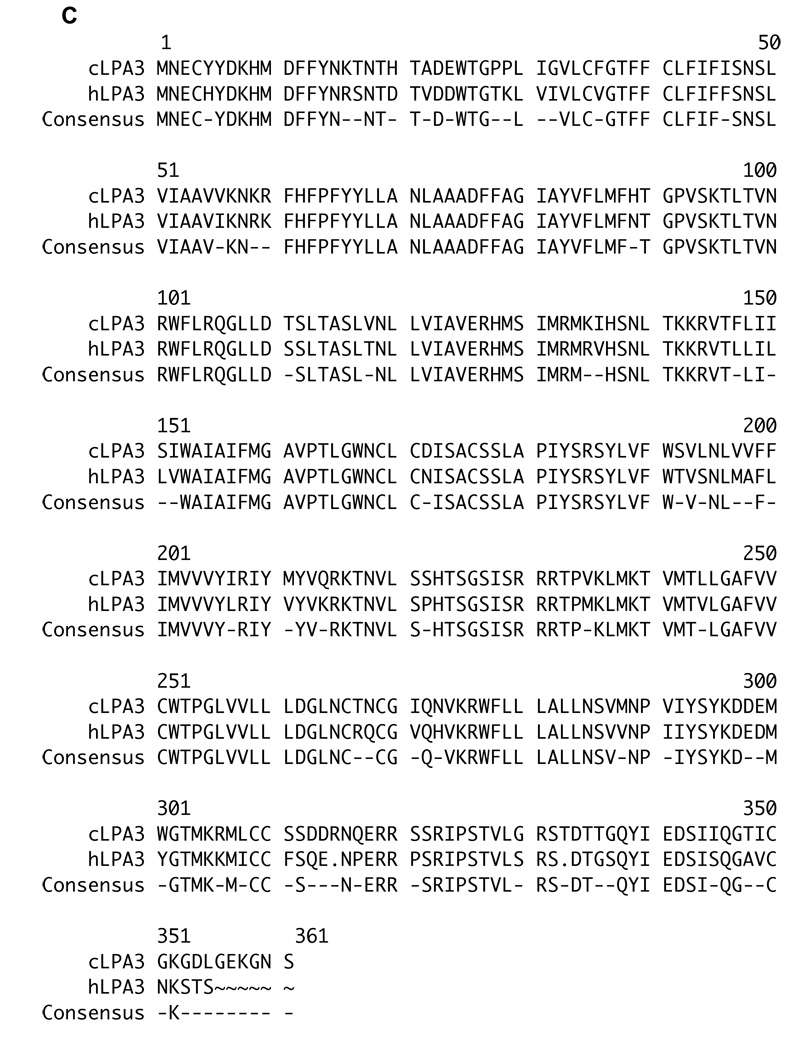
Because the CAM system is avian, we first determined the number and type of LPA/EDG receptors encoded in the chicken genome. Repeated queries of the chicken genome with the three human LPA/EDG receptor sequences using TBLASTN or FASTA algorithms revealed the existence of three orthologous genes, or fragments thereof. As described in the Experimental Methods section, we used this sequence information to clone the corresponding full-length chicken LPA receptor cDNAs. The amino acid sequence identities of the conceptualized human and chicken proteins over their full lengths are 95% (LPA1), 66% (LPA2) and 82% (LPA3) (Figure 1a–c). The LPA1 sequences are particularly highly conserved, indeed, there is very nearly complete conservation of amino acid sequence except at the amino termini of these proteins. We note in passing that this extraordinary degree of conservation among LPA1 receptor sequences extends to other non-mammalian vertebrates (e.g. the fish Tetraodon, the amphibian Xenopus) (data not shown). Such a high degree of similarity virtually assures that our LPA1 receptor-directed antagonist compounds (see below) are active at the orthologous chicken receptor. To determine which of the three LPA receptor genes are expressed in the chicken embryos, we analyzed RNA extracted from day E7 chicken embryos by RT/PCR using oligonucleotide primers specific for each of the three chicken receptors. We detected a signal for each of the three receptors using this method (data not shown).
Figure 1. LPA1, LPA2, and LPA3 receptors are expressed in chicken embryos.
Peptide sequence alignments of chicken lysophosphatidic acid receptors: cLPA1 (1A), cLPA2 (1B), and cLPA3 (1C) with the respective human LPA receptors (database accession numbers for human peptide sequences used are: LPA1: NM_001401, LPA2: NM_004720, LPA3: NM_012152). The “Pretty” sequence comparison program was used to align the peptide sequences and calculate a consensus. These newly identified chicken LPA receptor sequences have been deposited in GenBank. Their accession numbers are: cLPA1 (EU339317), cLPA2 (EU339318) and cLPA3 (EU339319).
