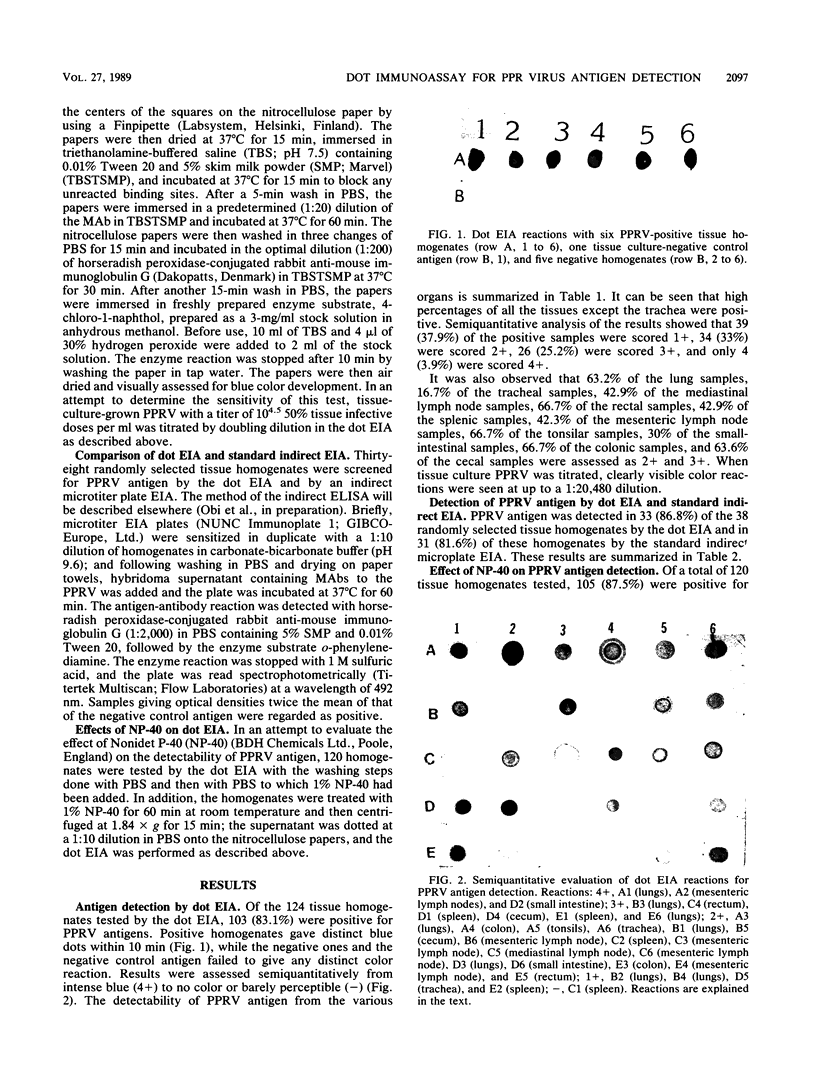
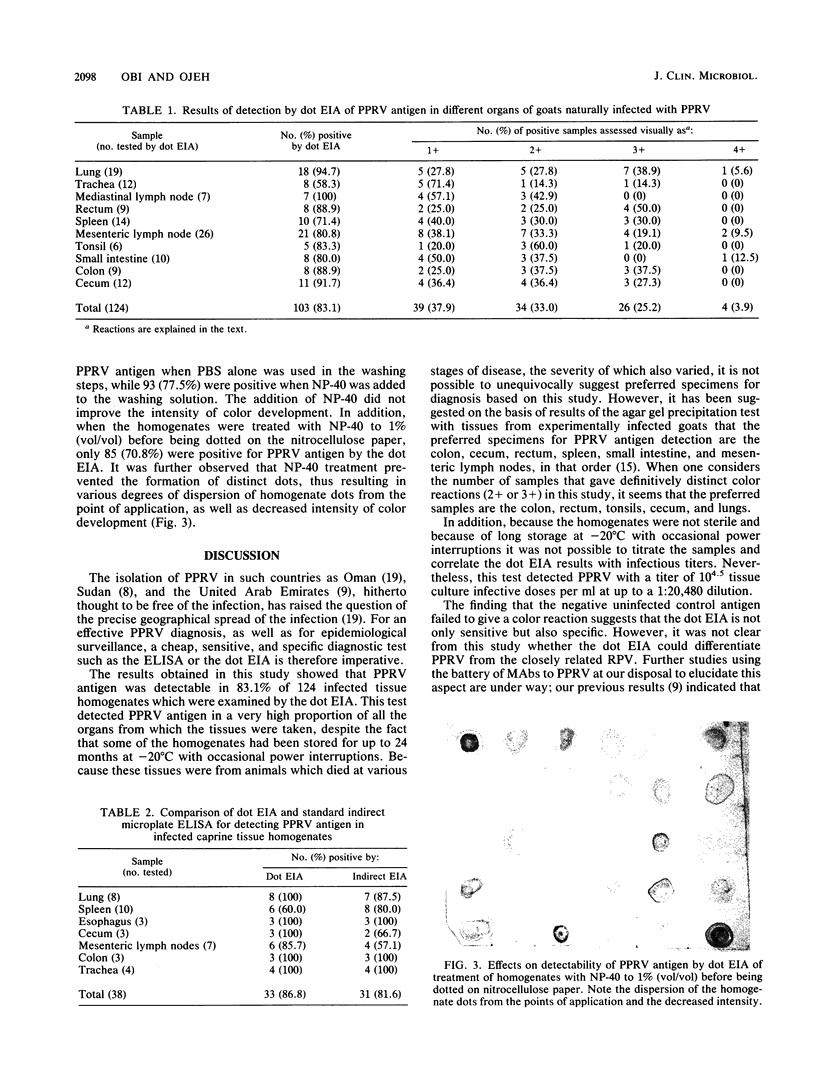
Abstract
An enzyme-linked immunosorbent microassay using nitrocellulose paper as the solid-phase support was developed for the detection of peste-des-petits-ruminants virus antigens in infected caprine tissue homogenates. Dots of tissue homogenates were applied to nitrocellulose papers, and any unreacted sites were blocked with 5% skim milk powder in triethanolamine-buffered saline. After incubation of the papers in tissue culture supernatant monoclonal antibody against the peste-des-petits-ruminants virus, the antigen-antibody reaction was detected with peroxidase-conjugated anti-mouse immunoglobulin G and the enzyme substrate 4-chloro-1-naphthol. Positive results were visualized as blue dots. Results of the dot enzyme immunoassay compared favorably with those of the standard enzyme-linked immunosorbent assay. Incorporation of Nonidet P-40 in the washing solution did not improve the sensitivity of the dot enzyme immunoassay, and pretreatment of homogenates with Nonidet P-40 before application to the nitrocellulose paper inhibited the binding of the antigen to the paper and reduced the intensity of the color development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afshar A., Myers D. J. Simple and rapid dot-enzyme immunoassay for visual detection of rinderpest antibodies in bovine and caprine sera. Trop Anim Health Prod. 1986 Nov;18(4):209–216. doi: 10.1007/BF02359536. [DOI] [PubMed] [Google Scholar]
- Afshar A., Wright P. F., Dulac G. C. Dot-enzyme immunoassay for visual detection of antibodies to pseudorabies virus in swine serum. J Clin Microbiol. 1986 Mar;23(3):563–567. doi: 10.1128/jcm.23.3.563-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. Use of monoclonal antibody in a blocking ELISA to detect group specific antibodies to bluetongue virus. J Immunol Methods. 1984 Nov 16;74(1):139–149. doi: 10.1016/0022-1759(84)90375-2. [DOI] [PubMed] [Google Scholar]
- Beutin L., Bode L., Richter T., Peltre G., Stephan R. Rapid visual detection of Escherichia coli and Vibrio cholerae Heat-labile enterotoxins by nitrocellulose enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Mar;19(3):371–375. doi: 10.1128/jcm.19.3.371-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C. F. A 'dot-immunobinding assay' on nitrocellulose membrane for the determination of the immunoglobulin class of mouse monoclonal antibodies. J Immunol Methods. 1984 Feb 24;67(1):79–87. doi: 10.1016/0022-1759(84)90087-5. [DOI] [PubMed] [Google Scholar]
