Abstract
Evolutionary biologists have long been fascinated by both the ways in which species respond to ecological conditions at the edges of their geographic ranges and the way that species' body sizes evolve across their ranges. Surprisingly, though, the relationship between these two phenomena is rarely studied. Here, we examine whether carnivore body size changes from the interior of their geographic range towards the range edges. We find that within species, body size often varies strongly with distance from the range edge. However, there is no general tendency across species for size to be either larger or smaller towards the edge. There is some evidence that the smallest guild members increase in size towards their range edges, but results for the largest guild members are equivocal. Whether individuals vary in relation to the distance from the range edges often depends on the way edge and interior are defined. Neither geographic range size nor absolute body size influences the tendency of size to vary with distance from the range edge. Therefore, we suggest that the frequent significant association between body size and the position of individuals along the edge-core continuum reflects the prevalence of geographic size variation and that the distance to range edge per se does not influence size evolution in a consistent way.
Keywords: abundance, Bergmann's rule, body size, Carnivora, range edge
1. Introduction
Range edges are usually thought to constitute suboptimal habitats. At the range edge at least one niche axis must be limiting to a peripheral population, or it would expand its range. Populations at range edges are therefore often seen as existing in unfavourable ecological conditions. If range edges are harsher environments for a species, climates may be less suitable and resource abundance may be lower (Brown 1984; Brown et al. 1995; Pitt et al. 2008), and hence body condition may be poorer (Perez-Tris et al. 2000).
Body size is a major factor influencing animal morphology, physiology, ecology, evolution and extinction probability (Haldane 1928; Stanley 1973; Peters 1983; Schmidt-Nielsen 1984; Cardillo et al. 2005), and has therefore been studied intensively. The ecology, genetics and evolution of populations at range edges are also the focus of much research (Brown 1984; Channell & Lomolino 2000; Sagarin & Gaines 2002b). The effects of range edges on ecological variables such as density have often been investigated (Brown 1984; Brown et al. 1995; Enquist et al. 1995; Blackburn et al. 1999). Populations near their range limits are thought to be facing harsh ecological conditions (such as different climate, abundance, competitors, prey and predator species). These factors are often thought to influence size evolution but, surprisingly, the relationship between body size and the gradient in niche optimality, which may exist from the core to the marginal areas of a species distribution, is seldom studied (e.g. Perez-Tris et al. 2000; Hallas et al. 2002; Fukui et al. 2005). Owing to the strong relationship between size and animal life history, ecology and evolution, studying the mode of size evolution near the limits of species' distributions may illuminate the ecological circumstances facing populations living on the edge. Furthermore, general patterns in the direction of body size evolution towards range margins might distinguish among the mechanisms by which the ranges of species become limited.
It is difficult, however, to hypothesize a priori about the direction and effect size of trends in body size towards the range edge, because plausible mechanisms from existing theory generate contrasting predictions. Applicable mechanisms are broadly categorized into inter- and intraspecific completion, resource and competitor density, climatic variation and effects of geographic and phenotypical scale.
Given a strong relationship between resource abundance and body size (Bonnet et al. 2002; Jessop et al. 2006; Raia & Meiri 2006), a plausible hypothesis may be that body sizes should decrease from core areas towards the edge of a species' range. Indeed, Allen (1876) hypothesized that body size should covary positively with abundance and increase towards the central distribution of the species.
However, both the relationship between abundance and position within the range, and abundance and body size, are much debated. Although it has been argued repeatedly that population abundances tend to be low at range edges, other patterns are often reported (e.g. Blackburn et al. 1999; Sagarin & Gaines 2002a; Sagarin et al. 2006). Furthermore, size is often thought to be positively correlated with density, because large size confers a competitive advantage within species (Melton 1982). However, low density may actually allow individuals access to more resources, and body size is sometimes negatively correlated with density (Boucher et al. 2004; Fiori & Defeo 2006). If peripheral populations inhabit areas where competitors are absent, then small guild members may increase in size and exploit the niche of a missing larger competitor (McNab 1971), whereas large species may decrease in size (Dayan & Simberloff 1998). However, if range edges are characterized by low resource abundance it may be that large species will decrease in size, while smaller species may be less affected (McNab 1971; Heaney 1978; Dayan & Simberloff 1998).
Core individuals may be larger than those living near range edges if core areas have more continental climates and if the higher seasonality of such areas selects for large body size (Brodie 1975; Boyce 1979; Millar & Hickling 1990; cf. Ferguson & McLoughlin 2000; Meiri et al. 2005b). Body size frequently varies clinally across space, often (in endotherms) in accordance with Bergmann's rule, which predicts that size will increase with decreasing temperatures and by proxy with increasing latitude (Mayr 1963; Freckleton et al. 2003; Meiri & Dayan 2003; Meiri et al. 2007). Therefore, size may be large at the colder edges of the range, small at the hotter edges, and, if core areas of the range are characterized by intermediate temperatures, intermediate there. The (few) studies that have explicitly examined intraspecific size variation in relation to population position along the core—range edge axis usually did not account for such overriding clinal variation (e.g. Hallas et al. 2002; Diaz et al. 2007).
Finally, the distance to range edges may not affect size evolution in any consistent manner but may appear to do so owing to factors that are not directly related to this distance. Thus, we would expect that species with larger ranges, where the scope for geographic variation in size is greater (Meiri et al. 2007), would more often show a significant relationship between size and distance to the range edge than would species with smaller ranges. Likewise, we would expect that species that vary more in size will more likely show a significant relationship between size and distance to the range edge.
