Summary
Originally described in insect viruses, cellular proteins with Baculoviral IAP Repeat (BIR) motifs have been thought to function primarily as inhibitors of apoptosis. The subsequent finding that a subset of IAPs that contain a RING domain have ubiquitin protein ligase (E3) activity implied the presence of other functions. It is now known that IAPs are involved in mitotic chromosome segregation, cellular morphogenesis, copper homeostasis, and intracellular signaling. Here we review the current understanding of the roles of IAPs in apoptotic and non-apoptotic processes, and explore the notion that the latter represent the primary physiologic activities of IAPs.
Introduction
Since the discovery 15 years ago by Miller and colleagues of iap, a baculovirus gene that inhibits apoptosis in virally-infected Spodoptera frugiperda insect cells (Crook et al., 1993), cellular homologs have been identified in yeast, nematodes, flies, and higher vertebrates. Members of this family of Inhibitors of Apoptosis (IAP) are characterized by the presence of a variable number of Baculoviral IAP Repeat (BIR) motifs, a sequence of approximately 70 amino acids that coordinates a zinc ion via histidine and cystine residues. Apoptosis is a genetically programmed process of controlled cell suicide that has critical roles in organismal development and homeostasis. Dysregulation of apoptosis contributes to the pathogenesis of a host of diseases including cancers, autoimmunity, and neurological disorders. The observation that some IAPs are highly expressed in several neoplasms, and the identification of a genetic translocation involving the gene encoding c-IAP2 in a subset of B cell lymphomas, also led to the idea that IAPs might contribute to the resistance to cell death that marks many cancers. The pursuit of this idea led to the discovery that the BIR domains bind directly and inhibit the proteolytic activity of caspases, central components of the apoptotic machinery (Eckelman et al., 2006). A significant body of biochemical, structural, and in vivo data now show that IAPs regulate caspases through distinct mechanisms, and that different family members serve different functions (Shi, 2004). Interestingly, even in plants, which possess programmed cell death mechanisms and inducible caspase-like activity, IAP-like proteins (ILPs) with BIR-like domains (BLDs) have been found (Higashi et al., 2005).
In addition to having distinct functional domains, posttranslational modifications and changes in the level of expression can be crucial factors in determining IAP function. For example, phosphorylation of some IAPs affects intracellular localization, protein-protein interactions, and IAP stability (Samuel et al., 2005; Kuranaga et al., 2006; Oshima et al., 2006). Likewise, cross-talk between IAP molecules can affect IAP levels (Dohi et al., 2004; Conze et al., 2005; Arora et al., 2007; Silke et al., 2005). Interestingly, molecules containing a particular IAP-binding motif (IBM) antagonize IAP function either by directly binding BIR domains and displacing bound caspases or by promoting IAP degradation (Yang and Du, 2004; Vaux and Silke, 2005). Because of its obvious clinical implications, early studies dealt mainly with the characterization of the anti-apoptotic functions of IAPs. However, like many other complex molecular families, IAPs defy a simple assignment to a particular functional category. There is now compelling evidence that IAPs have important roles in cell division, morphogenesis, heavy metal homeostasis, NF-κB activation, and MAP kinase signaling. In this review we will focus on Drosophila and mammalian IAPs, particularly but not exclusively the RING-containing subset, and explore the current appreciation of their roles not only in cell death but also these other functions, which may in fact prove to be more important in normal physiological processes. We will begin by describing the characteristic structural features of different BIRs and their interacting partners, and will then discuss the functional significance of these interactions in signaling.