LPA induces angiogenesis quantitatively similar to vascular endothelial growth factor (VEGF) and sphingosine 1-phosphate (S1P)
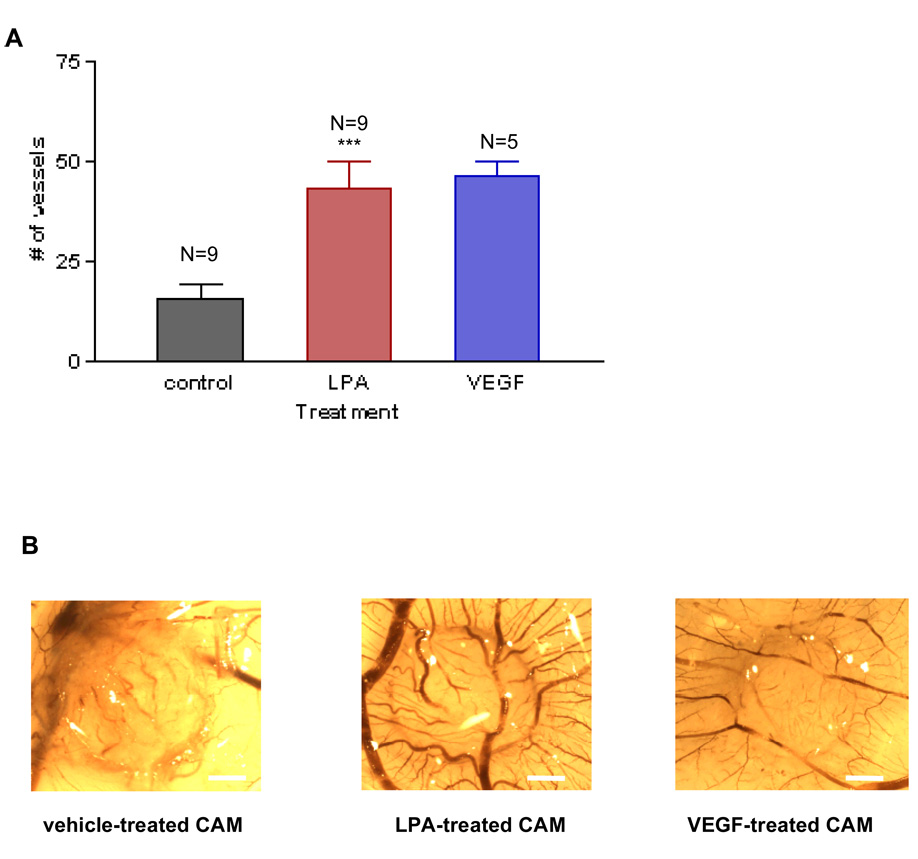
The CAM assay is a well-established system for gauging the angiogenic activity of small molecules, and thus is a logical first choice for assessing LPA in this regard. We began by treating eggs daily for three consecutive days with 1µM 1-oleoyl (18:1) LPA using sterile de-ionized water and 50 ng vascular endothelial growth factor-A (VEGF-A) as negative and positive controls, respectively. With this protocol, LPA evoked a robust angiogenic response quantitatively equivalent to that of VEGF (Figure 2A). Interestingly, LPA-induced vessels were larger than those induced by VEGF (Figure 2B), suggesting that LPA-induced vessels are more mature than VEGF-induced vessels.
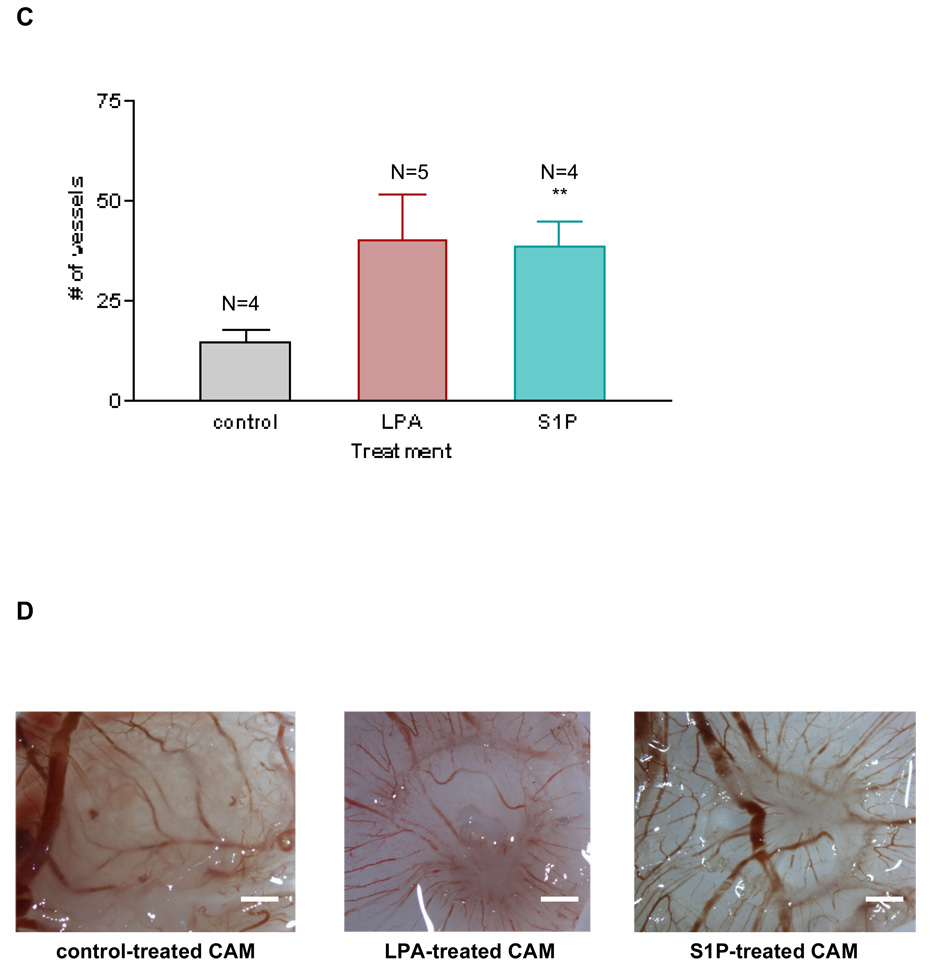
Figure 2. LPA induces angiogenesis in vivo that is quantitatively similar to VEGF and S1P.