Here, we examine the relationship between distance from the distribution edge and body size in carnivores (Mammalia: Carnivora) using digitized distribution maps and a large dataset of carnivore cranial measurements (Meiri et al. 2005c), while statistically accounting for clinal geographic variation. We test whether there is a general tendency for carnivores to be smaller towards the edges of their geographic range (Allen 1876). We further test whether (i) a tendency towards smaller sizes near the range edges is stronger in larger species, owing to low resource abundance (McNab 1971), (ii) small guild members tend to grow larger near their range edges, while larger species grow smaller owing to reduced interspecific competition near the range edges (McNab 1971; Dayan & Simberloff 1998); this hypothesis differs from the previous one in that small members of one guild (e.g. American black bears Ursus americanus) can be larger than even the largest members of other guilds (e.g. the wolverine, Gulo gulo), and vice versa, and (iii) sizes of species with larger geographic ranges, and greater size variability across their ranges, tend to respond to the distance from range ends more than to sizes of species with smaller ranges.
2. Material and methods
We use condylobasal skull length (CBL) as a measure of body size. This commonly used size index is associated with low measurement error, does not increase in adults and is independent of body condition (Gould 1974; Gittleman & Van Valkenburgh 1997; Dayan et al. 2002; Meiri et al. 2005c). We measured skulls in natural history museums (see acknowledgments) and use only wild-caught, sexed adult specimens. Log transforming CBL has no qualitative effect on our results (not shown), and we therefore use raw CBL values. Here, we use only species for which our sample size is greater than or equal to 100 specimens with relatively precise locality data (less than 1° error, although in the vast majority of cases precision was much higher; figure 1).
Figure 1.
Specimen data localities for all species plotted onto the Fuller Dymaxion projection used for distance calculations.
Some studies compare populations they consider to be peripheral with those they consider to inhabit core areas (e.g. Sexton et al. 1992; Fukui et al. 2005). Kark et al. (2008) have argued that such a dichotomy may miss important aspects of the between-population variation, and have used a third category of ‘sub-periphery’. They showed that genetic variability peaks at the sub-periphery (see also Schwartz et al. 2003). We use two ways to divide specimens into edge, sub-edge and interior categories. In the first, we find, for each species separately, the distance to range edge attained by the specimen furthest away from the edge. We then divide the logarithm of this distance by three, and note for each specimen of that species whether it falls in the furthest segment, the intermediate one or the one closest to the range edge. Under this classification, approximately 20, 29 and 51 per cent of the specimens are assigned to the edge, sub-edge and interior categories, respectively. In the second, we assign equal numbers of specimens to the three categories (but in case of ties, we classify specimens as edge and interior in preference to sub-edge). In a separate analysis, we treat distance to the nearest edge of the range (log transformed in all analyses) as a continuous predictor of size (following Blackburn et al. 1999; Komonen et al. 2004). Because specimen localities are often labelled in quite general terms we repeat this analyses at three levels of precision, with distances rounded up to the nearest 1, 5 and 30 km.
We treat populations of three species inhabiting both North America and the Palaearctic (Mustela erminea, Mustela nivalis and Vulpes vulpes) separately, owing to intraspecific size differences between North America and Eurasia (e.g. the first two species are larger in Eurasia, and response of their sizes to latitude differs between continents). We study Alopex lagopus, Canis lupus, G. gulo and Ursus arctos in their Nearctic range only, because we measured fewer than 100 Palaearctic specimens. While island populations are often peripheral, insularity may be associated with a host of selection pressures unrelated to position relative to the range end. Thus, at a similar distance to the near coast, even adjacent insular and mainland individuals may differ greatly in size. Furthermore, we have extensively examined the effects of insularity on body size elsewhere (Meiri et al. 2005a,b, 2006, 2008), so we opt to exclude all insular specimens from the present study.
Range edges can reflect either physiological–ecological or dispersal barriers such as mountain chains or oceans. Because we mainly measured specimens in order to compare island and mainland populations (see above), most (approx. 91%) of the specimens we study here were collected closer to coasts than to inland range edges. It is reasonable to believe that inland edges more likely reflect physiological or ecological barriers rather than dispersal barriers. If similar ecological or ecophysiological conditions affect body size, then the distance to inland edges may be a more informative size predictor than distance to coastal edges. We therefore introduce another analysis using only specimens that are closer to an inland than to a coastal edge. For this analysis, we use a minimum sample size of 20 specimens.
We digitized polygon range maps for each species used in Grenyer et al. (2006), modified, where necessary, using other published sources (see appendix 1 in the electronic supplementary material) and extended these range maps, where needed, to include specimen localities using ArcGIS v. 9.2 (ESRI, 2005). We used VMAP level 0 (NIMA 1997) to define the global extent of land. We generalized this outline and the digitized range maps to force 1 km between vertices to reduce computational demands.
Linear distances from specimen point localities were calculated to both the nearest coastal edge and the nearest inland edge. It proved computationally unfeasible to calculate geodesic distances (i.e. the ‘true’ distance between points on the surface of the Earth) between every specimen and the coastal or inland range edges. Consequently, the distances between specimens and range edges (and therefore the decision as to which point was nearest) were computed with the projected data. Since it was not feasible to produce over 7800 equidistant projections centred upon each specimen location, a compromise projection had to be chosen to minimize distortion across the analytical domain.
The Buckminster Fuller's Dymaxion projection (Fuller 1954) has two appealing properties for our purpose. Since it is a projection of the globe onto an icosahedron with each face subtending a separate centred gnomonic projection, both scale and conformal error are kept low across the global extent. The standard Dymaxion projection also positions the world's coastline such that it remains unbroken across discontinuities in the projection, and so facilitates the calculation of distances.