Structural and functional features
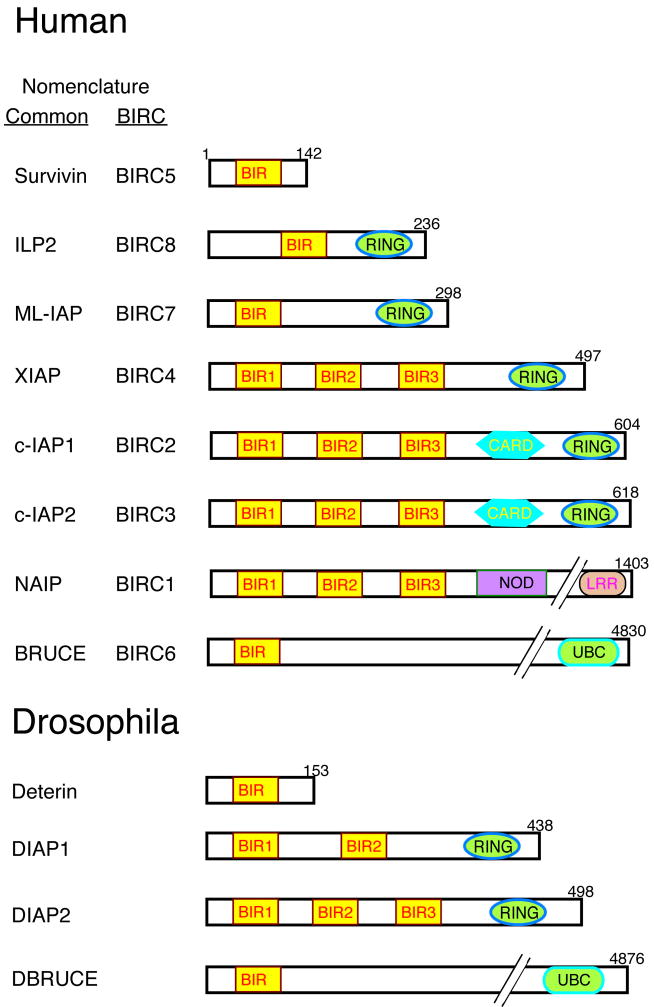
The BIR domain is the defining structural characteristic of IAP molecules. BIRs can be present in a single copy or an array of two to three repeats in the N-terminal portion of IAPs, which often include additional functional regions such as a RING or CARD (caspase-associated recruitment domain) domain near the C-terminus (Fig. 1). XIAP (X-linked IAP), c-IAP1, and c-IAP2, which are the most extensively characterized of the mammalian IAPs, each contain three BIRs and a RING domain. BIRs are regions of approximately 70 amino acids that contain the signature sequence CX2CX16HX6C (C = cysteine, H = histidine, X = any amino acid), and fold as three-stranded beta sheets surrounded by four alpha helices (Hinds et al., 1999; Sun et al., 1999; Sun et al., 2000; Verdecia et al., 2000). The BIR helices and beta strands pack tightly to form a hydrophobic core, at the center of which lies an atom of zinc coordinated by the three cysteines and the histidine.
Fig. 1. Schematic representation of the human and Drosophila IAP family of proteins.
The number of residues in each IAP is shown, as well as the functional motifs that they contain. The BIRC nomenclature equivalents are provided. ILP2, ML-IAP, and Deterin are shown in this figure but not discussed in the review because little information about their function is available. NCBI GenBank (http://www.ncbi.nlm.nih.gov/Genbank) accession numbers are survivin: O15392; ILP2 : Q96P09; ML-IAP : Q96CA5; XIAP : P98710; c-IAP1 : Q13490; c-IAP2 : Q13489; NAIP : Q13075; Bruce : Q9NR09; Deterin : NM_142351; DIAP1 : Q24306; DIAP2 : Q24307: DBruce : NP_649995. CARD, caspase-associated recruitment domain; UBC, ubiquitin-conjugation; NOD, nucleotide-binding oligomerization domain; LRR, leucine-rich repeats.
BIRs function principally by mediating protein-protein interactions. The presence of multiple copies in a molecule can increase the affinity for a given protein as well as the range of proteins with which the IAP can interact. Interestingly, the binding properties of sequentially-corresponding BIRs in multi-BIR-containing molecules is conserved, with BIR2 and -3 involved in the binding of caspases and apoptosis-regulatory molecules, and BIR1 interacting with a diverse array of signaling intermediates. For example, XIAP BIR2 and -3 share nearly 40% identity with each other and each have two distinct sites that are responsible for protein-protein interactions. One of these sites, common to both BIRs and conserved in BIRs from other IAPs, is an acidic surface grove that functions as an anchoring motif for caspases. This motif in BIR2, along with a short N-terminal flanking region of 18 amino acids that harbors the second interacting site, binds strongly to caspase-3 and -7 but not caspase-9 (Eckelman et al., 2006). By contrast, the common anchoring motif in BIR3, with the help of a helical region towards the C-terminus, selectively targets caspase-9 but not caspase-3 or -7 (Shiozaki and Shi, 2004). As a result, GST-BIR2 binds caspase-3 nearly 200 times more strongly than does GST-BIR3 (Takahashi et al., 1998). The BIR2 and -3 regions of c-IAP1 and c-IAP2 exhibit the same specificity with regard to caspase binding (Eckelman et al., 2006).
