Abstract
Studies indicate that higher sun exposure, especially in the recent past, is associated with reduced risk of non-Hodgkin lymphoma (NHL). Ultraviolet radiation-derived vitamin D may be protective against lymphomagenesis. We examined the relationship between pre-diagnostic serum 25-hydroxyvitamin D (25(OH)D) and lymphoid cancer risk in a case-control study nested within the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study cohort (1985–2002) of 29,133 Finnish male smokers (ages 50 to 69). We identified 270 incident lymphoid cancer cases and matched them individually with 538 controls by birth-year and month of fasting blood draw at baseline. In conditional logistic regression models for 10nmol/L increments or tertile comparisons, serum 25(OH)D was not associated with the risk of overall lymphoid cancers, NHL (N = 208), or multiple myeloma (N = 41). Odds ratios (OR) for NHL for higher tertiles were 0.75 (95% confidence interval (CI), 0.50, 1.14) and 0.82 (95% CI, 0.53, 1.26). The 25(OH)D-NHL association, however, differed by follow-up duration at diagnosis. Cases diagnosed less than 7 years from the baseline showed an inverse association (OR for highest versus lowest tertile = 0.43; 95% CI: 0.23, 0.83; p for trend = 0.01), but not later diagnoses (OR = 1.52; 95% CI: 0.82, 2.80; p for trend = 0.17). The inverse association found for close exposure to diagnosis was not confounded by other risk factors for lymphoma or correlates of 25(OH)D. Although our findings suggest that circulating 25(OH)D is not likely associated with overall lymphoid cancer, they indicate a potentially protective effect on short-term risk of NHL.
Keywords: 25-hydroxyvitamin D, diet, lymphoid neoplasms, nested case-control studies, non-Hodgkin lymphoma, vitamin D
The relationship between sunlight exposure and risk of lymphoma has been controversial (1). The potentially lymphomagenic role of solar ultraviolet (UV) radiation was first suggested by the parallel rise in non-Hodgkin lymphoma (NHL) and cutaneous melanoma over the past several decades (2–5). Although this hypothesis was supported by some ecologic studies in Europe (6) and other regions (7;8), which showed a positive association between lymphoma and estimated UV-B or its surrogates, U.S. studies revealed an inverse relationship (9;10). In addition, several case-control studies, though not all (11;12), found evidence for a protective effect of sunlight, based on residential sun exposure (13), outdoor hours on working (13) or non-working days (14;15), sun-seeking behavior such as vacations in sunny climate or sunbathing (14–17), history of sunburn (16;18), and host susceptibility (18). Finally, a recent pooled analysis of 10 case-control studies in the International Lymphoma Epidemiology Consortium found that recreational sun exposure was inversely associated with NHL in a dose-response manner (19): the highest category was associated with about 25% lower risk, and the association was particularly strong for sun exposure in the decade before diagnosis.
A plausible mechanism proposed for the apparently protective effects of sunlight on lymphoma is UV-induced cutaneous synthesis of vitamin D, which has shown anti-proliferative and pro-differentiative properties in experiments involving various organs, including lymphoid tissues (20). Consistently, human lymphoma studies revealed associations with common genetic polymorphisms in the vitamin D receptor (21;22), implicating altered receptor expression or function due to these polymorphisms or other linked variants.
There has been no prospective investigation of lymphoma reported to date in relation to circulating 25-hydroxyvitamin D (25(OH)D), the best indicator of vitamin D status that incorporates both solar and dietary exposures (23;24). To better understand the role of vitamin D in the etiology of lymphoid malignancies, we present here the results of a nested case-control study of pre-diagnostic fasting serum 25(OH)D. The study was conducted in older men living in Finland who may be particularly susceptible to vitamin D insufficiency because reduced cutaneous synthesis has been observed in people of older age or with residence in northern latitudes (25).
Methods
Study population
We conducted a nested case-control study within the cohort of the Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC) Study (26), which was a double-blinded randomized trial originally designed to investigate whether two antioxidant supplements reduce the incidence of lung and other cancers in male smokers. Participants were recruited from the southwest of Finland among men who were 50–69 years of age and cancer-free and smoked five or more cigarettes per day at baseline in 1985–1988. A total of 29,133 men were assigned to receive randomly either an intervention (supplements of alpha-tocopherol, 50 mg/day; beta-carotene, 20 mg/day; or both) or placebo in a 2 × 2 factorial design until April 1993. Questionnaires and blood samples after an overnight fast were collected at baseline, and cancer cases during the trial and thereafter were obtained through the nation-wide cancer registry (26). All study participants provided written informed consent, and handling of human subjects and data was approved by the institutional review boards at the National Public Health Institute in Finland and the U.S. National Cancer Institute.
Data collection
Through baseline questionnaires, study participants provided information on general characteristics and medical, smoking and dietary history. Diet was assessed by a self-administered comprehensive dietary history questionnaire that was developed specifically for the ATBC Study and validated against food records in a pilot study of middle-aged Finnish men (27). Frequency and usual portion size of 276 food items consumed over the previous 12 months were measured with the aid of portion size picture guides: for example, fish intake was estimated from questions on commonly consumed fish, such as herring and trout, and on dishes containing fresh, frozen, or processed fish. Vitamin D fortification in Finnish food stock was minimal during the follow-up and until 2003 when milk and fermented milk products started to be fortified (28). Dietary vitamin D intake was assessed from food sources and supplements: less than 7% of the study participants were taking multivitamin supplements, the majority of which contained 12.5μg (or 500IU) vitamin D. Nutrient intake was estimated through linkage with the food composition database of the Finnish National Public Health Institute (26). Leisure-time activity was categorized as sedentary, moderate, or heavy according to the types of activities performed regularly during the year prior to completion of the baseline survey. On-the-job activity was graded in five categories of “not working”, “very light”, through “heavy activity”. Study staff measured blood pressure, weight, and height, from which body mass index (BMI; kg/m2) was calculated. Certain information related to sun exposure (“how many weeks during the past ten years have you been on trips to south, such as Spain and Greece, and stayed out in the sun at least an hour daily?”) and host susceptibility (color of eye and hair and tendency for sunburn) was available among a subgroup of all participants (79% of participants included for the main analysis).