Because in carnivores males are almost always larger than females, and because both latitudinal and longitudinal size clines are common (Meiri et al. 2005d, 2007), we modelled CBL for each species as a function of sex, latitude, longitude and the distance to the edge of the range, or one of the three distance categories discussed above. Because specimen locality data are often imprecise, we repeated the analysis with distances binned to either 5 or 30 km intervals of distances from range edges. Slopes of CBL as a function of distance had identical signs for all three categories (see appendix 2 in the electronic supplementary material) in all species associated with significant (log) distance/CBL slopes. All the significant results (at p<0.05) obtained using 1 km bins remained significant using 5 and 30 km bins, and no result became significant using coarser distance measures. We are therefore confident that minor imprecision in locality data has negligible effects on our analysis, and we proceed using the 1 km precision data only.
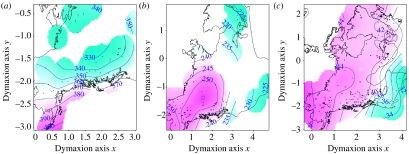
We performed exploratory data analysis with generalized additive models (GAMs). CBL was modelled as a function of specimen sex and a multidimensional smooth function of latitude and longitude (following Wood 2006), using a thin plate regression spline as the smooth function basis, and determining the degree of smoothing by generalized cross-validation. Model summaries and maps of the spatial components and ranges for all species are presented in the electronic supplementary material, appendix 3. Exemplar plots of the spatial components for three species showing opposing patterns are shown in figure 2.
Figure 2.
Three exemplar species from North America showing contrasting patterns of within-range variation in CBL. Contours and surfaces are marginal predicted CBL surfaces on the scale of the response variable from GAMs of CBL as a parametric function of sex and a smooth multidimensional function of position in projected (Fuller's Dymaxion) space (units are thousands of km, small–large gradient shaded as cyan to magenta). Only male specimen surfaces are shown. Points show specimen locations. (a) Ursus arctos, (b) Canus lupus and (c) Mustela erminea Nearctic.
We test whether the response of size to distance from range edge is related to carnivore body mass (data from Meiri et al. 2005e) or position along the large-to-small axis within guilds, defined on the basis of morphological similarities that reflect similarities in movement and killing behaviour (see Simberloff & Dayan (1991) for discussion of ecological guilds): we classify U. arctos, C. lupus and G. gulo as the largest members of their guilds, and A. lagopus, Urocyon procyonoides, Viverricula indica and M. nivalis as the smallest guild members. Other species are deemed intermediate and are not used in this analysis.
3. Results
(a) Distance to range edge as a continuous predictor
Our dataset contains 7871 specimens belonging to 25 species (three of which are examined separately in the Nearctic and Palaearctic; mean sample size 281±49 s.e., range 102–1227). The models of distance from range edge as a continuous predictor are presented in the electronic supplementary material, appendix 4A. Sex significantly affected CBL in all models (males were always larger). Latitude and longitude were significantly correlated with CBL in 16 and 19 of 28 cases, respectively. (CBL significantly increased with latitude in 12 cases and significantly decreased in 4 cases. It significantly increased eastwards in five cases and westwards in 14.) Slopes and probabilities for the effect of distance from range edge are shown in table 1. Twelve relationships were significant. Size increases towards the range interior in four cases and the range edge in eight cases. Adding a quadratic term to the log distance variable, to account for nonlinearity, resulted in significantly better models in 5 of 28 cases (determined by both ANOVA and AIC scores; see the electronic supplementary material appendices 4B and 4C).
Table 1.
Slopes and probabilities for the effect of distance from range edge (km) on CBL (mm). (The results are slopes and probabilities for a linear model of CBL as a function of sex, latitude, longitude and distance to range edge. P, Palaearctic sample; N, Nearctic sample. Coefficients for other variables are given in the electronic supplementary material, appendix 4A.)
| species | n | slope (mm CBL per log (km) distance) | p-value |
|---|---|---|---|
| Alopex lagopus | 153 | 0.087 | 0.900 |
| Canis aureus | 108 | −1.255 | 0.153 |
| Canis latrans | 176 | −0.251 | 0.741 |
| Canis lupus | 173 | 3.879 | 0.0002 |
| Gulo gulo | 128 | −0.201 | 0.689 |
| Herpestes edwardsii | 103 | 0.751 | 0.172 |
| Lontra canadensis | 153 | −1.868 | 0.0001 |
| Lutra lutra | 105 | −1.128 | 0.105 |
| Lynx canadensis | 197 | −0.145 | 0.748 |
| Martes americana | 307 | −0.678 | 0.001 |
| Martes foina | 264 | −0.190 | 0.482 |
| Martes martes | 145 | 0.232 | 0.637 |
| Meles meles | 328 | 0.730 | 0.061 |
| Mustela erminea (N) | 1227 | 0.199 | 0.014 |
| Mustela erminea (P) | 437 | 0.256 | 0.135 |
| Mustela frenata | 714 | −0.589 | <0.0001 |
| Mustela nivalis (N) | 149 | −0.473 | 0.001 |
| Mustela nivalis (P) | 823 | −1.492 | <0.0001 |
| Mustela putorius | 467 | 1.380 | <0.0001 |
| Mustela vison | 404 | 0.211 | 0.285 |
| Paradoxurus hermaphroditus | 109 | 3.181 | 0.001 |
| Procyon lotor | 131 | 0.902 | 0.123 |
| Spilogale gracilis | 102 | −1.839 | 0.002 |
| Urocyon cinereoargenteus | 174 | −0.063 | 0.906 |
| Ursus arctos | 151 | −8.817 | <0.0001 |
| Viverricula indica | 108 | −1.201 | 0.028 |
| Vulpes vulpes (N) | 235 | −0.332 | 0.481 |
| Vulpes vulpes (P) | 300 | 0.747 | 0.224 |
(b) Distance to range edge as a categorical predictor
The models with all predictor variables are shown in the electronic supplementary material, appendix 4D. With equal distances categorization, edge specimens are significantly larger than core ones in seven cases and smaller in five cases (table 2). Edge specimens are significantly larger than sub-edge ones in four cases and smaller in two cases. With equal numbers of specimen categories, edge specimens are significantly larger than core ones in eight cases and smaller in six cases. Edge specimens are significantly larger than sub-edge ones in four cases and smaller in four others (table 2).