Another example of BIR-interacting molecules includes the mitochondrial proteins SMAC (also known as DIABLO) and Omi (also known as HtrA2), which both possess an IBM motif. Both SMAC and Omi are synthesized as full-length precursors with N-terminal mitochondrial signal sequences. The N-terminal tetrapeptide (AVPI in human SMAC and AVPS in human Omi) is exposed upon mitochondrial import owing to cleavage of the mitochondrial targeting sequence. When a cell undergoes apoptosis these mature molecules are released from the intermembrane space into the cytosol, where they bind IAP BIR2 and -3 via the IBM. Biochemical and structural evidence has provided a molecular explanation for XIAP BIR3/IBM binding (Shiozaki and Shi, 2004; Srinivasula et al., 2000). The first N-terminal residue, alanine, is inserted into a hydrophobic pocket on the BIR3 surface and forms hydrogen bonds with neighboring residues. Modeling has suggested that only a free alanine can form a stable interaction. Consistent with this finding, replacement of the alanine with methionine or glycine in the SMAC IBM abrogates its interactions with BIRs. Moreover, the same acidic surface groove on BIRs interacts with IBM molecules and caspases in a mutually exclusive manner. For example, recombinant mature SMAC, or short peptides containing the SMAC IBM, block caspase-9 binding to BIR3 (Srinivasula et al., 2001). Importantly, the presence of BIR2 and BIR3 in tandem in XIAP enhances its binding to SMAC many-fold. SMAC, which exists as a dimer in solution, makes a stable complex with XIAP in a 2:1 ratio by interacting simultaneously with both BIR2 and -3 in a single IAP molecule. As a result, surface plasma resonance measurements showed that recombinant SMAC interacts nearly 1000 times more strongly with XIAP containing both BIRs than with isolated BIR2 or BIR3 (Huang et al., 2003). This mode of interaction between BIRs, caspases, and IBM molecules is conserved amongst species, including the fly. Unlike mammalian XIAP, which contains three BIRs, Drosophila IAP1 (DIAP1) contains only two BIRs, its BIR2 being structurally similar to XIAP BIR3 and having a precisely matching array of interacting residues. Correspondingly, DIAP1 BIR2 functions like XIAP BIR3 and binds strongly to the IBM-containing Drosophila molecules Reaper, Grim, Hid, Sickle, and the caspase Dronc. Interestingly, another mitochondrial protein that is released during apoptosis, AIF (apoptosis-inducing factor), interacts with the XIAP BIR2 despite lacking the IBM binding motif (Wilkinson et al., 2008).
BIRs mediate interactions with molecules not directly involved in signaling for, or executing, the apoptotic program. Unlike BIR2 and -3, the BIR1 domain of XIAP, c-IAP1, and c-IAP2, does not bind caspases or IBM proteins. Rather, it functions in several signaling pathways via oligomerization of binding partners. Overexpressed XIAP BIR1 interacts with TAB1 (TGF-β activating kinase 1 (TAK1) associating subunit 1), an event that has been suggested to be of importance for XIAP-induced MAPK kinase kinase TAK1 activation and TGF-β signaling (Lu et al., 2007). Structural analysis of BIR1 alone or in a complex with TAB1 shows that BIR1 exists as a dimer, indicating that BIR1 probably functions via proximity-induced activation of signaling molecules in the oligomerized complex. Consistent with this idea, point mutations that disrupt either BIR1-BIR1 dimerization or BIR1-TAB1 interactions reduce XIAP-induced signaling (Lu et al., 2007). This mechanism applies to other IAP BIR1 motifs as well, as both c-IAP1 and -2 interact with TRAF-1 and -2 via their BIR1 regions (Samuel et al., 2006; Varfolomeev et al., 2006). Additionally, BIRs mediate binding between members of the IAP family itself. For example, the XIAP BIR1 and BIR3 domains interact with the single BIR of survivin, and upon being signaled for apoptosis these molecules form higher order complexes (Dohi et al., 2004). In addition to its involvement in homodimerization, the survivin BIR binds Aurora B, a serine/threonine kinase that regulates processes during cell division. Point mutations in the acidic patch of the BIR abrogate the survivin-Aurora B interaction. The survivin BIR, along with a short C-terminal proximal region, also binds Borealin, and the three proteins form the chromosomal passenger complex (CPC) (Jeyaprakash et al., 2007).
Although most of our structural knowledge of BIR-protein interactions has been obtained using isolated domains, a few biochemical approaches have unraveled the complex nature of these interactions with full-length IAP molecules. One example is mammalian NAIP (neuronal apoptosis-inhibitory protein), which in addition to three BIRs contains a unique central nucleotide binding oligomerization domain (NOD) and a C-terminal leucine-rich repeat (LRR) domain (Fig. 1). The NOD and LRR domains regulate the NAIP BIR interaction with caspase-9 in a manner not seen in any other members of the family (Davoodi et al., 2004). The full length NAIP binds caspase-9 only in the presence of ATP or in the absence the LRR domain, indicating that access to BIRs is spatially blocked by the C-terminal domains and that ATP-mediated conformational changes are required for caspase-9 binding. In another example, SMAC binding to XIAP BIR2 and -3 gives rise to changes that affect BIR1 interactions, in particular preventing full-length XIAP BIR1/TAB1 interactions (Lu et al., 2007). Unlike full-length SMAC, dimeric SMAC peptides (dAVPI) fail to disrupt XIAP/TAB1 interactions, suggesting that the inhibitory effect is likely a result of steric exclusion rather than direct competition (Lu et al., 2007). Therefore, despite their overall similarity, individual BIRs recognize distinct intermediates involved in diverse apoptotic and signaling pathways.