To determine the effects of LPA in angiogenesis, we treated the chorio-allantoic membranes (CAMs) with 1µM 18:1 LPA, sterile water as a negative control or 50 ng vascular endothelial growth factor-A (VEGF-A) as a positive control. We also treated CAMs with S1P, another angiogenic factor that is structurally related to LPA. A piece of GF/C filter paper (0.5 cm diameter) was used as the drug carrier. After three consecutive days of treatment, we counted the number of vessels that intersected the filter paper disc at an angle greater than 45°. These vessels are the new vessels that represent the angiogenic response. 2A LPA induces angiogenesis, compared to the control. Interestingly, LPA-induced angiogenesis is comparable with VEGF-induced angiogenesis in terms of the number of new vessels induced. 2C LPA-induced angiogenesis is also comparable with S1P-induced angiogenesis. One-Way analysis of variance (ANOVA) test was performed between groups. *** P< 0.0001. ** P< 0.001. Error bars represent standard deviation (SD). Images were captured using a dissecting microscope (Makroscope) connected to a digital camera, at a magnification of 6 ×. In Figures 2B and 2D we show one membranes representative of each group. Scale bar: 0.125 cm.
We also tested another known angiogenic molecule, sphingosine 1-phosphate (S1P)23, which is a phospholipid that is structurally similar to LPA. As presented in Figures 2C and 2D, 1µM of S1P induced an angiogenic response similar to the same concentration of LPA, confirming that S1P, like LPA, is an angiogenic molecule in vivo.
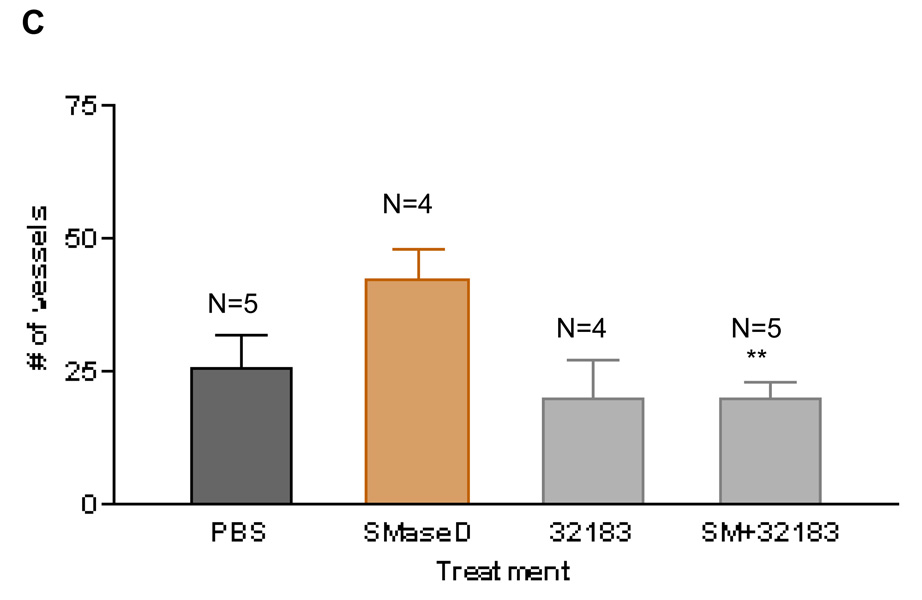
LPA-induced angiogenesis is blocked by an LPA1,3 antagonist
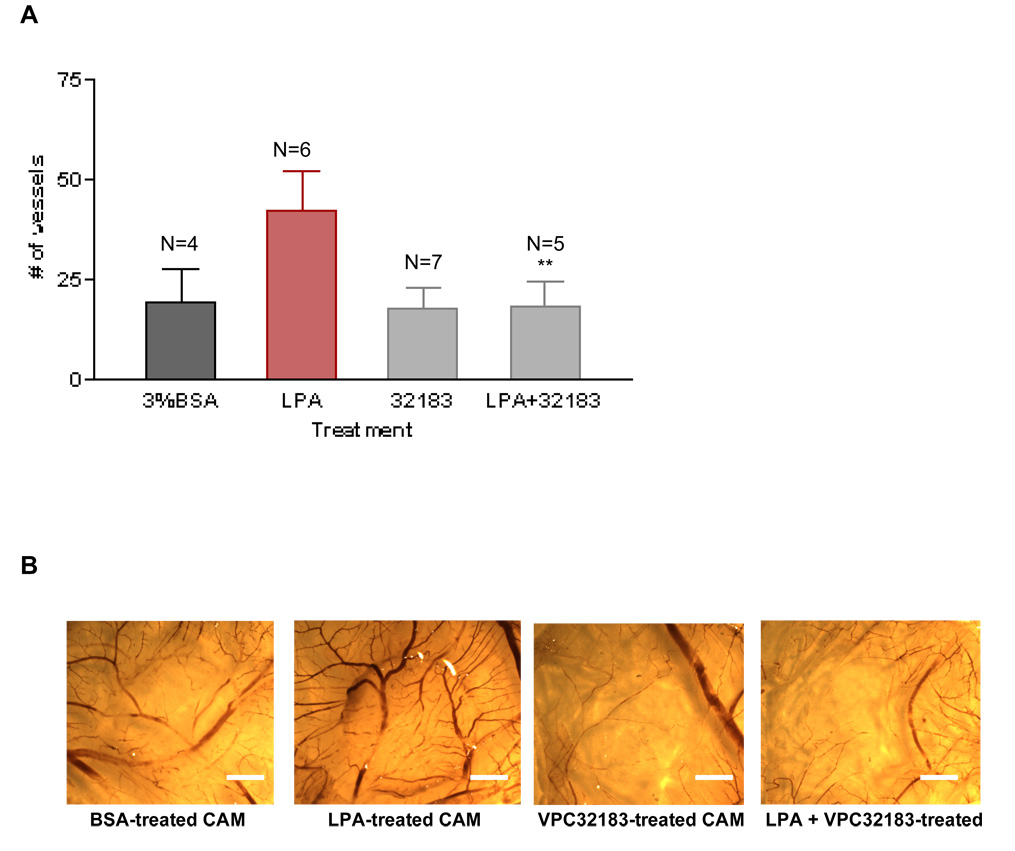
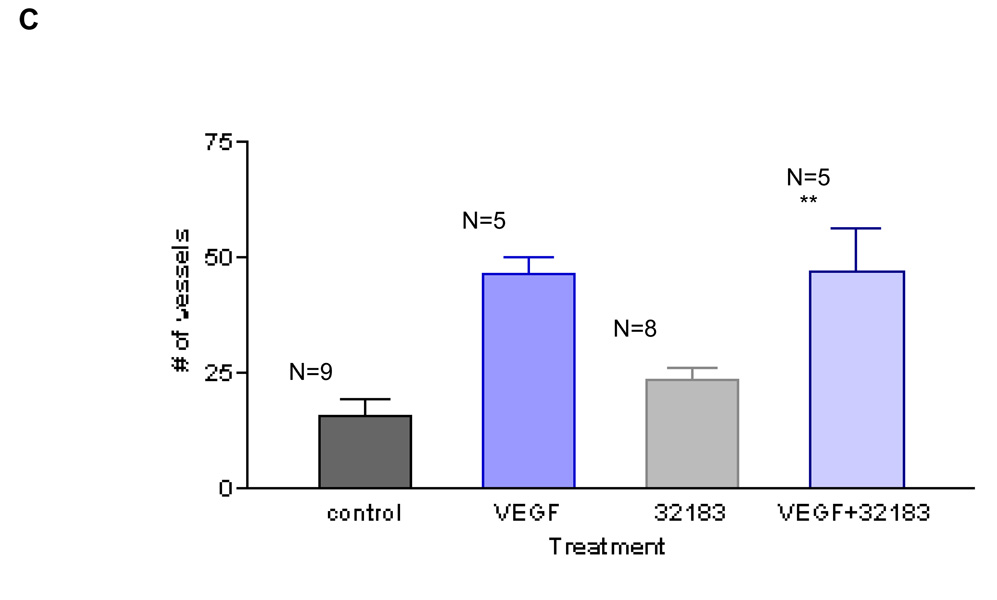
Although there is not yet a complete set of LPA receptor selective antagonists, several antagonists active at both the LPA1 and LPA3 receptors have been developed. After determining that LPA induces angiogenesis in vivo, we determined if the response was blocked by an antagonist for LPA1 and LPA3 receptors, VPC3218324. This compound does not have agonist activity at the LPA1, LPA2 or LPA3 receptors, rather it is antagonist for LPA1 and LPA3 receptors24. We treated the