Table 2.
Comparison of edge individuals to those at the sub-edge and core. (Sample sizes as in Table 1. For each species, categories of edge, sub-edge and interior were based on either (i) equal distances, or (ii) equal numbers of specimens. Edge specimens are compared with (a) interior ones, and (b) sub-edge ones. P, Palaearctic sample; N, Nearctic sample. The results are t values from a linear model of CBL as a function of sex latitude, longitude and distance to range edge categories. Sample sizes in Table 1. Precise p values and coefficients for other variables are given in appendix 4D in the electronic supplementary material. *p<0.05; **p<0.01; ***p<0.001.)
| (a) comparison of edge to interior | (b) comparison of edge to sub-edge | |||
|---|---|---|---|---|
| (i) categories based on equal distances | (ii) categories based on equal sample sizes | (i) categories based on equal distances | (ii) categories based on equal sample sizes | |
| Alopex lagopus | −0.601 | 0.614 | 1.090 | −0.842 |
| Canis aureus | −1.083 | −1.440 | −1.027 | −0.945 |
| Canis latrans | 0.305 | 0.126 | −0.688 | −0.979 |
| Canis lupus | 3.454** | 2.877** | 1.227 | 0.952 |
| Gulo gulo | 0.141 | −0.236 | 0.290 | 0.625 |
| Herpestes edwardsii | 1.451 | 0.114 | 1.609 | −0.266 |
| Lontra canadensis | −4.421*** | −3.571*** | −0.835 | −2.335* |
| Lutra lutra | −1.123 | −1.028 | 0.237 | 0.461 |
| Lynx canadensis | −0.911 | 0.681 | −1.486 | 0.517 |
| Martes americana | −4.911*** | −4.354*** | 1.466 | −0.238 |
| Martes foina | −1.342 | 1.090 | −2.127* | 0.480 |
| Martes martes | −0.303 | 0.698 | −0.569 | −0.595 |
| Meles meles | 2.162* | 2.916** | 0.524 | 0.222 |
| Mustela erminea (N) | 2.383* | 2.762** | −0.743 | 2.557* |
| Mustela erminea (P) | 0.484 | 1.394 | 0.971 | −0.732 |
| Mustela frenata | −2.896** | −3.672*** | −2.414* | −2.442* |
| Mustela nivalis (N) | −3.343** | −3.006** | −1.979* | −1.925 |
| Mustela nivalis (P) | −11.171*** | −13.445*** | −2.996** | −8.470*** |
| Mustela putorius | 4.695*** | 5.318*** | 2.699** | 2.190* |
| Mustela vison | 0.481 | 2.053* | −0.674 | −0.515 |
| Paradoxurus hermaphroditus | 3.136** | 2.250* | 1.480 | 2.467* |
| Procyon lotor | 0.653 | 0.900 | 1.322 | 1.668 |
| Spilogale gracilis | −3.369** | −3.247** | 2.469* | 2.477* |
| Urocyon cinereoargenteus | −0.219 | −1.093 | 0.790 | 0.673 |
| Ursus arctos | −5.454*** | −4.788*** | −1.848 | −2.551* |
| Viverricula indica | −0.866 | −2.561* | 1.434 | −1.856 |
| Vulpes vulpes (N) | −1.054 | −0.204 | −0.794 | −1.715 |
| Vulpes vulpes (P) | 1.749 | 0.241 | 0.788 | −0.996 |
In four species, all five tests (distance as a continuous variable, edge versus sub-edge and edge versus interior with both equal distances and equal number of specimens) are significant. Mustela putorius is larger in the interior of its range, whereas Mustela frenata and Palaearctic M. nivalis are larger towards the edges. Specimens of Spilogale gracilis increase linearly in size towards the range edge, and edge specimens are larger than interior ones. However, they are smaller than sub-edge ones (although a quadratic term for distance in this species was not significant). Ursus arctos, Lontra canadensis and Nearctic M. nivalis are significantly larger closer to the range edge in four of five tests, while Paradoxurus hermaphroditus and Nearctic M. erminea are significantly smaller closer to the edge in four tests.
Twelve species showed no significant response to range edge in any test (including V. vulpes in both the Nearctic and the Palaearctic). The other six species showed significant change in size in relation to range edges in one to three of five tests.
By examining the effect of distance to range edge using only specimens closer to an inland edge than to a coastal one (table 3, see appendix 4E in the electronic supplementary material), we found that size significantly increased towards range edges in 3 of 11 cases and significantly decreased in two others. In just one case (M. frenata) was the direction of size change similar to that found for the whole dataset. In one case (Nearctic M. erminea), the direction of size changes was opposite to that found in the whole dataset, and three species showing no significant response to distance in the whole dataset showed significant size changes when only specimens closer to an inland edge than to a coastal one were considered.
Table 3.