Case ascertainment and control selection
Incident lymphoid malignancies were identified from the Finnish Cancer Registry, which provides close to 100 percent case ascertainment in Finland (29;30). The hospital records of the identified cases were reviewed by an experienced study oncologist for confirmation of the lymphoma diagnosis. There were 280 men with confirmed primary incident lymphoid malignancy during the 17.4 years of follow-up until December 31, 2002 (31). After excluding 10 cases without available baseline serum, our case-control study nested within the cohort included 270 lymphoid cancer cases.
Based on the histology information coded in the International Classification of Disease-Oncology second edition (ICD-O-2) and following the proposed adaptation (32) of the World Health Organization classification (33) for epidemiologic studies, we grouped all hematopoietic cancers of lymphoid origin into NHL (ICD-O-2: 9590–9595, 9670–9677, 9680–9688, 9690–9698, 9700–9717, 9760–9764, 9820–9828, 9940–9941; N = 208), multiple myeloma (ICD-O-2: 9731–9732; N = 41), and Hodgkin lymphoma (ICD-O-2: 9650–9667; N = 12). NHL was further classified into the five most common subtypes: diffuse large B-cell lymphoma (N = 41), follicular lymphoma (N = 23), chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL; N = 64), mantle cell lymphoma (N = 21), and T-cell lymphoma (N = 22).
Two controls (N = 540) for each case were randomly selected from the risk set of individuals who had matching birth-year (± 1 year) and month of baseline blood draw (± 30 days) and who had not developed lymphoid cancers by the time of case diagnosis. Two control subjects with extreme serum 25(OH)D values were excluded as described below.
Serum vitamin D assay
Serum samples were stored at −70°C until analyzed for 25(OH)D at Heartland Assay, Inc., Iowa, using radioimmunoassay (DiaSorin, Inc., Stillwater, MN). Assay reproducibility was monitored by inserting 6% (N = 52) blinded quality control samples from 3 individuals (2 from unselected ATBC subjects and one from a pooled sample of U.S. volunteers) randomly among study samples that were batched in matched case-control triplets. Percent coefficient of variation (CV), estimated by variance components analysis that assigned the total variance in serum 25(OH)D into within- and between-subject variability and measurement error, yielded 14% overall CV and 10% CV for the two ATBC quality control samples that had 25(OH)D values in the low range comparable to the study samples.
Statistical analysis
Characteristics of matched cases and controls were compared using the Wilcoxon nonparametric test for continuous variables and the chi-square test for categorical variables. Spearman rank correlation was performed among controls for the relationship between serum 25(OH)D and potential determinants. Serum 25(OH)D showed close to a normal distribution, and two controls with high outlier values, 179.3 and 158.5nmol/L each, were excluded from the analysis to further improve the normality and to generate more representative summary statistics of the study population. Findings were similar when we did not exclude the two outliers. Predicted levels of serum 25(OH)D across weeks of blood draw at baseline were estimated non-parametrically from controls using generalized additive modeling (GAM) and the option of locally weighted scatterplot smoothing. Food items and nutrients, except alcohol, were adjusted for energy intake by the nutrient density method (34).
The association between serum 25(OH)D and lymphoid cancers was estimated by odds ratios (OR) and 95% confidence intervals (CI) using conditional logistic regression that accounted for the matched status. Serum 25(OH)D was modeled as both a continuous and categorical variable. Continuous analysis is presented for 10nmol/L increments. We also considered models that used log-transformed or the square term of 25(OH)D, but these models did not indicate strong non-linear associations. In categorical analysis, tertiles of serum 25(OH)D were defined in three different ways to examine any residual confounding by seasonal variation at baseline blood draw. Cutoff points of tertiles were determined either (1) from serum 25(OH)D values among all controls regardless of season at baseline; (2) from serum 25(OH)D values among controls that were stratified into two season-specific groups at baseline (blood draw in winter/spring months with median 25(OH)D for each month < 50nmol/L or in summer/fall months with monthly median ≥50nmol/L); or (3) from season-adjusted residuals obtained in the GAM analysis as described above. Linear trends were assessed using the score variable that contained median values of tertile categories.
Because the pooled analysis of NHL case-control studies detected a somewhat stronger association for sun exposure close in time to diagnosis (19), we examined the 25(OH)D-NHL association by time to diagnosis. Analyzing the data at 2-year intervals or in regression models that included an interaction term between 25(OH)D and time to diagnosis, we found differential associations for diagnoses with shorter versus longer follow-up from the baseline. We present the stratified analysis findings using a median cutoff point of 7 years.
Distribution of case subtypes and potential confounding factors were examined by time to diagnosis to assess whether their differential distribution explained the disparate associations. These factors included residential latitude, smoking, BMI, physical activity, sun exposure or related host susceptibility, consumption of alcohol, multivitamin supplements, vitamin D, and fish, and baseline serum levels of retinol and total and high-density lipoprotein cholesterol (HDL-C). We also examined the study intervention of antioxidants, education, rural/urban residence, diastolic and systolic blood pressure, and intake of fat, fruits, vegetables, whole grain, folate, and calcium (35). None of these factors, when individually controlled for in the regression model, changed the association between serum 25(OH)D and NHL by 10% or more, and thus, the final model adjusted for matching factors only. We also assessed the significance (p interaction < 0.10) of the product term between serum 25(OH)D and aforementioned factors to detect any effect modification.
Results
Compared to controls, lymphoid cancer cases had lower serum HDL-C (Table 1). Cases and controls were otherwise similar at baseline with regard to the matching factors of age and season of blood draw and other characteristics of residential latitude, smoking history, BMI, physical activity, sun exposure, host susceptibility, consumption of alcohol, multivitamin use, total vitamin D or fish intake, and serum retinol and total cholesterol. The study intervention agents, alpha-tocopherol and beta-carotene, were also evenly distributed by case status (data not shown). Controls showed a wide but low range of serum 25(OH)D values, between 6.3 and 124.8nmol/L (or 2.5–49.9ng/ml), and a low median (48.8nmol/L), indicating limited levels of sun exposure and vitamin D intake.
Table 1.