- Chand P., Batra H. V., Sadana J. R. Detection of brucella specific protein-A reactive antibodies in buffaloes by dot-enzyme-linked immunosorbent assay. Vet Rec. 1988 Feb 13;122(7):162–163. doi: 10.1136/vr.122.7.162. [DOI] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Furley C. W., Taylor W. P., Obi T. U. An outbreak of peste des petits ruminants in a zoological collection. Vet Rec. 1987 Nov 7;121(19):443–447. doi: 10.1136/vr.121.19.443. [DOI] [PubMed] [Google Scholar]
- Gibbs E. P., Taylor W. P., Lawman M. J., Bryant J. Classification of peste des petits ruminants virus as the fourth member of the genus Morbillivirus. Intervirology. 1979;11(5):268–274. doi: 10.1159/000149044. [DOI] [PubMed] [Google Scholar]
- Hamdy F. M., Dardiri A. H., Nduaka O., Breese S. S., Jr, Ihemelandu E. C. Etiology of the stomatitis pneumoenteritis complex in Nigerian dwarf goats. Can J Comp Med. 1976 Jul;40(3):276–284. [PMC free article] [PubMed] [Google Scholar]
- Heberling R. L., Kalter S. S. Rapid dot-immunobinding assay on nitrocellulose for viral antibodies. J Clin Microbiol. 1986 Jan;23(1):109–113. doi: 10.1128/jcm.23.1.109-113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majiyagbe K. A., Nawathe D. R., Abegunde A. Rapid diagnosis of peste des petits ruminants (PPR) infection, application of immunoelectroosmophoresis (IEOP) technique. Rev Elev Med Vet Pays Trop. 1984;37(1):11–15. [PubMed] [Google Scholar]
- Obi T. U., Ojo M. O., Durojaiye O. A., Kasali O. B., Akpavie S., Opasina D. B. Peste des petits ruminants (PPR) in goats in Nigeria: clinical, microbiological and pathological features. Zentralbl Veterinarmed B. 1983 Dec;30(10):751–761. doi: 10.1111/j.1439-0450.1983.tb01900.x. [DOI] [PubMed] [Google Scholar]
- Obi T. U., Patrick D. The detection of peste des petits ruminants (PPR) virus antigen by agar gel precipitation test and counter-immunoelectrophoresis. J Hyg (Lond) 1984 Dec;93(3):579–586. doi: 10.1017/s0022172400065165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Porter H. G. A glucose oxidase immunoenzyme stain for the detection of viral antigen or antibody on nitrocellulose transfer blots. J Immunol Methods. 1984 Aug 3;72(1):1–9. doi: 10.1016/0022-1759(84)90428-9. [DOI] [PubMed] [Google Scholar]
- Suresh M. R., Milstein C. A direct antigen-binding assay to screen hybridoma supernatants. Anal Biochem. 1985 Nov 15;151(1):192–195. doi: 10.1016/0003-2697(85)90071-5. [DOI] [PubMed] [Google Scholar]