Data of specimens closer to inland than to coastal edges. (Slopes and probabilities for the effect of distance from range edge (km) on CBL (mm). Only specimens inhabiting areas closer to an inland edge than to a coastal edge are used. The results are slopes and probabilities for a linear model of CBL as a function of sex, latitude, longitude and distance to range edge. P, Palaearctic sample; N, Nearctic sample. Coefficients for other variables are given in the electronic supplementary material, appendix 4E.)
| species | n | slope | p-value |
|---|---|---|---|
| Herpestes edwardsii | 54 | 0.603 | 0.575 |
| Martes americana | 53 | −1.669 | 0.488 |
| Meles meles | 22 | 6.744 | 0.008 |
| Mustela erminea (N) | 143 | −1.996 | 0.0002 |
| Mustela erminea (P) | 37 | 5.091 | 0.031 |
| Mustela frenata | 111 | −0.869 | 0.033 |
| Mustela nivalis (N) | 28 | 0.399 | 0.701 |
| Mustela nivalis (P) | 23 | −0.644 | 0.851 |
| Mustela vison | 28 | −11.607 | 0.0001 |
| Viverricula indica | 30 | 2.435 | 0.337 |
| Vulpes vulpes (N) | 78 | −3.145 | 0.117 |
(c) Correlates of response to range ends
Body size per se does not influence the response of CBL to distance from range edges: of three large (more than 10 kg) species in our dataset, one (C. lupus) increases in size away from range edges (all comparisons here refer to distance treated as a continuous variable; table 1); one (U. arctos) decreases (figure 2); and a third (the coyote, Canis latrans) shows no significant change. Small (up to approx. 1 kg) species either increase in size towards the range edge (M. nivalis in both Palaearctic and Nearctic, M. frenata, S. gracilis and Martes americana), increase in size away from the range edge (Nearctic M. erminea and M. putorius) or show no response (Palaearctic M. erminea, Mustela vison and Eurasian Martes). The standardized slope for the distance to range edge/CBL relationship is not correlated with body mass (n=28, t=0.70, R2=0.019, p=0.49), nor is the absolute value of this standardized slope correlated with mass (t=0.10, R2<0.001, p=0.92).
One largest guild member (C. lupus) decreases in size towards the edge of its range, in agreement with the prediction of McNab (1971), while one other (the wolverine, G. gulo) shows no trend and a third (U. arctos) shows the opposite pattern. Two of the smallest members of their guilds (M. nivalis and V. indica) increase in size towards the edges of their range, in accordance with the predictions of McNab (1971), whereas the two small members of the canid guild, the arctic and grey foxes (A. lagopus and Urocyon cinereoargenteus), show no response.
Range size (log transformed) did not differ between carnivores that show a significant linear relationship between CBL and distance to range edge (mean log range size=6.97) and those that did not (mean log range size=7.09 t=−1.22, p=0.23). Furthermore, there was no correlation between range size and the standardized slope for the distance to range edge/CBL relationship (slope −0.056±0.10, R2=0.011, p=0.59) or the absolute value of this slope (slope −0.05±0.07, R2=0.019, p=0.48).
The range sizes of the carnivores we examine are uniformly large, with just one order of magnitude covering the entire range size (2.9 million km2 for S. gracilis to 42 million km2 for Palaearctic red foxes (V. vulpes); see appendix 5 in the electronic supplementary material).
Carnivores showing a significant linear relationship between CBL and distance to range edge have higher overall geographic variation in size (measured as the average of the coefficients of variation for male and female CBL, to control for sexual dimorphism) to those that do not (mean CV=5.99 versus 4.59; t=2.41, p=0.027).
4. Discussion
Size change in response to distance from range edge is relatively common: between 21 and 50 per cent of our results in the different analyses are significant. We are unsure, however, whether this high prevalence of significant results truly reflects an effect of range edge per se. Apart from species' tendencies to vary in size, none of the factors we examined seem to predict the direction and magnitude of size evolution consistently. There does not seem to be an excess of species in which body size is smaller near the range edges (cf. Allen 1876). In fact, individuals inhabiting range edges are often larger than core-area conspecifics (Thurber & Peterson 1991; Law et al. 2002; Goltsman et al. 2005; this study), but again this pattern is not general. Neither does there seem to be a general tendency for similar patterns to be obtained in relation to range size or absolute body size. There is some indication that small carnivores and small guild members may increase in size towards the edges of their ranges in line with McNab's (1971) hypothesis that small-bodied members of guilds are likely to increase in size away from their mid-latitudinal distribution. However, even these results are equivocal, and there is no general pattern of size decrease towards the edge of the range in the largest guild members.
Body sizes may often differ greatly between range edges and core area in parallel with clinal responses to latitude or temperature (i.e. according to Bergmann's rule, Mayr 1963; Meiri & Dayan 2003). Such a pattern, for example, is seen in Nearctic M. erminea (figure 2). However, whether an animal will be small or large at the range edge depends on which edge it inhabits and is not a response to living on the edge per se. The common occurrence of insular dwarfs and giants (Foster 1964; Clegg & Owens 2002; Meiri et al. 2008) also probably depends on an interaction between the biology of a given species and the autecological conditions present on each island (Case 1978; Lawlor 1982; Raia & Meiri 2006; Meiri 2007).
The high variability of responses to range edge in body size of carnivores suggests the occurrence of different forces that block range expansion in different species (see, for example, Hersteinsson and Macdonald 1992) and differing directions, and a probable diversity in causes of size variation. In brown bears, for example, Ferguson & McLoughlin (2000) found that coastal populations with access to salmon had large body sizes, whereas inland bears were smaller (see also Meiri et al. 2007). However, barren-ground bears were smaller still, despite also living near the species' range edge. Thus, Ferguson & McLoughlin (2000) concluded that food abundance determines bear body size. We think body size variation often reflects autecological conditions interacting with species' biology.
We therefore believe that the common but somewhat idiosyncratic response of body size to distance from range edge we report here is more of a manifestation of the remarkable variability of carnivore body size than a reflection of a common response of size to position along the range edge–core continuum. Our finding that carnivores that vary more geographically also tend to show greater response to distance from the range edge supports this view. We hope that the finding that size often evolves in relation to the position of individuals within the geographic range will lead to further efforts to reveal the mechanisms that underpin such an impressive tendency for geographic variation in size.