CAMs for three consecutive days with 1µM LPA or 1µM LPA with 10µM VPC32183. In these experiments, VPC32183 (Figures 3A and 3B) blocked the angiogenic response obtained with 1µM 18:1 LPA. At this antagonist concentration (10µM), the compound alone does not have any significant effect in the number of vessels, as compared to the vehicle control (Figures 3A and 3B). Further, VPC32183 (at 10µM) had no effect in VEGF-induced angiogenesis (Figure 3C). Together, these results suggest that the angiogenic response obtained with LPA was due to the activation of LPA1 and/or LPA3.
Figure 3. LPA-induced angiogenesis is blocked by an LPA1,3 receptor antagonist.
To analyze the role of LPA receptors in LPA-induced angiogenesis we used the LPA1 and LPA3 receptor antagonist, VPC32183. Using the same approach aforementioned, we show in 3A and 3B that LPA-induced angiogenesis is blocked completely by this compound at 10µM. Importantly, VPC32183 alone (at 10µM) had no discernable effect in angiogenesis and does not block VEGF-induced angiogenesis (Figure 3C). One-Way ANOVA test was performed between groups. ** P< 0.001. Error bars represent standard deviation (SD). In figure 3B (scale bar: 0.125 cm), we present the image of a single membrane representative of each group.
An LPA3-selective agonist induces angiogenesis
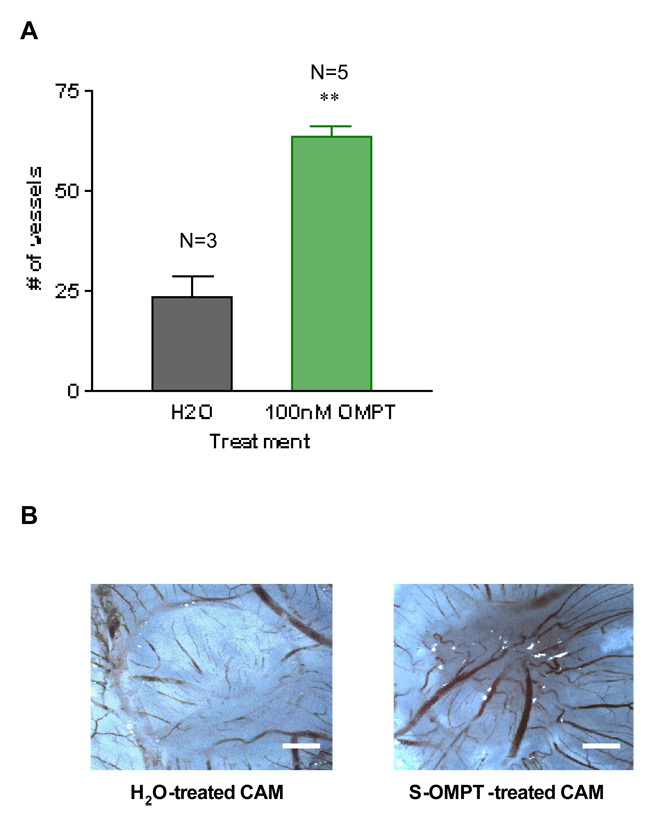
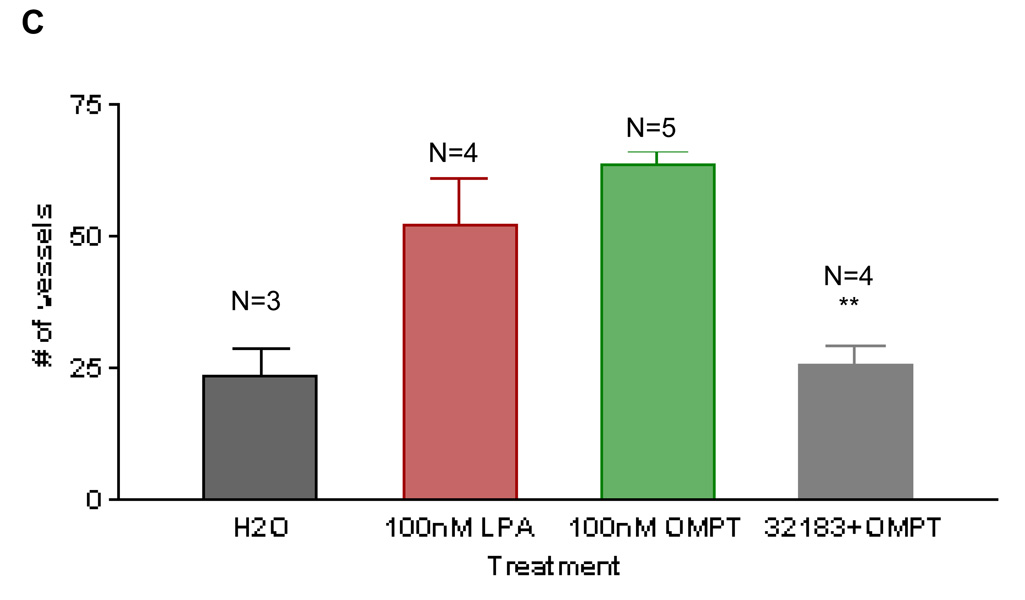
There is a paucity of LPA receptor selective compounds, particularly agonists; the few that have been discovered are directed to the LPA3 receptor. One of these is 1-oleoyl-2-O-methylglycerophosphothionate (OMPT). S-OMPT is a selective agonist for the LPA3 receptor if used at a relatively low concentration (i.e. 100nM)25. We used S-OMPT to study the role of LPA3 receptor in angiogenesis in the CAM system, applying compound daily to eggs for three days. Due to the low solubility of this compound in pure water, we used water containing 3% fatty acid free BSA as a vehicle. As documented in Figures 4A and 4B, S-OMPT was angiogenic at this concentration. When we collided the LPA3 receptor selective agonist, S-OMPT, with the antagonist VPC32183, the angiogenic effect of this LPA3-selective agonist was not observed (Figure 4C).