Caspases are a family of cysteine-dependent aspartate-specific proteases that are synthesized as inactive precursors (procaspases). Generally, procaspases are activated by proteolytic processing that yields two unequal subunits that form a hetero-tetramer containing two copies of each (Eckelman et al., 2006). Caspase activity is either essential for (e.g., Type I, or death receptor-induced) or at least a major component of (Type II, or mitochondrial) apoptotic pathways. Initial biochemical studies using GST-IAP fusion proteins and overexpression analyses demonstrated that human survivin, XIAP, c-IAP1, and c-IAP2 bind and effectively inhibit caspase-3, -7, and -9. However, subsequent and more quantitative studies have made it clear that, with the exception of XIAP, no IAP in this group contains all the elements required for potent caspase inhibition. The elucidation of the precise structural elements of BIRs that are involved in caspase inhibition has revealed that XIAP inhibits caspase-3/7 and caspase-9 by unrelated mechanisms. XIAP BIR2 and a short preceding peptide strand bind the substrate binding cleft of caspase-3/7, with the peptide strand in reverse orientation to that of substrate (‘back to front’) (Eckelman et al., 2006). By contrast, BIR3 inhibits caspase-9 by a novel allosteric mechanism in which its distal helix forces caspase-9 into an inactive monomeric conformation by interposition between the caspase dimerization interfaces (Shiozaki and Shi, 2004). This provides an example of diversity within BIRs, with two closely related BIRs acquiring the ability to inhibit different caspases by fundamentally distinct mechanisms. This finding also illustrates that caspase binding is by itself insufficient to inhibit enzymatic activity, as both c-IAP1 and c-IAP2 bind caspases efficiently but are weak caspase inhibitors because they lack the structural features present in the XIAP BIRs (Eckelman and Salvesen, 2006).
In addition to the family-defining BIRs, some IAPs contain another more widely distributed region called a RING domain (an acronym for Really Interesting New Gene). RING domains are characterized by the presence of six to seven cysteines and one or two histidines that form a cross-brace architecture and coordinate two zinc ions (Weissman, 2001). RING domains often function as modules that confer ubiquitin protein ligase (E3) activity, and in conjunction with a ubiquitin activating enzyme (E1) and a ubiquitin conjugating enzyme (E2) catalyze the transfer of ubiquitin to target proteins (Lorick et al., 1999). Ubiquitin moieties themselves are subject to ubiquitination, and in an iterative process involving the conjugation of the C-terminal glycine of one ubiquitin to a lysine in another, extended ubiquitin chains can form, and proteins bearing chains with ubiquitins connected via lysine 48 linkages are usually recognized by proteasomes and degraded (Weissman, 2001). All known RING-containing IAPs have E3 activity, and the range of substrates includes molecules involved in apoptosis and signaling, and even themselves in a homo- or heterotypic fashion. These substrates will be discussed in context in the following sections.
Apoptosis
Apoptosis is a process in which a cascade of proteolytic events involving caspases results in the elimination of damaged or unwanted cells. It was anti-apoptotic activity that gave IAPs their name, and it is therefore not surprising that it is this aspect of IAP biology that has received the most attention. Even before the identification of caspases as positive regulators of apoptosis, a baculovirus Autographa californica protein called p35 was known to prevent cell death, its deletion resulting in apoptosis of insect cells during viral infection (Clem et al., 1991). Efforts to identify other such molecules using genetic complementation resulted in the initial identification of two viral IAPs: Cp-IAP (from Cydia pomonella granulosis virus) and Op-IAP (from Orgyia pseudotsugata nuclear polyhydrosis virus) (Birnbaum et al., 1994; Crook et al., 1993). Overexpression studies found that IAPs from virus to human can inhibit apoptosis induced by a variety of stimuli, leading to the belief that the primary role of IAPs is to prolong cell survival. There is in fact strong genetic evidence from gene deletion or mutation analyses that for at least some IAPs this hypothesis is correct. For example, flies harboring DIAP1 mutations that disrupt caspase binding exhibited increased cell death and embryonic lethality (Martin, 2002). BRUCE (also known as Apollon) is a very large (530 kDa) IAP molecule with an N-terminal BIR and a C-terminal UBC (ubiquitin conjugating enzyme) domain. Gain-of-function mutations in DBRUCE potently inhibited apoptosis induced by Reaper and GRIM (Vernooy et al., 2002), and targeted disruption of BRUCE in mice caused embryonic or neonatal lethality, with BRUCE-deficient embryonic fibroblasts (MEFs) exhibiting greater sensitivity to cell death by the combination of TNF and a cell-permeable N-terminal peptide derived from SMAC (Hao et al., 2004). Contrary to expectations, the BRUCE BIR domain alone was not sufficient for anti-apoptotic function, and in both fly and mouse deletion of the C-terminal half containing the UBC domain resulted in mortality owing to excessive apoptosis (Vernooy et al., 2002; Ren et al., 2005).