Acknowledgments
We thank Robert Asher (Museum für Naturkunde, Humboldt Universität zu Berlin), H. Baagøe (Zoological Museum, University of Copenhagen), D. Balkwill and M. Gosselin (Canadian Museum of Nature), Josefina Barreiro (Museo Nacional de Ciencias Naturales), Yang Chang Man (Raffles Museum of Biodiversity Research), Judith Chupasko (Museum of Comparative Zoology), Judith Eger (Royal Ontario Museum), H. Endo (National Science Museum, Tokyo), D. Harrison and M. Perch (HZM), Thor Holmes (University of Kansas Museum of Natural History), Louise Tomsett and Daphne Hills (Natural History Museum, London), Lesley M. Kennes (Royal BC Museum), G. Jarrell (University of Alaska, Museum of Natural History), Richard Kraft (Zoologische Staatsamlung München), Eileen A. Lacey (MVZ), Georges Lenglet (Institut Royal des Sciences Naturelles de Belgique), Suzanne McLaren (Carnegie Museum of Natural History), Adri Rol (University of Amsterdam Zoological Museum), Byrdena Shepherd, Linda Gordon, Don Wilson and Kris Helgen (National Museum of Natural History Smithsonian Institution), Tsila Shariv (Tel-Aviv University Zoological Museum), Chris Smeenk (National Museum of Natural History ‘Naturalis’), William Stanley (Field Museum), Clara Stefen (Staatliche Naturhistorische Sammlungen, Dresden), Ray Symonds (Zoology Museum of Cambridge University), Geraldine Veron (Musee National d'Histoire Naturelle, Paris) and Darrin Lunde, Nancy Simmons and Eileen Westwig (American Museum of Natural History) for their invaluable help during data collecting. We thank Gavin Thomas, Kris Helgen, Joaquin Hortal and Ally Phillimore for advice and discussion, John Gittleman and Kamran Safi for help on an earlier version of the manuscript, and Miguel-Angel Olalla-Tarraga, Rodolphe Bernard and Meirion Hopkins for help with GIS.
Footnotes
One contribution of 17 to a Special Issue ‘Geographic range limits of species’.
Supplementary Material
Appendix 1—origin of carnivore range maps; appendix 2—sensitivity analysis with three distance categories; appendix 3—general additive model maps of the spatial components of size for all species; appendix 4—full result tables for all analyses; appendix 5—species level data
References
- Allen J.A. Geographical variation among North American mammals, especially in respect to size. Bull. U.S. Geol. Geogr. Surv. Territories. 1876;2:309–344. [Google Scholar]
- Blackburn T.M., Gaston K.J., Quinn R.M., Gregory R.D. Do local abundances of British birds change with proximity to range edge? J. Biogeogr. 1999;26:493–505. doi:10.1046/j.1365-2699.1999.00298.x [Google Scholar]
- Bonnet X., Pearson D., Ladyman M., Lourdais O., Bradshaw D. ‘Heaven’ for serpents? A mark-recapture study of tiger snakes (Notechis scutatus) on Carnac Island, Western Australia. Austral Ecol. 2002;27:442–450. doi:10.1046/j.1442-9993.2002.01198.x [Google Scholar]
- Boucher S., Crete M., Ouellet J.P., Daigle C., Lesage L. Large-scale trophic interactions: white-tailed deer growth and forest understory. Ecoscience. 2004;11:286–295. [Google Scholar]
- Boyce M.S. Seasonality and patterns of natural selection for life histories. Am. Nat. 1979;114:569–583. doi:10.1086/283503 [Google Scholar]
- Brodie P.F. Cetacean energetics, an overview of intraspecific size variation. Ecology. 1975;56:152–161. doi:10.2307/1935307 [Google Scholar]
- Brown J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984;124:255–279. doi:10.1086/284267 [Google Scholar]
- Brown J.H., Mehlman D.W., Stevens G.C. Spatial variation in abundance. Ecology. 1995;76:2028–2043. doi:10.2307/1941678 [Google Scholar]
- Cardillo M., Mace G.M., Jones K.E., Bielby J., Bininda-Emonds O.R.P., Sechrest W., Orme C.D.L., Purvis A. Multiple causes of high extinction risk in large mammal species. Science. 2005;309:1239–1241. doi: 10.1126/science.1116030. doi:10.1126/science.1116030 [DOI] [PubMed] [Google Scholar]
- Case T.J. A general explanation for insular body size trends in terrestrial vertebrates. Ecology. 1978;59:1–18. doi:10.2307/1936628 [Google Scholar]
- Channell R., Lomolino M.V. Dynamic biogeography and conservation of endangered species. Nature. 2000;403:84–86. doi: 10.1038/47487. doi:10.1038/47487 [DOI] [PubMed] [Google Scholar]
- Clegg S.M., Owens I.P.F. The ‘island rule’ in birds: medium body size and its ecological explanation. Proc. R. Soc. B. 2002;269:1359–1365. doi: 10.1098/rspb.2002.2024. doi:10.1098/rspb.2002.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan T., Simberloff D. Size patterns among competitors: ecological character displacement and character release in mammals, with special reference to island populations. Mamm. Rev. 1998;28:99–124. doi:10.1046/j.1365-2907.1998.00029.x [Google Scholar]
- Dayan T., Wool D., Simberloff D. Variation and covariation of skulls and teeth: modern carnivores and the interpretation of fossil mammals. Paleobiology. 2002;28:508–526. doi:10.1666/0094-8373(2002)028<0508:VACOSA>2.0.CO;2 [Google Scholar]