Figure 4. S-OMPT, an LPA3-selective agonist, is also an inducer of angiogenesis in vivo and its effect is antagonized by VPC32183.
S-1-oleoyl-2-O-methylglycerophosphothionate (S-OMPT), at 100nM, is able to induce a strong angiogenic response, similar to the LPA response at this concentration (4A–4C). CAMs treated with S-OMPT (100nM) together with VPC32183 (10µM) document that the response is completely blocked by inclusion of this antagonist (4C). For statistical analysis, Student t test was performed between groups. ** P< 0.001. Error bars represent standard deviation (SD). Figure 4B is the image of a single membrane that is representative of each group. Scale bar: 0.125 cm.
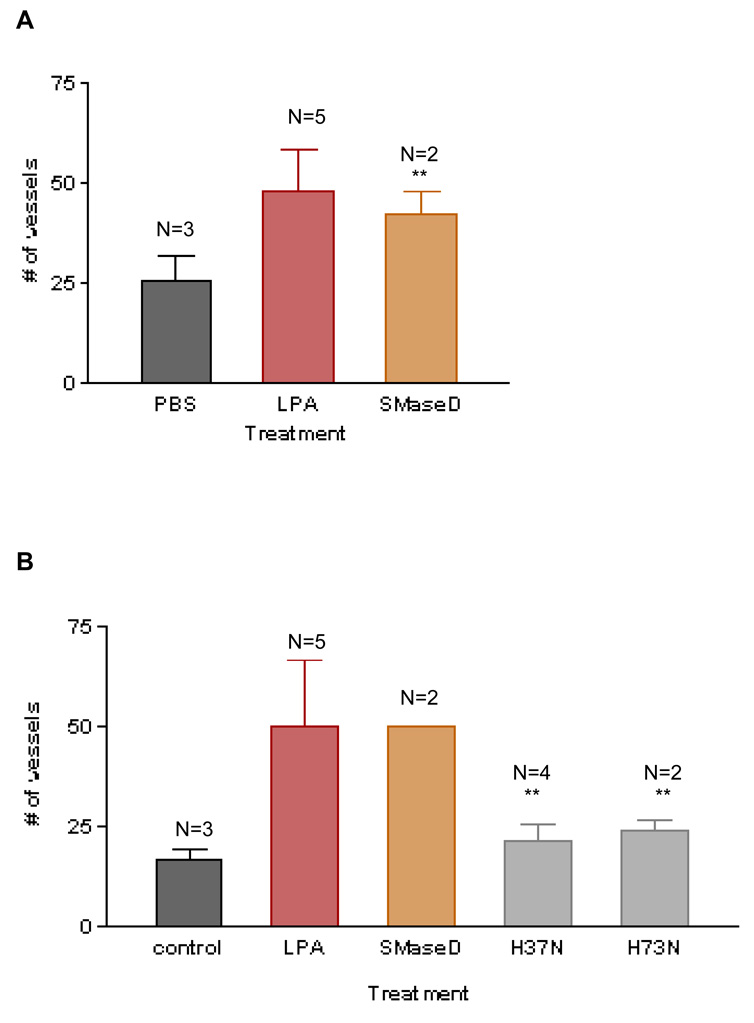
Arachnid lysoPLD is an angiogenic factor in vivo and its action is blocked by VPC32183
An arachnid sphingomyelinase D (“SMaseD”) that is a component of the venom of Loxosceles reclusa spiders was demonstrated recently by us26 and others27 to also catalyze the formation of LPA from lysophosphatidylcholine (LPC). Indeed, this enzyme activity is far more robust than that of the mammalian lysoPLD, autotaxin (ATX), and it lacks the broad-spectrum nucleotide phosphodiesterase and pyrophosphatase activities of autotaxin. Since the spider enzyme generates LPA, we wanted to determine if it is able to induce angiogenesis in vivo, similar to autotaxin (known to be angiogenic in mice21). We tested recombinant SMaseD produced in our laboratory and compared its effects with two catalytically inactive mutant SMaseD proteins (H37N, H73N)26. As documented in Figure 5A, the brown recluse spider enzyme evokes an angiogenic response, and the magnitude of its effect is comparable to the response induced by LPA. In contrast, the two catalytically inactive mutants did not mimic the angiogenic response observed with the wild type enzyme (Figure 5B). Importantly, the angiogenesis that results from the treatment with the wild type spider enzyme is blocked fully by the LPA1, 3 receptor antagonist, VPC32183 at 10µM (Figure 5C). Combined, these results further suggest that LPA is an angiogenic factor in vivo and that its actions are due to the activation of the LPA1 receptor, the LPA3 receptor, or both.