Unlike BRUCE, none of the common RING-containing IAPs (XIAP, c-IAP1, and c-IAP2) is required in mice for survival or normal development (Harlin et al., 2001; Conze et al., 2005; Conte et al., 2006) (Table I). In fact, given the large amount of evidence from overexpression studies in cells and transgenic mice (Conte et al., 2001), the lack of obvious abnormal apoptotic phenotypes was remarkable. In the initial report using immortalized XIAP-deficient MEFs it was suggested that this resulted from compensatory upregulation of c-IAP1 and c-IAP2 (Harlin et al., 2001) but studies using primary cells subsequently showed that c-IAP1 and c-IAP2 levels are normal in XIAP-deficient animals (Conze et al., 2005). Despite the absence of major abnormalities, a few stimulus-specific and tissue-dependent defects in apoptosis have been identified in IAP knockout mice. For example, sympathetic neurons and postmitotic cardiomyocytes from XIAP-null mice were reported to be more sensitive to cell death induced by cytochrome c microinjection (Potts et al., 2005; Potts et al., 2003). In addition, macrophages derived from c-IAP2-deficient mice were hypersensitive to Fas-induced apoptosis after LPS treatment (Conte et al., 2006). The resistance of wild-type cells in this case might be due to the transcriptional regulation of c-IAP2, which unlike XIAP and c-IAP1, is upregulated 30-fold in LPS-stimulated macrophages. This idea is consistent with other observations showing that constitutive IAP levels are insufficient to antagonize apoptotic signals, but that IAPs can have this activity when expression is upregulated.
Table 1.
Summary of IAP gene mutation or ablation phenotypes in human and mouse
| Protein | Mutation in human | Knockout in mice | Ref |
|---|---|---|---|
| Survivin | NR* | Lethal at early embryonic stage. Loss in thymocytes causes mitotic defects. | (Okada et al., 2004; Uren et al., 2000) |
| XIAP | Loss of expression causes an X-linked lymphoproliferative syndrome with low numbers of NKT cells. | Viable. Delayed lobuloalveolar development in mammary gland. | (Rigaud et al., 2006; Harlin et al., 2001; Olayioye et al., 2005) |
| c-IAP1 | Deletion of c-IAP1/c-IAP2 associated with increased activation of the non-canonical NF-κB pathway in multiple myeloma patients. | Viable. Loss of c-IAP1 causes elevated levels of c-IAP2 expression. | (Conze et al., 2005; Keats et al., 2007) |
| c-IAP2 | c-IAP2/MALT1 fusion protein, generated by chromosomal translocation associated with MALT lymphoma, activates NF-κB. Deletion of c-IAP1/c-IAP2 associated with increased activation of the non-canonical NF-κB pathway in multiple myeloma patients. |
Viable. Mice are resistant to LPS-induced shock. Protects macrophages from LPS-induced death. | (Conte et al., 2006; Zhou et al., 2005; Keats et al., 2007) |
| NAIP | NR | Multiple alleles of NAIP are present in different strains of mice. Mice lacking NAIP5 are susceptible to Legionella pneumophila infection. | (Wright et al., 2003) |
| BRUCE | NR | Embryonic lethal. Apollon-deficient MEFs are sensitive to SMAC-induced apoptosis. Knockout of UBC domain alone causes apoptosis in placenta and yolk sac. | (Hao et al., 2004; Ren et al., 2005) |
No mutation reported
Another factor to consider when interpreting the lack of an obvious phenotype in the knockout mice is the post-translational regulation of IAP expression in normal cells. XIAP, c-IAP1, and c-IAP2 catalyze their own ubiquitination in RING-dependent manner in cells subjected to a pro-apoptotic stimulus (Yang et al., 2000; Li et al., 2002). This autoubiquitination and degradation is an important regulatory step, because RING-mutant XIAP that lacks E3 activity is relatively stable and confer resistance to apoptotic stimuli (Yang et al., 2000). The reason that apoptotic stimuli cause IAP autoubiquitination in mammalian cells is likely due to the release of IAP antagonists containing the IBM motif. Direct evidence for this idea comes from studies in Drosophila, in which the IBM protein HID causes DIAP1 degradation in embryos and wing compartments with a corresponding increase in caspase activity and apoptosis (Martin, 2002). Similarly, expression of Reaper or GRIM in the eye promotes DIAP1 degradation and organ ablation. Addition of Reaper to Xenopus laevis egg extracts, or expression of Reaper in mammalian cells, along with DIAP1, XIAP, or c-IAP1, destabilized the IAP-level in a RING-dependent manner, suggesting that this mechanism for IAP E3 activation is conserved across species (Holley et al., 2002; Yoo et al., 2002). Consistent with this idea, mammalian SMAC promotes c-IAP1 and c-IAP2 polyubiquitination and degradation (Vaux and Silke, 2005; Yang and Du, 2004). Although SMAC binding triggers XIAP E3 activity, its effect on XIAP levels in cells remains controversial, with some groups finding degradation and others not (Vaux and Silke, 2005). One possible function of IAP autodegradation in healthy cells is to target accidentally-released IBM proteins from the mitochondria for co-degradation to prevent toxic consequences. In cells committed to apoptosis, however, substantial loss of IAPs as a result of a large release of IBM proteins would ensure minimal resistance to cell death. Therefore, because RING IAPs are downregulated in some normal cells that receive an apoptotic stimulus, it is not surprising that deficiencies in these proteins do not have a marked apoptotic phenotype.