Descriptive baseline characteristics (median or proportion) among lymphoid cancer cases and controls: the ATBC Study (1985–2002).
| Cases, N = 270 | Controls, N = 538 | |
|---|---|---|
| Serum 25-hydroxyvitamin D, nmol/L (ng/mL) | 47.9 (19.2) | 48.8 (19.5) |
| Age | 58 | 58 |
| Season of blood draw†, % | ||
| Winter/Spring/Fall/Summer | 33/37/24/6 | 33/37/24/6 |
| Latitude (60.1–62.5°) | 60.5 | 60.5 |
| Smoking | ||
| Average cigarettes/day | 20 | 20 |
| Years of smoking | 39 | 39 |
| Pack-years | 37 | 36 |
| Body mass index, kg/m2 | 26.1 | 26.0 |
| < 25, % | 34 | 38 |
| 25 29.9 | 47 | 48 |
| ≥ 30 | 18 | 13 |
| Leisure-time physical activity, % | ||
| Intensity | ||
| Low | 42 | 39 |
| Moderate | 53 | 53 |
| Heavy | 5 | 8 |
| Frequency | ||
| <1/week | 51 | 49 |
| 1–2/week | 26 | 30 |
| 3+/week | 23 | 21 |
| On-job physical activity, % | ||
| Not working | 45 | 47 |
| Very light | 12 | 11 |
| Light | 19 | 20 |
| Moderate | 16 | 15 |
| Heavy | 8 | 6 |
| Weeks of staying out in sun for ≥ 1hour/day on trips to south in past 10 years | 5 | 6 |
| Eye color, % | ||
| Blue | 64 | 65 |
| Green | 6 | 6 |
| Gray | 18 | 19 |
| Brown | 12 | 11 |
| Hair color, % | ||
| Fair | 13 | 11 |
| Red | 2 | 3 |
| Light brown | 39 | 42 |
| Dark brown | 34 | 34 |
| Black | 11 | 9 |
| Skin tendency to burn, % | ||
| It burns easily and does not get tanned. | 5 | 11 |
| It burns easily but gets tanned. | 27 | 28 |
| It burns slightly and gets tanned slowly and evenly. | 27 | 25 |
| It does not burn and gets tanned easily. | 6 | 6 |
| It does not burn easily but gets tanned. | 35 | 30 |
| Dietary intake | ||
| Alcohol, g/day | 9.4 | 11.2 |
| Multivitamin supplement use, % | 6.7 | 7.1 |
| Calories, kcal/day | 2759 | 2675 |
| Total vitamin D, μg/day (μg/1000kcal/day) | 4.6 (1.7) | 4.8 (1.8) |
| Fish‡, g/day (g/1000kcal/day) | 28.8 (11.1) | 28.6 (10.7) |
| Serum metabolites | ||
| Retinol, ug/L | 568 | 568 |
| Total cholesterol, mg/dL | 237 | 239 |
| High-density lipoprotein cholesterol, mg/dL | 42* | 44 |
p < 0.05 for contrast between cases and controls by Wilcoxon nonparametric test
Season was coded as 1 for winter months (December, January, February), 2 for spring (March, April, May), 3 for fall (September, October, November), and 4 for summer (June, July, August).
Dietary intake estimated from all queried fish items was combined: fresh (e.g., rainbow trout, Baltic herring, other types), frozen (e.g., cod or sea perch), processed (e.g., salted/spiced, canned), or mixed.
Fasting serum 25(OH)D levels varied across the seasons at blood collection (Figure 1), with mean values among controls at 47nmol/L in winter and spring and 63nmol/L in summer and fall. Serum 25(OH)D was also correlated positively with leisure-time physical activity, frequent vacation in the sunny south, multivitamin use, intake of total vitamin D and fish, and serum retinol and total cholesterol and negatively with cigarette smoking (Table 2).
Figure 1.
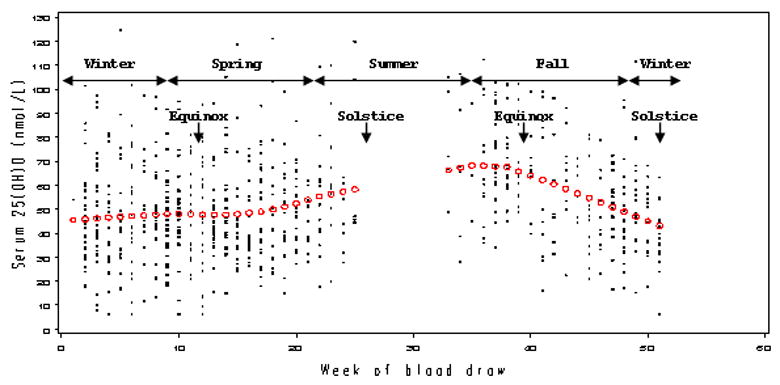
Serum 25-hydroxyvitamin D (25(OH)D) distribution across the week at baseline blood draw among controls in the ATBC Study (1985–2002). Predicted or “season-adjusted” levels are depicted in open circles, using locally weighted scatterplot smoothing in a generalized additive model. Note that no recruitments were made during the month of July.
Table 2.
Correlations between baseline characteristics and serum 25(OH)D among controls: the ATBC Study (1985–2002).
| N* | r | P | |
|---|---|---|---|
| Age at blood draw | 538 | 0.04 | NS |
| Season of blood draw† | 538 | 0.24 | <0.0001 |
| Latitude | 538 | −0.07 | NS |
| Cigarette smoking | |||
| Average cigarettes/day | 538 | −0.13 | 0.002 |
| Pack-years | 536 | −0.16 | 0.0003 |
| Body mass index, kg/m2 | 538 | 0.01 | NS |
| Leisure-time activity intensity | 538 | 0.17 | 0.0001 |
| Leisure-time activity frequency | 536 | 0.09 | 0.03 |
| Physical activity on the job | 538 | −0.05 | NS |
| Sunny vacation, weeks† | 426 | 0.25 | <0.0001 |
| Eye color† | 427 | 0.03 | NS |
| Hair color† | 428 | −0.06 | NS |
| Sunburn tendency† | 427 | 0.09 | NS |
| Dietary intake | |||
| Alcohol consumption, g/day | 499 | −0.04 | NS |
| Multivitamin supplement use | 499 | 0.20 | <0.0001 |
| Total vitamin D (foods, supplements), mg/1000kcal/day | 499 | 0.32 | <0.0001 |
| Fish, g/1000kcal/day | 499 | 0.26 | <0.0001 |
| Serum metabolites | |||
| Retinol, μg/L | 538 | 0.09 | 0.03 |
| Total cholesterol, mg/dl | 538 | 0.13 | 0.003 |
| High-density lipoprotein cholesterol, mg/dl | 538 | 0.03 | NS |
NS: not significant (p ≥ 0.05)
Spearman correlation coefficients were obtained from varying numbers of controls based on the availability of each baseline information.