- Diaz J.A., Perez-Tris J., Bauwens D., Perez-Aranda D., Carbonell R., Santos T., Telleria J.L. Reproductive performance of a lacertid lizard at the core and the periphery of the species' range. Biol. J. Linn. Soc. 2007;92:87–96. doi:10.1111/j.1095-8312.2007.00877.x [Google Scholar]
- Enquist B.J., Jordan M.A., Brown J.H. Connections between ecology, biogeography, and paleobiology: relationship between local abundance and geographic distribution in fossil and recent molluscs. Evol. Ecol. 1995;9:586–604. doi:10.1007/BF01237657 [Google Scholar]
- Ferguson S.H., McLoughlin P.D. Effect of energy availability, seasonality, and geographic range on brown bear life history. Ecography. 2000;23:193–200. doi:10.1111/j.1600-0587.2000.tb00275.x [Google Scholar]
- Fiori S., Defeo O. Biogeographic patterns in life-history traits of the yellow clam, Mesodesma mactroides, in sandy beaches of South America. J. Coast. Res. 2006;22:872–880. doi:10.2112/04-0409.1 [Google Scholar]
- Foster J.B. Evolution of mammals on islands. Nature. 1964;202:234–235. doi:10.1038/202234a0 [Google Scholar]
- Freckleton R.P., Harvey P.H., Pagel M. Bergmann's rule and body size in mammals. Am. Nat. 2003;161:821–825. doi: 10.1086/374346. doi:10.1086/374346 [DOI] [PubMed] [Google Scholar]
- Fukui D., Maeda K., Hill D.A., Matsumura S., Agetsuma N. Geographical variation in the cranial and external characters of the little tube-nosed bat, Murina silvatica in the Japanese archipelago. Acta Therio. 2005;50:309–322. [Google Scholar]
- Fuller, R. B. 1954 Fluid Geography, a Primer for the Airocean [sic] World. North Carolina State School of Design Journal, 41–48.
- Gittleman J.L., Van Valkenburgh B. Sexual dimorphism in the canines and skulls of carnivores: effects of size, phylogeny and behavioural ecology. J. Zool. 1997;242:97–117. [Google Scholar]
- Goltsman M., Kruchenkova E.P., Sergeev S., Volodin I., Macdonald D.W. ‘Island syndrome’ in a population of Arctic foxes (Alopex lagopus) from Mednyi Island. J. Zool. 2005;267:405–418. doi:10.1017/S0952836905007557 [Google Scholar]
- Gould S.J. The origin and function of ‘bizarre’ structures: antler size and skull size in the ‘Irish elk’, Megaloceros giganteus. Evolution. 1974;28:191–220. doi: 10.1111/j.1558-5646.1974.tb00740.x. doi:10.2307/2407322 [DOI] [PubMed] [Google Scholar]
- Grenyer R., et al. Global distribution and conservation of rare and threatened vertebrates. Nature. 2006;444:93–96. doi: 10.1038/nature05237. doi:10.1038/nature05237 [DOI] [PubMed] [Google Scholar]
- Haldane J.B.S. Possible worlds. Harper; New York, NY: 1928. On being the right size. pp. 20–28. [Google Scholar]
- Hallas R., Schiffer M., Hoffmann A.A. Clinal variation in Drosophila serrata for stress resistance and body size. Genet. Res. 2002;79:141–148. doi: 10.1017/s0016672301005523. doi:10.1017/S0016672301005523 [DOI] [PubMed] [Google Scholar]
- Heaney L.R. Island area and body size of insular mammals: evidence from the tri-colored squirrel (Callosciurus prevosti) of southeast Asia. Evolution. 1978;32:29–44. doi: 10.1111/j.1558-5646.1978.tb01096.x. doi:10.2307/2407408 [DOI] [PubMed] [Google Scholar]
- Hersteinsson P., Macdonald D.W. Interspecific competition and the geographical distribution of red and arctic foxes Vulpes vulpes and Alopex lagopus. Oikos. 1992;64:505–515. doi:10.2307/3545168 [Google Scholar]
- Jessop T.S., Madsen T., Sumner J., Rudiharto H., Phillips J.A., Ciofi C. Maximum body size among insular Komodo dragon populations covaries with large prey density. Oikos. 2006;112:422–429. doi:10.1111/j.0030-1299.2006.14371.x [Google Scholar]
- Kark S., Hadany L., Safriel U.N., Noy-Meir I., Eldredge N., Tabarroni C., Randi E. How does genetic diversity change towards the range periphery? An empirical and theoretical test. Evol. Ecol. Res. 2008;10:391–414. [Google Scholar]
- Komonen A., Grapputo A., Kaitala V., Kotiaho J.S., Paivinen J. The role of niche breadth, resource availability and range position on the life history of butterflies. Oikos. 2004;105:41–54. doi:10.1111/j.0030-1299.2004.12958.x [Google Scholar]
- Law B.S., Reinhold L., Pennay M. Geographic variation in the echolocation calls of Vespadelus spp. (Vespertilionidae) from New South Wales and Queensland, Australia. Acta Chiro. 2002;4:201–215. [Google Scholar]
- Lawlor T.E. The evolution of body size in mammals: evidence from insular populations in Mexico. Am. Nat. 1982;119:54–72. doi:10.1086/283890 [Google Scholar]
- Mayr E. Belknap Press; Cambridge, MA: 1963. Animal species and evolution. [Google Scholar]
- McNab B.K. On the ecological significance of Bergmann's rule. Ecology. 1971;52:845–854. doi:10.2307/1936032 [Google Scholar]
- Meiri S. Size evolution in island lizards. Glob. Ecol. Biogeogr. 2007;16:702–708. doi:10.1111/j.1466-8238.2007.00327.x [Google Scholar]
- Meiri S., Dayan T. On the validity of Bergmann's rule. J. Biogeogr. 2003;30:331–351. [Google Scholar]