Figure 5. Arachnid PLD is an angiogenic factor in vivo and its action is blocked by VPC32183.
CAMs were treated with recombinant sphingomyelinase D (SMaseD) and the number of vessels that intersected the disc was counted. This enzyme is able to induce an angiogenic response (5A) similar to the LPA response. Two catalytically inactive forms of this enzyme (H37N and H73N) were unable to induce angiogenesis (5B), suggesting that the response obtained is dependent on the catalytic activity (lyso-PLD) of the arachnid enzyme. Moreover, SMaseD-induced angiogenesis is blocked by the LPA receptor antagonist VPC32183 at 10µM (5C), suggesting that the positive response obtained is dependent on the activation of LPA1,3 receptors. One-Way ANOVA test was performed between groups. ** P< 0.001. Error bars represent standard deviation (SD).
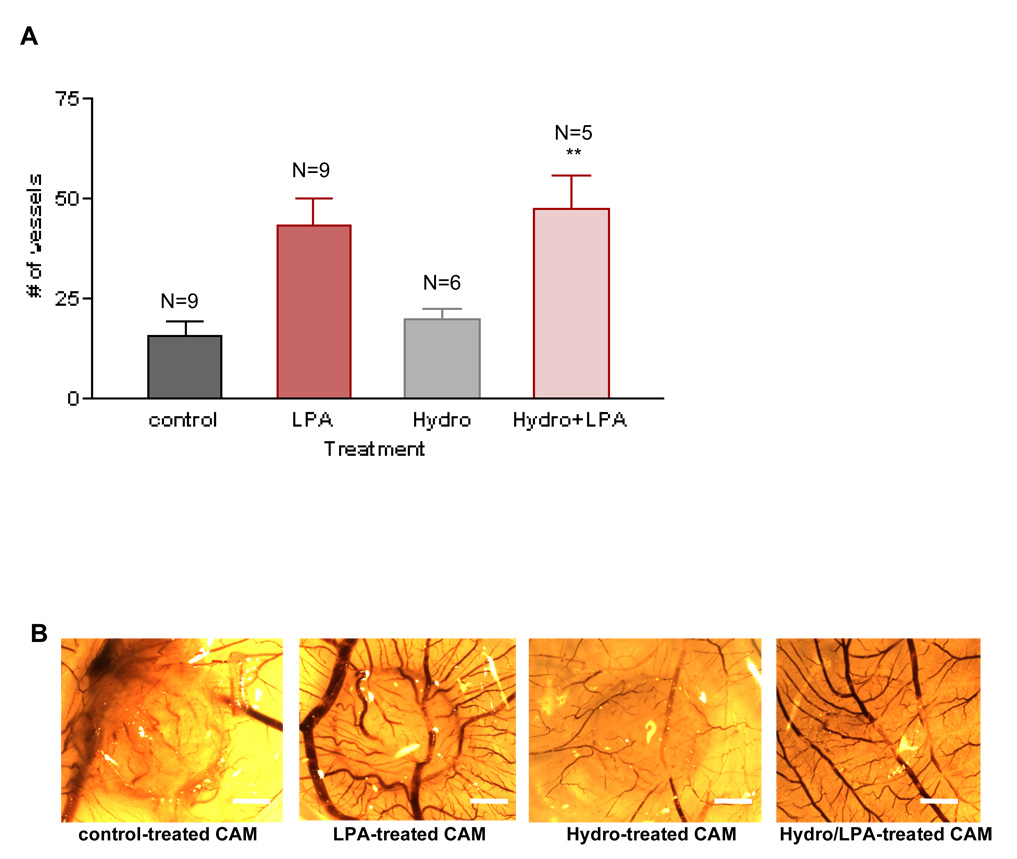
LPA-induced angiogenesis is not due to a non-specific inflammatory response
LPA has been shown to be involved in some inflammatory responses after activation of its receptors28. To discount the possibility that the angiogenesis obtained with LPA in the CAM membranes is a result of a non-specific inflammatory response, we treated them with hydrocortisone, an anti-inflammatory corticosteroid. CAMs were treated for three days with LPA, hydrocortisone alone or LPA together with 30 ng hydrocortisone and then the number of new vessels was determined29. We found that hydrocortisone is unable to block LPA-induced angiogenesis (Figures 6A and 6B), suggesting that the response we observed with LPA is not due to a non-specific inflammatory response. Importantly, hydrocortisone alone has no effect in the normal angiogenesis/development of the CAMs.