Season was coded as 1 for winter months (December, January, February), 2 for spring (March, April, May), 3 for fall (September, October, November), and 4 for summer (June, July, August). Weeks on sunny vacations were assessed by “how many weeks during the past ten years have you been on trips to south, such as Spain and Greece, and stayed out in the sun at least an hour daily?” Eye color was coded in an ascending order for blue, green, gray, and brown; hair color for fair, red, light brown, dark brown, and black; and sunburn tendency in 5 categories ranging from “skin burns easily without getting tanned” to “skin will not burn but gets tanned” to prolonged direct sunlight.
In continuous and categorical analyses, the association of serum 25(OH)D with overall lymphoid cancers was null as were the associations with individual types: NHL, multiple myeloma, or Hodgkin lymphoma (Table 3). Common subtypes of NHL showed either reduced (diffuse, CLL/SLL, and T-cell lymphoma) or increased risk (follicular, mantle cell lymphoma) with increasing levels of 25(OH)D, but none reached statistical significance. Also, similarly null associations were observed using tertiles that were determined either from controls stratified into season-specific groups (winter/spring versus summer/fall months) or from controls’ distribution of season-adjusted residuals (Figure 1) based on serum 25(OH)D across weeks of baseline blood draw (data not shown). ORs for NHL comparing the highest to the lowest category were 0.83 (95% CI: 0.55, 1.27; p trend = 0.35) for the season-specific tertiles and 0.80 (95% CI: 0.53, 1.20; p trend = 0.35) for the tertiles of season-adjusted residuals.
Table 3.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between serum 25-hydroxyvitamin D and incident lymphoid cancers, overall and subtypes: the ATBC Study (1985–2002).
| Serum 25-hydroxy vitamin D |
||||
|---|---|---|---|---|
| Continuous (per 10nmol/L increase) | Tertile 1 (6.3 40nmol/L) | Tertile 2 (40.1 59.4nmol/L) | Tertile 3 (59.5 124.8nmol/L) | |
| All lymphoid cancers | ||||
| N, case/control | 270/538 | 95/179 | 84/179 | 91/180 |
| OR (95% CI) | 0.99 (0.92, 1.06) | 1.0 | 0.89 (0.62, 1.27) | 0.95 (0.65, 1.40) |
| P trend | 0.70 | 0.82 | ||
| Non-Hodgkin lymphoma (NHL) | ||||
| N, case/control | 208/424 | 75/132 | 63/146 | 70/146 |
| OR (95% CI) | 0.96 (0.88, 1.04) | 1.0 | 0.75 (0.50, 1.14) | 0.82 (0.53, 1.26) |
| P trend | 0.28 | 0.41 | ||
| Diffuse NHL | ||||
| N, case/control | 41/83 | 14/27 | 14/26 | 13/30 |
| OR (95% CI) | 0.97 (0.81, 1.16) | 1.0 | 1.08 (0.43, 2.72) | 0.85 (0.33, 2.14) |
| P trend | 0.74 | 0.71 | ||
| Follicular NHL | ||||
| N, case/control | 23/50 | 7/19 | 10/19 | 6/12 |
| OR (95% CI) | 1.08 (0.81, 1.46) | 1.0 | 1.32 (0.42, 4.21) | 1.21 (0.31, 4.72) |
| P trend | 0.60 | 0.76 | ||
| Chronic lymphocytic leukemia/Small lymphocytic lymphoma | ||||
| N, case/control | 64/136 | 28/39 | 13/41 | 23/56 |
| OR (95% CI) | 0.89 (0.77, 1.03) | 1.0 | 0.42 (0.18, 0.97) | 0.49 (0.22, 1.08) |
| P trend | 0.12 | 0.10 | ||
| Mantle Cell NHL | ||||
| N, case/control | 21/45 | 5/15 | 9/19 | 7/11 |
| OR (95% CI) | 1.06 (0.78, 1.43) | 1.0 | 1.24 (0.33, 4.62) | 1.67 (0.39, 7.20) |
| P trend | 0.70 | 0.49 | ||
| T-cell NHL | ||||
| N, case/control | 22/46 | 7/11 | 6/17 | 9/18 |
| OR (95% CI) | 0.93 (0.72, 1.21) | 1.0 | 0.58 (0.15, 2.21) | 0.73 (0.16, 3.33) |
| P trend | 0.60 | 0.80 | ||
| Multiple myeloma | ||||
| N, case/control | 41/88 | 12/39 | 15/20 | 14/29 |
| OR (95% CI) | 1.11 (0.94, 1.32) | 1.0 | 2.38 (0.94, 6.01) | 1.55 (0.58, 4.17) |
| P trend | 0.22 | 0.37 | ||
| Hodgkin lymphoma | ||||
| N, case/control | 12/28 | 6/16 | 6/12 | – |
| OR (95% CI) | 1.26 (0.83, 1.92) | 1.0 | 1.37 (0.29, 6.62) | – |
| P trend | 0.28 | 0.67 | ||
Conditional logistic regression model adjusted for the matching factors (age, month of blood collection) only and compared above versus below median for Hodgkin lymphoma due to small numbers.