- Meiri S., Dayan T., Simberloff D. Area, isolation, and size evolution in insular carnivores. Ecol. Lett. 2005a;8:1211–1217. doi: 10.1111/j.1461-0248.2005.00825.x. doi:10.1111/j.1461-0248.2005.00825.x [DOI] [PubMed] [Google Scholar]
- Meiri S., Dayan T., Simberloff D. Biogeographic patterns in the western Palearctic: the fasting-endurance hypothesis and the status of Murphy's rule. J. Biogeogr. 2005b;32:369–375. doi:10.1111/j.1365-2699.2005.01197.x [Google Scholar]
- Meiri S., Dayan T., Simberloff D. Variability and correlations in carnivore crania and dentition. Funct. Ecol. 2005c;19:337–343. doi:10.1111/j.1365-2435.2005.00964.x [Google Scholar]
- Meiri S., Dayan T., Simberloff D. Variability and sexual size dimorphism in carnivores: testing the niche variation hypothesis. Ecology. 2005d;86:1432–1440. doi:10.1890/04-1503 [Google Scholar]
- Meiri S., Simberloff D., Dayan T. Insular carnivore biogeography: island area and mammalian optimal body size. Am. Nat. 2005e;165:505–514. doi: 10.1086/428297. doi:10.1086/428297 [DOI] [PubMed] [Google Scholar]
- Meiri S., Dayan T., Simberloff D. The generality of the island rule reexamined. J. Biogeogr. 2006;33:1571–1577. doi:10.1111/j.1365-2699.2006.01523.x [Google Scholar]
- Meiri S., Yom-Tov Y., Geffen E. What determines conformity to Bergmann's rule? Glob. Ecol. Biogeogr. 2007;16:788–794. doi:10.1111/j.1466-8238.2007.00330.x [Google Scholar]
- Meiri S., Cooper N., Purvis A. The island rule: made to be broken? Proc. R. Soc. B. 2008;275:141–148. doi: 10.1098/rspb.2007.1056. doi:10.1098/rspb.2007.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton R.H. Body size and island Peromyscus: a pattern and a hypothesis. Evol. Theor. 1982;6:113–126. [Google Scholar]
- Millar J.S., Hickling G.J. Fasting endurance and the evolution of mammalian body size. Funct. Ecol. 1990;4:5–12. doi:10.2307/2389646 [Google Scholar]
- NIMA 1997 Vector Map Level 0 (VMAP0) 4th ed. Fairfax, VA: National Imagery and Mapping Agency (NIMA).
- Perez-Tris J., Carbonell R., Telleria J.L. Abundance distribution, morphological variation and juvenile condition of robins, Erithacus rubecula (L.), in their Mediterranean range boundary. J. Biogeogr. 2000;27:879–888. doi:10.1046/j.1365-2699.2000.00457.x [Google Scholar]
- Peters H.R. Cambridge University Press; New York, NY: 1983. The ecological implications of body size. [Google Scholar]
- Pitt J.A., Lariviere S., Messier F. Survival and body condition of raccoons at the edge of the range. J. Wildl. Manag. 2008;72:389–395. doi:10.2193/2005-761 [Google Scholar]
- Raia P., Meiri S. The island rule in large mammals: paleontology meets ecology. Evolution. 2006;60:1731–1742. doi:10.1554/05-664.1 [PubMed] [Google Scholar]
- Sagarin R.D., Gaines S.D. Geographical abundance distributions using 1-dimensional ranges to test biogeographic hypotheses. J. Biogeogr. 2002a;29:985–998. doi:10.1046/j.1365-2699.2002.00705.x [Google Scholar]
- Sagarin R.D., Gaines S.D. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecol. Lett. 2002b;5:137–147. doi:10.1046/j.1461-0248.2002.00297.x [Google Scholar]
- Sagarin R.D., Gaines S.D., Gaylord B. Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol. Evol. 2006;21:524–530. doi: 10.1016/j.tree.2006.06.008. doi:10.1016/j.tree.2006.06.008 [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; Cambridge, MA: 1984. Scaling. Why is animal size so important? [Google Scholar]
- Schwartz M.K., Mills L.S., Ortega Y., Ruggiero L.F., Allendorf F.W. Landscape location affects genetic variation of Canada lynx (Lynx canadensis) Mol. Ecol. 2003;12:1807–1816. doi: 10.1046/j.1365-294x.2003.01878.x. doi:10.1046/j.1365-294X.2003.01878.x [DOI] [PubMed] [Google Scholar]
- Sexton O.J., Andrews R.M., Bramble J.E. Size and growth rate characteristics of a peripheral population of Crotaphytus collaris (Sauria: Crotaphytidae) Copeia. 1992;1992:968–980. doi:10.2307/1446626 [Google Scholar]
- Simberloff D., Dayan T. The guild concept and the structure of ecological communities. Annu. Rev. Ecol. Syst. 1991;22:115–143. doi:10.1146/annurev.es.22.110191.000555 [Google Scholar]
- Stanley S.M. An explanation for Cope's rule. Evolution. 1973;27:1–26. doi: 10.1111/j.1558-5646.1973.tb05912.x. doi:10.2307/2407115 [DOI] [PubMed] [Google Scholar]
- Thurber J.M., Peterson R.O. Changes in body size associated with range expansion in the coyote (Canis latrans) J. Mammal. 1991;72:750–755. doi:10.2307/1381838 [Google Scholar]
- Wood S.N. Chapman and Hall/CRC Press; Boca Raton, FL: 2006. Generalized additive models: an introduction with R. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1—origin of carnivore range maps; appendix 2—sensitivity analysis with three distance categories; appendix 3—general additive model maps of the spatial components of size for all species; appendix 4—full result tables for all analyses; appendix 5—species level data