Given the lack of an overt phenotype in XIAP-deficient mice, the severe consequences of XIAP mutations in humans came as a surprise. Studies of patients with X-linked lymphoproliferative syndrome (XLP) manifested by splenomegaly, lymphomas, hypogammaglobulinaemia, and lymphohystiocytosis identified male individuals from three families with mutations in XIAP (Nichols et al., 2005). Lymphocytes from these XLP patients had no detectable XIAP protein expression and showed enhanced apoptosis in response to stimulation with TRAIL (TNF-related apoptosis-inducing ligand), anti-Fas (CD95), and anti-CD3 (Rigaud et al., 2006). Introduction of XIAP into XIAP-deficient T cells by lentiviral infection rendered them resistant to activation-induced apoptosis. In addition, these patients had low numbers of natural killer T cells (NKT cells), perhaps suggesting that XIAP is required for survival of this lymphocyte subset, despite the fact that NKT cell numbers were normal in XIAP-deficient mice. The reasons for differences in the phenotype exhibited by XIAP-deficiency in human and mouse are unknown. Although unlikely, it is possible that XIAP has acquired functions in humans that it does not have in mice. It is also possible that in mice, but not in humans, other IAPs perform XIAP-overlapping activities.
Cancer
Suppression of apoptotic programs is often a key factor in tumor formation because it allows cells to survive and grow in microenvironments deficient in oxygen and necessary growth factors, conditions that are hostile to normal cells. Cancer therapy strategies based on the activation of apoptotic pathways therefore are an attractive means of intervention. In fact, many anti-cancer therapies that have long been in clinical use, such as ionizing radiation and chemotherapy, kill cells primarily by activating intrinsic death programs (Brown and Wilson, 2003). These therapies often, however, offer modest discrimination between normal and neoplastic tissue. Another approach that is currently under clinical investigation is the use of TNF ligand family members, such as TRAIL, to induce apoptosis by triggering extrinsic death-receptor pathways (Duiker et al., 2006). A major impediment to such treatments is that tumor cells often express high levels of anti-apoptotic proteins, including IAP family members.
The observation that survivin is elevated in cancers (e.g. breast, colorectal, esophageal and gastric carcinoma, lymphoma, and neuroblastoma) out of proportion to the number of mitotic cells, and that its expression correlates with poor prognosis, led to the hypothesis that IAP overexpression might be an oncogenic event (Altieri, 2003; Duffy et al., 2007). Although the prognostic significance of elevated XIAP levels is not clear (Fulda, 2007), correlative genetic evidence suggests that c-IAP1 and c-IAP2 have a role in mammalian cancers (Hunter et al., 2007; Zender et al., 2006). The genes encoding c-IAP1 and c-IAP2 are closely linked on the chromosome, and this region (11q21-Q23 in human and 9qA1 in mouse), which also contains a transcription factor known as Yap, is amplified in a variety of human malignancies, including esophageal, liver, lung, and ovarian carcinomas, and in mouse hepatocellular carcinoma (HCC). mRNA for all three genes is elevated in tumor samples, but only c-IAP1 and Yap protein levels are increased (Zender et al., 2006). The lack of an increase in c-IAP2 protein levels likely occurs because c-IAP1, via its ubiquitin protein ligase activity, promotes c-IAP2ubiquitination and degradation, as shown in c-IAP1 knockout mice and in c-IAP1 overexpression studies (Conze et al., 2005). In agreement with this idea, the addition of proteasome inhibitors to murine HCC cells with an amplified 9qA1 region increased c-IAP2 protein levels (Zender et al., 2006). These findings suggest that high levels of c-IAP1, but not c-IAP2, promote tumorigenesis. Interestingly, in mouse HCC models 9qA1 amplification was identified in tumors derived from p53-/- hepatoblasts expressing Myc, but not Ras or Akt, indicating that c-IAP1 and Yap might cooperate with Myc. More importantly, c-IAP1 and Yap acted synergistically with Myc in p53-null ES cells to cause hepotomas in nude mice (Zender et al., 2006). The answer to how c-IAP1 might affect Myc signaling came from a screen for c-IAP1-mediated ubuiquitination of genes involved in tumorigenesis (Xu et al., 2007). Mad1 (Max-dimerization protein-1), an important cellular Myc antagonist, was identified as a c-IAP1 substrate, which triggers its degradation in cells. Unlike c-IAP1, XIAP did not ubiquitinate Mad1, which is consistent with the localization of c-IAP1 in the nucleus where it can ubiquitinate Mad1, whereas XIAP is mostly found in the cytosol (Samuel et al., 2005). When expressed in cell lines, c-IAP1 increased Mad1 degradation and cooperated with Myc to enhance proliferation in an E3-dependent manner (Xu et al., 2007). These studies suggest that suppression of c-IAP1 expression or E3 activity might have some beneficial effects in the treatment of Myc-mediated oncogenesis.