Because lower risk of NHL has been associated with sun exposure close in time to diagnosis (19), we examined the 25(OH)D-NHL association by the follow-up duration at diagnosis and found differential associations. A significant inverse association was detected between 25(OH)D and NHL for cases diagnosed before the median point of 7 years from baseline, but not the cases diagnosed later in the follow-up (Table 4; p for interaction = 0.007). The inverse association was stronger for blood collection during winter/spring months than for collection in summer/fall months. Findings based on season-specific and season-adjusted tertiles showed a similarly disparate pattern of associations for early versus late diagnoses (Online Supplements 1 & 2). When diagnoses in the first 2 years (N = 23 cases) were excluded, the early inverse association was only slightly attenuated and no longer significant: OR for higher tertiles were 0.67 (95% CI: 0.34, 1.33) and 0.52 (95% CI: 0.25, 1.10; p trend = 0.10).
Table 4.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between serum 25-hydroxyvitamin D (25(OH)D) and incident non-Hodgkin lymphoma, stratified by time to diagnosis: the ATBC Study (1985–2002).
| Diagnosis at <7 years after baseline (101 cases, 205 controls) | Diagnosis at ≥7 years after baseline (107 cases, 219 controls) | |||||||
|---|---|---|---|---|---|---|---|---|
| Serum 25(OH)D tertiles based on overall cutoff points, range in nmol/L | Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | ||
| 6.3–40.0 | 40.1–59.4 | 59.5–124.8 | 6.3–40.0 | 40.1–59.4 | 59.5–124.8 | |||
| Combined seasons | ||||||||
| Median among controls (nmol/L) | 34.1 | 47.5 | 72.0 | 29.4 | 49.0 | 73.3 | ||
| N, case/control | 41/56 | 33/71 | 27/78 | 34/76 | 30/75 | 43/68 | ||
| OR | 1.0 | 0.60 | 0.43 | 1.0 | 0.89 | 1.52 | ||
| 95% CI | — | 0.33, 1.10 | 0.23, 0.83 | P trend = 0.01 | — | 0.49, 1.61 | 0.82, 2.80 | P trend = 0.17 |
| Winter & spring | ||||||||
| N, case/control | 35/46 | 25/51 | 14/55 | 31/65 | 17/57 | 24/27 | ||
| OR | 1.0 | 0.61 | 0.32 | 1.0 | 0.57 | 1.92 | ||
| 95% CI | — | 0.30, 1.22 | 0.15, 0.70 | P trend = 0.004 | — | 0.28, 1.19 | 0.95, 3.89 | P trend = 0.13 |
| Summer & fall | ||||||||
| N, case/control | 6/10 | 8/20 | 13/23 | 3/11 | 13/18 | 19/41 | ||
| OR | 1.0 | 0.66 | 0.93 | 1.0 | 2.70 | 1.67 | ||
| 95% CI | — | 0.18, 2.48 | 0.25, 3.44 | P trend = 0.90 | — | 0.62, 11.88 | 0.39, 7.19 | P trend = 0.93 |
Overall serum 25(OH)D tertiles were determined based on values among all controls regardless of season at blood draw. Conditional logistic regression model adjusted for the matching factors (age, month of blood collection) only.
The inverse association in early but not late diagnoses of NHL appeared to be stronger for CLL/SLL (OR for above versus below median 25(OH)D = 0.41; 95% CI: 0.15, 1.09 for <7 years; 1.15; 95% CI: 0.44, 3.01 for ≥7 years) than other common subtypes examined. Risk estimates for NHL excluding CLL/SLL were 0.67 (95% CI: 0.37, 1.23) and 1.34 (95% CI: 0.74, 2.42). Based on polytomous regression analysis, the difference between the ORs for CLL/SLL and all other NHL was significant for earlier diagnoses (p for Wald chi-square test = 0.03) but not for later diagnoses (p = 0.44). Multiple myeloma risk associated with high versus low serum 25(OH)D was non-significantly elevated for both early (N = 21; OR = 2.10; 95% CI: 0.68, 6.49) and later diagnoses (N = 20; OR =1.49; 95% CI: 0.51, 4.34). T-cell NHL and Hodgkin lymphoma cases were too few to examine by time to diagnosis.
Case histology and most baseline characteristics did not differ comparing cases, and their matched controls, by time to diagnosis (Online Supplement 3). The only significant difference was that the NHL cases with a shorter follow-up had lower serum HDL-C and that serum HDL-C was lower for cases compared to controls only during the early follow-up, latter of which was reported previously (31). However, adjusting for HDL-C did not explain the differential 25(OH)D-NHL associations across follow-up: HDL-adjusted ORs for combined seasons comparing the highest versus lowest tertile was 0.46 (95% CI: 0.24, 0.88) and 1.53 (95% CI: 0.83, 2.84) for NHL cases with short and long time to diagnosis from baseline, respectively. The 25(OH)D-NHL association was minimally affected by stratifying by the latitude, smoking history, obesity, physical activity, serum retinol, or the study treatments of alpha-tocopherol and beta-carotene (p interaction > 0.10 for all).
Discussion
We found no overall association between serum 25(OH)D and lymphoid cancers or their components of NHL, multiple myeloma, and Hodgkin lymphoma. Higher serum 25(OH)D levels were, however, associated with lower incidence of NHL cases with shorter time to diagnosis, especially among participants whose baseline blood was drawn in less sunny seasons.
It is biologically plausible that higher levels of serum 25(OH)D may reduce the risk of lymphomagenesis. Activated lymphocytes express 1α-hydroxylase, which converts the circulating inert form, 25-hydroxyvitamin D (25(OH)D), to the functional hormone, 1,25-dihydroxyvitamin D (1,25(OH)2D) (36). Lymphocytes contain nuclear receptors for the hormone as well, which suggests their autocrine/paracrine capacity to respond to the hormone’s anti-neoplastic effects (37;38). Vitamin D receptor knockout mice have shown an accelerated rate of mitogen-induced malignant transformations in the thymus gland and lymph nodes (39). In humans, a small clinical trial of 1,25(OH)2D analog showed ~25% remission of low-grade NHL (40). In addition, the active vitamin D may be protective through its immunity-modulating effects (41;42), such as selectively down-regulating helper T1 cells (Th1) and suppressing their systemic release of pro-inflammatory cytokines (20;43), and thereby, reducing the autoimmunity-induced risk of lymphoma (44). Alternatively, UV-B exposure, which is the principal source of vitamin D levels, may exert similar immunosuppression independently of vitamin D, by, for example, lowering the secretion of melatonin and elevating melanocyte stimulating hormone, which in turn attenuates Th1-mediated immunity (45–47).