Figure 6. LPA-induced angiogenesis is not due to a non-specific inflammatory response.
To test the possibility of LPA producing a non-specific inflammatory response, we treated the CAMs with hydrocortisone (30ng). As shown in Figures 6A and 6B, hydrocortisone did not block the LPA-induced angiogenesis in the CAMs, suggesting that the LPA response is not due to a non-specific inflammatory response. The One-Way ANOVA test was performed between groups. ** P< 0.001. Error bars represent standard deviation (SD). Figure 6B shows one membrane representative of each group. The vehicle and LPA images in this figure are replicated from Figure 2B because it was one large experiment wherein we used the same controls (LPA and vehicle) to analyze two results. Scale bar: 0.125 cm.
DISCUSSION
Angiogenesis is the sprouting and growth of new capillaries from pre-existing blood vessels. It is a tightly regulated process that controls a number of normal biological functions such as normal development and reproduction, but also regulates pathological conditions such as tumor growth and cancer metastasis30. Angiogenesis has been studied for many years and the discovery of new angiogenic (stimulants) and angiostatic (inhibitors) factors makes it an active area of research. Two essential processes that are involved in the growth of new blood vessels are proliferation and migration of endothelial cells to start lining the new vessels. Lysophosphatidic acid (LPA) has been shown to induce both of these processes in vitro using endothelial cell cultures. Because of these results obtained in the last few years, we were interested in studying LPA and its role in angiogenesis in vivo. Using the chicken chorio-allantoic membrane (CAM) assay, we document that LPA is a direct angiogenic factor in vivo. Importantly, this response is completely blocked by an LPA1 and LPA3 receptor antagonist, VPC32183, suggesting that the response obtained with LPA is due to the activation of either of these receptors or both working in a synergistic manner. To study further the role of individual LPA receptors in LPA-induced angiogenesis, we tested an LPA3 receptor-selective agonist, S-OMPT. At a concentration where this compound does not activate other LPA receptor subtypes, S-OMPT induces a strong angiogenic response in the CAMs. Moreover, its effect is completely blocked by the LPA1/LPA3 antagonist VPC32183. This suggests that LPA3 could be inducing the angiogenic response, but we cannot discard the possibility that LPA1 is also involved in the chicken assay. A better tool kit of LPA receptor-selective compounds is required to test such hypotheses, but such molecules are lacking at present.
In 2001 a group of researchers showed that autotaxin (ATX; one of the LPA-producing enzymes) is angiogenic in vivo21 while, another group showed that this enzyme is essential for normal vascular development22. To complement these results, we tested a similar LPA-producing enzyme present in brown recluse spider (Loxosceles reclusa) venom. This enzyme also has lyso-PLD activity and robustly generates LPA from LPC. As we documented herein, the spider enzyme is able to induce angiogenesis as well. Interestingly, the angiogenic response obtained from the enzyme is dependent on its lyso-PLD activity, since two different catalytically-inactive mutants are not able to produce the same response. Moreover, the response of the wild type construct appears to be dependent on the activation of LPA receptors, because it is completely abolished by the antagonist VPC32183. In toto, our results suggest that LPA is an angiogenic factor in vivo and that the response is via the activation of LPA1, LPA3 or both.
An interesting detail that we noticed in the course of our studies is that LPA-induced vessels appear larger and more robust than VEGF-induced vessels. It is known that VEGF induces vasculogenesis and angiogenesis and also that it produces immature/leaky vessels that need to continue a maturation process induced by other factors (arteriogenic factors)31, 32. Perhaps LPA is an angiogenic as well as arteriogenic (or vessel maturation) factor in vivo. However, this hypothesis needs to be studied in detail with other arteriogenesis-specific experiments. It is clear now that LPA induces angiogenesis in vivo in the CAM assay. However, more experiments using other angiogenic models in rodents are needed to determine the biological effects of blocking LPA receptors and LPA-induced angiogenesis. Also, there is a need for new and more selective compounds to target (positively or negatively) individual LPA receptors. Another option is to use LPA1 and/or LPA3 null mice to study further the role of each of these receptors in angiogenesis and angiogenesis-dependent diseases such as tumor metastasis. If we are able to determine the role that each of these receptors play in angiogenesis, then it would be helpful to have a receptor-selective antagonist that blocks the unwanted response and that could be used in the future to treat angiogenesis-related diseases.
Acknowledgements
We want to thank Sangderk Lee, Ph.D. (Lynch laboratory) for providing us with helpful advice for cloning the chicken LPA receptors.
Funding sources: This work was supported by research grants from the National Institutes of Health (R01 GM052722 (KRL) and F31 HL079881 (CRL).
Footnotes
Disclosures: VPC32183 is sold by Avanti Polar Lipids under license from the University of Virginia.