c-IAP2 is involved in a particular form of neoplasia known as MALT (mucosa-associated lymphoid tissue) lymphomas, a disease in which there is an accumulation of B-lineage cells, often in extranodal locations (Inagaki, 2007). c-IAP2 gene rearrangements are found in approximately 50% of extranodal MALT lymphomas, which results in the formation of a fusion protein between c-IAP2 BIR domains (that is, excluding the RING domain) and the C-terminal portion of MALT1, the latter being an E3 that is a critical mediator of T cell receptor-stimulated activation of NF-κB (Oeckinghaus et al., 2007). The chimeric c-IAP2/MALT1 fusion protein, but not wild type c-IAP2 or MALT1, constitutively activates NF-κB, which might be critical for B cell transformation and lymphoma progression. c-IAP2/MALT1 self-oligomerizes via BIR1, resulting in increased MALT1 E3 activity, increased NEMO ubiquitination, and NF-κB activation (Varfolomeev et al., 2006; Zhou et al., 2005). This finding establishes a feedback loop for transcriptional activation of the c-IAP2 promoter, a known NF-κB target, which could result in uncontrolled cell proliferation. Another as yet untested possibility is that the missing c-IAP2 E3 activity might contribute to B-cell survival/proliferation.
Cancer cells might also acquire resistance to apoptosis by downregulating molecules that suppress the caspase-inhibitory activity of XIAP. One example is the mitochondrial protein ARTS (apoptosis-related protein in the TGF-beta signaling pathway), whose expression is lost owing to defective methylation in more than 70% of patients with childhood acute lymphoblastic leukemia (ALL) (Gottfried et al., 2004); resistant leukemic cell lines that lack ARTS can be killed by chemotherapeutic agents once ARTS has been reintroduced (Elhasid et al., 2004). Similarly, expression of another suppressor of XIAP activity, XAF1 (XIAP-associating factor 1), is reduced in some tumor cells and cell lines (Liston et al., 2001; Plenchette et al., 2007). Together, these data provide provocative associations between cancer and IAP expression and/or activity. Whether they are mechanistically involved in the etiology of de novo human neoplasms or simply promote tumor maintenance by providing a survival advantage remains to be determined.
Targets of anticancer therapy
Given their possible importance in tumorigenesis, strategies have been developed to target IAPs by downregulating their expression with RNA interference technologies, or directly neutralizing their functions with compounds that mimic naturally-occurring IAP antagonists (Wright and Duckett, 2005). XIAP downregulation using antisense oligonucleotides sensitized various tumor cell types to death caused by γ-irradiation, chemotherapy, or TRAIL both in vitro and in vivo (Fulda, 2007; Hunter et al., 2007). More importantly, antisense oligonucleotides against XIAP by themselves exhibited potent anti-tumor activity in several preclinical cancer xenograft solid tumor models (Lacasse et al., 2005). Similarly, survivin or c-IAP1 suppression also sensitized cancer cells to death by chemotherapeutic agents and death receptors (Altieri, 2003; Gordon et al., 2002; McEleny et al., 2004). Preclinical data obtained from human tumor samples and animal models suggest that targeting IAP expression with antisense oligonucleotides is a viable treatment option in combination with other anti-cancer modalities (Cummings et al., 2006). Antisense compounds against XIAP (AEG35156, Aegera Therapeutic Inc.), and survivin (YM-155, Astellas Pharma, Inc. and LY-2181308, ISIS Pharmaceuticals, Eli Lilly & Company) currently are being evaluated in Phase I/II trials for treatment of a variety of cancers (http://clinicaltrials.gov).
The strategy of neutralizing IAP function with reagents that are structural mimics of the SMAC N-terminal IBM motif is appealing for several reasons. As discussed above, SMAC binds the BIRs of many IAPs including XIAP, c-IAP1, and c-IAP2, and neutralizes their antiapoptotic activity (Shiozaki and Shi, 2004). Thus, unlike IAP-specific antisense oligonucleotides, SMAC mimetics can in principle target multiple IAPs simultaneously. More importantly, SMAC binding triggers autoubiquitination and downregulation of c-IAP1 and c-IAP2, but not XIAP, suggesting that SMAC could promote apoptosis by at least two mechanisms: preventing caspase-inhibition (e.g. XIAP), and targeting of c-IAP1 and c-IAP2 for degradation (Yang and Du, 2004). The crystallization of XIAP BIR3 in a complex with SMAC led to the design of small compounds or peptides that mimic SMAC function (Shiozaki and Shi, 2004; Nikolovska-Coleska et al., 2007). SMAC peptides not only neutralized IAP inhibition of caspase activity in vitro, they also sensitized primary cancer cells to death-receptor or chemotherapy-induced apoptosis (Srinivasula et al., 2000; Fulda et al., 2002). SMAC peptides also strongly enhanced anti-tumor activity of TRAIL in a mouse model of malignant glioma (Fulda et al., 2002). Additionally, a number of non-peptide SMAC mimetics have been shown to be effective potentiators of cell death induced by anti-neoplastic therapies (Fulda, 2007; Hunter et al., 2007). Although many mechanistic