A possible explanation for a protective association between serum 25(OH)D and NHL with shorter, but not longer, time to diagnosis from baseline is that exposure to vitamin D close to diagnosis has a stronger protective effect than earlier exposures. Our finding is consistent with the pooled analysis in the InterLymph Consortium, where sunlight exposure in the last decade prior to NHL diagnosis had a stronger inverse association with NHL than sun exposure in teen years or early to mid-adulthood (19). Similarly, studies of other cancers have reported stronger protective associations for vitamin D indicators in relation to cancer diagnoses that are close temporally: e.g., baseline dietary vitamin D and breast cancer in the first 5 years than later years of a follow-up (48) and serum 25(OH)D and colon cancer in the first 10 years compared with later follow-up (49;50). If a protective effect was limited to exposures close in time to the diagnosis, we would not expect to see a uniform relationship over time unless 25(OH)D levels in individuals were fairly constant over time. However, intra-individual variability of serum 25(OH)D over time was evidenced in a study of adult women who were followed up for repeat measures at 1, 2 and 5 years from the baseline (51). The 19% variation detected in the study was not entirely accounted for by the measurement error in the enzyme immunoassay (10% CV), supporting the notion that a one-time measure of serum 25(OH)D may poorly predict vitamin D status many years after blood collection.
Alternatively, a covariate that changes over the several years prior to diagnosis may account for the disparate results. Because abnormal lipid metabolism is common in latent lymphoma (52), and because we previously observed an inverse association between serum HDL cholesterol and NHL in diminishing magnitude with follow-up time (31), we considered HDL as such a covariate. However, the 25(OH)D-NHL association was confounded by neither HDL nor total serum cholesterol, and the strength of the association remained consistent for the early follow-up. Likewise, adjusting for other risk factors for NHL or correlates of serum 25(OH)D did not alter the association. We, however, cannot rule out possible vitamin D-lowering effects of latent lymphoma, although normal levels of 25(OH)D with high 1,25(OH)2D in some patients have been clinically observed (53). We also cannot disregard the possibility of residual confounding by known or unknown risk factors or, in this observational study, that the disparate pattern of associations might have been due to chance.
The 25(OH)D-NHL association was more inverse for CLL/SLL than other common subtypes of NHL among cases with shorter time to diagnosis from baseline. Whether this is an indication of true heterogeneity, i.e., a stronger involvement of vitamin D status in CLL/SLL etiology over other subtypes’ (54), or due to chance requires further research. The literature is inconsistent on the effects of sun exposure or vitamin D on multiple myeloma (15;55), and the non-significant positive association we observed regardless of time to diagnosis remains inconclusive due to small numbers.
Our findings were based on a population with lower serum 25(OH)D levels compared to the US and other populations (56;57), likely due to the high latitude, older age, and smoking status. For example, levels below 37.5nmol/L were found in 27% of this versus <10% of the U.S. population among white men of 60–79 years (56). Similarly, levels above 62.5nmol/L were found in 28% versus ~65% of the respective populations. This may or may not have contributed to a stronger inverse association in our data if serum 25(OH)D exhibits a threshold for protective effects. In addition, our observation of a stronger inverse association for 25(OH)D measured in winter/spring compared with the summer/fall months implicates the importance of considering year-round levels, and not simply high values limited to a part of the year.
Some of the suggested confounding or modifying associations (25) were not observed in our study population. For example, the range of the latitude from mostly Southwestern counties of Finland was narrow (60.1–62.5°) and was not correlated with serum 25(OH)D. Physical activity, especially intensity of leisure-time activity, was positively correlated with serum 25(OH)D, but was not associated with NHL. The proposed antagonistic or modifying effects of obesity and high levels of serum retinol on vitamin D (25) were not evident in this study. This may be because the heavy smokers’ BMI was likely distorted as an indicator of positive energy balance or obesity-related etiology and their baseline serum retinol levels were low to exhibit such modifying effects (58). Also, we did not detect any influence of beta-carotene or alpha-tocopherol intervention treatments, which were randomized and evenly distributed across baseline serum 25(OH)D levels. Although substantially lower serum 25(OH)D levels have been reported among smokers (59), smoking has not shown to modify the 25(OH)D association with NHL or any cancers, to the best of our knowledge, and thus, we do not have a reason to question the generalizability of our findings.
As the first investigation of serum 25(OH)D and lymphoma risk, this study was conducted within a prospective cohort with excellent case ascertainment and reliable histology information, which minimized selection bias and allowed subtype analyses. We applied different analytic approaches to better adjust for seasonal variation in serum 25(OH)D at blood draw and found confirmatory estimates for those obtained from using overall tertile cutpoints on all raw measurement values. Nevertheless, this study had some limitations. Serum 25(OH)D derives from solar and dietary exposures in recent weeks (24), and thus is not necessarily correlated with historic 25(OH)D levels. We could not determine how well the baseline serum 25(OH)D was correlated with individuals’ typical or cumulative sun exposure or sun-seeking behavior in the past, with only limited information available in a subgroup of all participants. For example, the number of vacation days in the sunny south, but not host susceptibility, was correlated with serum 25(OH)D, and adjustment of this antecedent factor did not change the 25(OH)D-NHL association.
These results warrant further evaluation in other prospective data, ideally with a large number of lymphoid neoplasm cases to examine histological and molecular subtypes, lifetime history of sunlight exposure, and DNA to enable assessment of genes involved in the metabolism and functional pathways of vitamin D. In addition, the differences in our findings by proximity to blood collection calls for further investigations of repeat measures of 25(OH)D to determine the intra-individual variability over time and to clarify the potential influence of disease progression on the marker.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health at the National Cancer Institute and additionally by U.S. Public Health Service contracts N01-CN-45165, N01-RC-45035, and N01-RC-37004 from the National Cancer Institute, Department of Health and Human Services.
We gratefully acknowledge the contributions of Drs. Tom Fears, Sholom Wacholder, and Mitchell Gail for statistical consulting, Jackie King at BioReliance and Karon Drew at Fisher Bioservices for biospecimen handling, Susan Dorozinsky at the Information Management Services, Inc. for computing assistance, and Tawanda Roy and Cherise Banks in the Division of Cancer Epidemiology and Genetics for research assistance.