REFERENCES
- 1.English D, Kovala AT, Welch Z, Harvey KA, Siddiqui RA, Brindley DN, Garcia JG. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by LPA, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res. 1999;8(6):627–634. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- 2.Duriex ME, Lynch KR. Signaling properties of lysophosphatidic acid. Trends of Pharmacological Sciences. 1993;14:249–254. doi: 10.1016/0165-6147(93)90021-b. [DOI] [PubMed] [Google Scholar]
- 3.Goodemote KA, Mattie ME, Berger A, Spiegel S. Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine-1-phosphate. Journal of Biological Chemistry. 1995;270:10272–10277. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- 4.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 5.Postma FR, Jalink K, Hengeveld T, Moolenar WH. Sphingosine-1-Phosphate rapidly induces rho-dependent neurite retraction: action through a specific cell surface receptor. The EMBO Journal. 1996;15:2388–2395. [PMC free article] [PubMed] [Google Scholar]
- 6.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. Journal of Biological Chemistry. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 7.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. Journal of Biological Chemistry. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 8.Van Koppen CJ, Heringdorf DMZ, Laser KT, Zhang CY, Jakobs KH, Bunemann M, Pott L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine 1-phosphate. Journal of Biological Chemistry. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- 9.Fromm C, Coso OA, Montaner S, Xu N, Gutkind JS. The small GTP-binding protein Rho links G protein-coupled receptors and Galpha 12 to the serum response element and to cellular transformation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10098–10103. doi: 10.1073/pnas.94.19.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S, Strum JC, Bell RM. Lipid Biochemistry: functions of glycerolipids and sphingolipids in cellular signaling. FASEB Journal. 1997;11:45–50. doi: 10.1096/fasebj.11.1.9034165. [DOI] [PubMed] [Google Scholar]
- 11.Bian D, Su S, Mahanivong C, Cheng RK, Han Q, Pan ZK, Sun P, Huang S. Lysohphosphatidic acid stimulates ovarian cancer cell migration via a Ras-MEK Kinase 1 pathway. Cancer Research. 2004;64:4209–4217. doi: 10.1158/0008-5472.CAN-04-0060. [DOI] [PubMed] [Google Scholar]
- 12.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa2 (Edg4) and lpa1/lpa2 (Edg4/Edg2) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa2. Molecular and Cellular Biology. 2002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signaling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Analytical Biochemistry. 2001;292:287–295. doi: 10.1006/abio.2001.5063. [DOI] [PubMed] [Google Scholar]
- 15.Saulnier-Blache JS, Girard A, Simon MF, Lafontan M, Valet P. A simple and highly sensitive radioenzymatic assay for lysophosphatidic acid quantification. Journal of Lipid Research. 2000;41:1947–1951. [PMC free article] [PubMed] [Google Scholar]
- 16.Pages C, Simon MF, Valet P, Saulnier-Blache JS. Lysophosphatidic acid synthesis and release. Prostaglandins and Other Lipid Mediators. 2001;64:1–10. doi: 10.1016/s0090-6980(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 17.Saba JD. Lysophospholipids in Development: Miles Apart and Edging In. Journal of Cellular Biochemistry. 2004;92:967–992. doi: 10.1002/jcb.20128. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Goetzl EJ, An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. American Journal of Physiology and Cell Physiology. 2000;278:C612–C618. doi: 10.1152/ajpcell.2000.278.3.C612. [DOI] [PubMed] [Google Scholar]
- 19.English D, Kovala AT, Welch Z, Harvey KA, Siddiqui RA, Brindley DN, Garcia JG. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. Journal of Hematotherapy Stem Cell Research. 1999;8(6):627–634. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- 20.Wu WT, Chen CN, Lin CI, Chen JH, Lee H. Lysophospholipids enhance matrix metalloproteinase-2 expression in human endothelial cells. Endocrinology. 2005;146:3387–3400. doi: 10.1210/en.2004-1654. [DOI] [PubMed] [Google Scholar]
- 21.Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, Stracke ML. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Research. 2001;61:6938–6944. [PubMed] [Google Scholar]
- 22.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJO, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Molecular and Cellular Biology. 2006;26(13):5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabbadini RA. Targeting sphingosine-1-phosphate for cancer therapy. British Journal of Cancer. 2006;95:1131–1135. doi: 10.1038/sj.bjc.6603400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heasley BH, Jarosz R, Lynch KR, Macdonald TL. Initial structure-activity relationships of lysophosphatidic acid receptor antagonist: discovery of a high-affinity LPA1/LPA3 receptor antagonist. Bioorg Med Chem Letter. 2004;14:2735–2740. doi: 10.1016/j.bmcl.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa Y, Erickson JR, Goddard GJ, Yu S, Liu S, Cheng KW, Eder A, Bandoh K, Aoki J, Jarosz R, Schrier AD, Lynch KR, Mills GB, Fang X. Identification of a phosphothionate analogue of lysophosphatidic acid (LPA) as a selective agonist of the LPA3 receptor. Journal of Biological Chemistry. 2003;278:11962–11969. doi: 10.1074/jbc.M209168200. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Lynch KR. Brown Recluse spider (Loxosceles reclusa) venom phospholipase D (PLD) generates lysophosphatidic acid (LPA) Biochemical Journal. 2005;391:317–323. doi: 10.1042/BJ20050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Meeteren LA, Frederiks F, Giepmans BN, Pedrosa MF, Billington SJ, Jost BH, Tambourgi DV, Moolenaar WH. Spider and bacterial sphingomyelinases D target cellular lysophosphatidic acid receptors by hydrolyzing lysophosphatidylcholine. Journal of Biological Chemistry. 2004;279(12):10833–10836. doi: 10.1074/jbc.C300563200. [DOI] [PubMed] [Google Scholar]
- 28.Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. The FASEB Journal. 1998;12:1589–1598. [PubMed] [Google Scholar]
- 29.Koutrafouri V, Leondiadis L, Avgoustakis K, Livaniou E, Czarnecki J, Ithakissios DS, Evangelatos GP. Effect of thymosin peptides on the chick chorioallantoic membrane angiogenesis model. Biochimica et Biophysica Acta. 2001;1568:60–66. doi: 10.1016/s0304-4165(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 30.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Reviews: Drug Discovery. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 31.Chen CH, Walterscheid JP. Plaque angiogenesis versus compensatory arteriogenesis in atherosclerosis. Circulation Research. 2006;99:787–789. doi: 10.1161/01.RES.0000247758.34085.a6. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]