Abbreviations
- ATBC Study
Alpha-Tocopherol Beta-Carotene Cancer Prevention Study
- CI
confidence interval
- ICD-O
International Classification of Disease-Oncology
- OR
odds ratio
Footnotes
Conflict of Interest
RLH is president and chief executive officer of Heartland Assays, Inc., and BWH is a consultant to DiaSorin Corp, which conducted the assays for this analysis.
Reference List
- 1.Armstrong BK, Kricker A. Sun exposure and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):396–400. doi: 10.1158/1055-9965.EPI-06-1068. [DOI] [PubMed] [Google Scholar]
- 2.Zheng T, Mayne ST, Boyle P, Holford TR, Liu WL, Flannery J. Epidemiology of non-Hodgkin lymphoma in Connecticut. 1935–1988. Cancer. 1992;15(70(4)):840–9. doi: 10.1002/1097-0142(19920815)70:4<840::aid-cncr2820700420>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright R, McNally R, Staines A. The increasing incidence of non-Hodgkin’s lymphoma (NHL): the possible role of sunlight. Leuk Lymphoma. 1994;14(5–6):387–94. doi: 10.3109/10428199409049694. [DOI] [PubMed] [Google Scholar]
- 4.Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin’s lymphoma and skin cancer. BMJ. 1995;10(310(6993)):1491–5. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lens MB, Newton-Bishop JA. An association between cutaneous melanoma and non-Hodgkin’s lymphoma: pooled analysis of published data with a review. Ann Oncol. 2005;16(3):460–5. doi: 10.1093/annonc/mdi080. [DOI] [PubMed] [Google Scholar]
- 6.Bentham G. Association between incidence of non-Hodgkin’s lymphoma and solar ultraviolet radiation in England and Wales. BMJ. 1996;4(312(7039)):1128–31. doi: 10.1136/bmj.312.7039.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMichael AJ, Giles GG. Have increases in solar ultraviolet exposure contributed to the rise in incidence of non-Hodgkin’s lymphoma? Br J Cancer. 1996;73(7):945–50. doi: 10.1038/bjc.1996.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uehara M, Takahashi K, Hoshuyama T, Pan G, Feng Y. Geographical correlation between ambient UVB level and mortality risk of leukemia in Japan. Environ Res. 2003;92(2):78–84. doi: 10.1016/s0013-9351(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 9.Hartge P, Devesa SS, Grauman D, Fears TR, Fraumeni JF., Jr Non-Hodgkin’s lymphoma and sunlight. J Natl Cancer Inst. 1996;6(88(5)):298–300. doi: 10.1093/jnci/88.5.298. [DOI] [PubMed] [Google Scholar]
- 10.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;15(94(6)):1867–75. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 11.Adami J, Gridley G, Nyren O, Dosemeci M, Linet M, Glimelius B, Ekbom A, Zahm SH. Sunlight and non-Hodgkin’s lymphoma: a population-based cohort study in Sweden. Int J Cancer. 1999;1(80(5)):641–5. doi: 10.1002/(sici)1097-0215(19990301)80:5<641::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Holford TR, Leaderer B, Boyle P, Zhu Y, Wang R, Zou K, Zhang B, Wise JP, Sr, Qin Q, Kilfoy B, Han J, et al. Ultraviolet radiation exposure and risk of non-Hodgkin’s lymphoma. Am J Epidemiol. 2007;1(165(11)):1255–64. doi: 10.1093/aje/kwm020. [DOI] [PubMed] [Google Scholar]
- 13.Freedman DM, Zahm SH, Dosemeci M. Residential and occupational exposure to sunlight and mortality from non-Hodgkin’s lymphoma: composite (threefold) case-control study. BMJ. 1997;17(314(7092)):1451–5. doi: 10.1136/bmj.314.7092.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes AM, Armstrong BK, Vajdic CM, Turner J, Grulich AE, Fritschi L, Milliken S, Kaldor J, Benke G, Kricker A. Sun exposure may protect against non-Hodgkin lymphoma: a case-control study. Int J Cancer. 2004;10(112(5)):865–71. doi: 10.1002/ijc.20470. [DOI] [PubMed] [Google Scholar]
- 15.Boffetta P, Hel OV, Kricker A, Nieters A, Sanjose SD, Maynadie M, Cocco PL, Staines A, Becker N, Font R, Mannetje AT, Goumas C, et al. Exposure to ultraviolet radiation and risk of malignant lymphoma and multiple myeloma--a multicentre European case-control study. Int J Epidemiol. 2008:29. doi: 10.1093/ije/dyn092. [DOI] [PubMed] [Google Scholar]
- 16.Smedby KE, Hjalgrim H, Melbye M, Torrang A, Rostgaard K, Munksgaard L, Adami J, Hansen M, Porwit-Macdonald A, Jensen BA, Roos G, Pedersen BB, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;2(97(3)):199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 17.Weihkopf T, Becker N, Nieters A, Mester B, Deeg E, Elsner G, Blettner M, Seidler A. Sun exposure and malignant lymphoma: a population-based case-control study in Germany. Int J Cancer. 2007;1(120(11)):2445–51. doi: 10.1002/ijc.22492. [DOI] [PubMed] [Google Scholar]
- 18.Hartge P, Lim U, Freedman DM, Colt JS, Cerhan JR, Cozen W, Severson RK, Davis S. Ultraviolet radiation, dietary vitamin D, and risk of non-Hodgkin lymphoma (United States) Cancer Causes Control. 2006;17(8):1045–52. doi: 10.1007/s10552-006-0040-8. [DOI] [PubMed] [Google Scholar]
- 19.Kricker A, Armstrong BK, Hughes AM, Goumas C, Smedby KE, Zheng T, Spinelli JJ, De SS, Hartge P, Melbye M, Willett EV, Becker N, et al. Personal sun exposure and risk of non Hodgkin lymphoma: A pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;1(122(1)):144–54. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 20.Hewison M, Gacad MA, Lemire J, Adams JS. Vitamin D as a cytokine and hematopoetic factor. Rev Endocr Metab Disord. 2001;2(2):217–27. doi: 10.1023/a:1010015013211. [DOI] [PubMed] [Google Scholar]
- 21.Purdue MP, Lan Q, Kricker A, Vajdic CM, Rothman N, Armstrong BK. Vitamin D receptor gene polymorphisms and risk of non-Hodgkin’s lymphoma. Haematologica. 2007;92(8):1145–6. doi: 10.3324/haematol.11053. [DOI] [PubMed] [Google Scholar]
- 22.Purdue MP, Hartge P, Davis S, Cerhan JR, Colt JS, Cozen W, Severson RK, Li Y, Chanock SJ, Rothman N, Wang SS. Sun exposure, vitamin D receptor gene polymorphisms and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2007;18(9):989–99. doi: 10.1007/s10552-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 23.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135(2):317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. The use and interpretation of assays for vitamin D and its metabolites. J Nutr. 1990;120(Suppl 11):1464–9. doi: 10.1093/jn/120.suppl_11.1464. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16(2):83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 26.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 27.Pietinen P, Hartman AM, Haapa E, Rasanen L, Haapakoski J, Palmgren J, Albanes D, Virtamo J, Huttunen JK. Reproducibility and validity of dietary assessment instruments. I A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655–66. doi: 10.1093/oxfordjournals.aje.a115013. [DOI] [PubMed] [Google Scholar]
- 28.Valimaki VV, Loyttyniemi E, Valimaki MJ. Vitamin D fortification of milk products does not resolve hypovitaminosis D in young Finnish men. Eur J Clin Nutr. 2007;61(4):493–7. doi: 10.1038/sj.ejcn.1602550. [DOI] [PubMed] [Google Scholar]
- 29.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994;33(4):365–9. doi: 10.3109/02841869409098430. [DOI] [PubMed] [Google Scholar]
- 30.Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, Virtamo J. The Finnish Cancer Registry as follow-up source of a large trial cohort--accuracy and delay. Acta Oncol. 2002;41(4):381–8. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- 31.Lim U, Gayles T, Katki HA, Stolzenberg-Solomon R, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Serum high-density lipoprotein cholesterol and risk of non-hodgkin lymphoma. Cancer Res. 2007;1(67(11)):5569–74. doi: 10.1158/0008-5472.CAN-07-0212. [DOI] [PubMed] [Google Scholar]
- 32.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, Jack A, Cozen W, Maynadie M, Spinelli JJ, Costantini AS, Rudiger T, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;15(110(2)):695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 34.Willett WC. Nutritional epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 35.Cross AJ, Lim U. The role of dietary factors in the epidemiology of non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006;47(12):2477–87. doi: 10.1080/10428190600932927. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;1(179(3)):1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 37.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 38.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;16(221(4616)):1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 39.Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97(1–2):153–64. doi: 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Raina V, Cunningham D, Gilchrist N, Soukop M. Alfacalcidol is a nontoxic, effective treatment of follicular small-cleaved cell lymphoma. Br J Cancer. 1991;63(3):463–5. doi: 10.1038/bjc.1991.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 42.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;19(98(13)):7498–503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–45. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 44.Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, Sundstrom C, Akerman M, Melbye M, Glimelius B, Adami HO. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;4(98(1)):51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 45.Ponsonby AL, McMichael A, van dM I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002;27(181–182):71–8. doi: 10.1016/s0300-483x(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 46.Schade N, Esser C, Krutmann J. Ultraviolet B radiation-induced immunosuppression: molecular mechanisms and cellular alterations. Photochem Photobiol Sci. 2005;4(9):699–708. doi: 10.1039/b418378a. [DOI] [PubMed] [Google Scholar]
- 47.Norval M. The mechanisms and consequences of ultraviolet-induced immunosuppression. Prog Biophys Mol Biol. 2006;92(1):108–18. doi: 10.1016/j.pbiomolbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Robien K, Cutler GJ, Lazovich D. Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women’s Health Study. Cancer Causes Control. 2007;18(7):775–82. doi: 10.1007/s10552-007-9020-x. [DOI] [PubMed] [Google Scholar]
- 49.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;18(2(8673)):1176–8. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 50.Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Colon cancer and serum vitamin D metabolite levels 10–17 years prior to diagnosis. Am J Epidemiol. 1995;15(142(6)):608–11. doi: 10.1093/oxfordjournals.aje.a117682. [DOI] [PubMed] [Google Scholar]
- 51.Rejnmark L, Lauridsen AL, Brot C, Vestergaard P, Heickendorff L, Nexo E, Mosekilde L. Vitamin D and its binding protein Gc: long-term variability in peri- and postmenopausal women with and without hormone replacement therapy. Scand J Clin Lab Invest. 2006;66(3):227–38. doi: 10.1080/00365510600570623. [DOI] [PubMed] [Google Scholar]
- 52.Blackman JD, Cabana VG, Mazzone T. The acute-phase response and associated lipoprotein abnormalities accompanying lymphoma. J Intern Med. 1993;233(2):201–4. doi: 10.1111/j.1365-2796.1993.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 53.Seymour JF, Gagel RF. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood. 1993;1(82(5)):1383–94. [PubMed] [Google Scholar]
- 54.Pepper C, Thomas A, Hoy T, Milligan D, Bentley P, Fegan C. The vitamin D3 analog EB1089 induces apoptosis via a p53-independent mechanism involving p38 MAP kinase activation and suppression of ERK activity in B-cell chronic lymphocytic leukemia cells in vitro. Blood. 2003;1(101(7)):2454–60. doi: 10.1182/blood-2002-07-1984. [DOI] [PubMed] [Google Scholar]
- 55.Kumagai T, O’Kelly J, Said JW, Koeffler HP. Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst. 2003;18(95(12)):896–905. doi: 10.1093/jnci/95.12.896. [DOI] [PubMed] [Google Scholar]
- 56.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–7. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 57.Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4(3):e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stephensen CB, Gildengorin G. Serum retinol, the acute phase response, and the apparent misclassification of vitamin A status in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2000;72(5):1170–8. doi: 10.1093/ajcn/72.5.1170. [DOI] [PubMed] [Google Scholar]
- 59.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–7. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