details remain to be explored, a number of studies using SMAC mimetics have revealed a novel apoptotic mechanism. Treatment of some transformed cell lines with SMAC mimetics resulted in NF-κB activation and autocrine TNF production (Petersen et al., 2007; Vince et al., 2007; Varfolomeev et al., 2007; Gaither et al., 2007). NF-κB is sequestered in the cytosol by a family of inhibitors called IκBs (Inhibitor of NF-κB) and can be activated by two distinct mechanisms. In the canonical NF-κB pathway, activation via receptors such as TNF-R1 (TNF receptor 1) results in IκB degradation and subsequent NF-κB translocation to the nucleus. In the non-canonical pathway, receptors such as CD40 activate NIK (NF-κB inducing kinase), which results in processing of the NF-κB family member p100 to p52 and its translocation, with other NF-κB components, to the nucleus. SMAC mimetics initiated both NF-κB pathways by triggering c-IAP1 autoubiquitination and degradation, and to a lesser extent that of c-IAP2, with no effect on XIAP or TRAF proteins. Loss of c-IAP1 induced the canonical NF-κB pathway by increasing recruitment of RIP to TNF-R1 and subsequent IκB phosphorylation and degradation, and the non-canonical pathway by stabilizing and increasing levels of NIK and subsequent processing of p100 (Vince et al., 2007; Varfolomeev et al., 2007). Blocking NF-κB activation reduced TNF production and protected cells from SMAC mimetic-induced death, and knockdown either TNF or TNF-R1 with siRNA or treatment with TNF-blocking antibodies protected cells from death (Petersen et al., 2007; Vince et al., 2007; Varfolomeev et al., 2007). In addition, c-IAP1 silencing or treatment with SMAC mimetics sensitized cells to death induced by exogenous TNF. SMAC mimetic-induced cell death required caspase-8 but not, surprisingly, IAP-inhibitable caspase-9, consistent with the fact that caspase-8 is the primary initiator caspase in TNF-induced apoptosis. Because its acute loss might result in enhanced TNF-induced apoptosis, c-IAP1 degradation might also contribute to sensitization of SMAC mimetic-treated cells to TNF (Deng et al., 2003; Partheniou et al., 2001; Wang et al., 1998). Thus, SMAC mimetics appear to kill transformed cells by a mechanism far different from that for which they were originally developed. Although SMAC mimetics are known to have anti-tumor effects in mice, it is not known whether they act in this case by causing autocrine TNF-mediated cell death. It remains to be seen whether SMAC mimetic-induced TNF production occurs in humans, and if so at concentrations that are effective for tumor elimination without causing inflammatory responses. It is important to consider the potential deleterious consequences of SMAC-mimetics on B-cell homeostasis, especially in light of the association between deletion of c-IAP1/2 and constitutive activation of the non-canonical NF-κB pathway in some multiple myloma cell lines (Keats et al., 2007).
Non-apoptotic functions
Although the initial presumption was that IAPs function mainly by antagonizing cell death pathways, there is a rapidly growing body of evidence that an important, if not a predominant, role for IAPs is the regulation of a diverse set of non-apoptotic signaling pathways, including those involved in heavy metal metabolism, cell division, morphogenesis, MAP kinase activation, and NF-κB activation. This is perhaps not surprising, because many of the components of the apoptotic machinery also participate in non-cell death pathways, as exemplified by the regulation of cell proliferation and differentiation by caspases (Yuan, 2006).
Receptor signaling
TNF elicits diverse biological responses by binding to two members of its receptor superfamily: TNF-R1 (p55/p60) and TNF-R2 (p75/p80) (MacEwan, 2002). TNF-R1, which contains an intracellular death domain, is ubiquitiously expressed and the better studied of the two, and initiates signaling via activation of caspase-8, IκB kinase (IKK), and mitogen-activated protein kinases (MAPKs) such as p38 and JNK (c-Jun N-terminal kinase). TNF-R2, which is expressed primarily in immune cells and lacks a death domain, also promotes activation of IKK and MAPKs, but not caspase-8. The finding that c-IAP1 and c-IAP2 are components of the TNF-R1 and TNF-R2 signaling complexes led to the suggestion that they regulate TNF-R function (Rothe et al., 1995; Shu et al., 1996). TNF-R-associated factor-2 (TRAF-2) is a component of both receptor complexes and binds and recruits c-IAP1 and c-IAP2. The interaction between c-IAPs and TRAF2 occurs via a motif in BIR1 (PXXER) (Samuel et al., 2006). c-IAP1 has a unique role in regulating the duration of TNF-R2-initiated signaling and an indirect effect on TNF-R1-mediated death signals. Upon stimulation via TNF-R2, TRAF2 and c-IAP1 translocate to an ER-associated perinuclear compartment, where c-IAP1 and its cognate E2, the ER transmembrane protein Ubc6, ubiquitinate and target TRAF2 for degradation (Li et al., 2002; Wu et al., 2005). Another signaling intermediate, the MAPK kinase kinase ASK1, is also in a complex with TRAF2 and c-IAP1 and is targeted for ubiquitination and degradation. Notably, stimulation of primary c-IAP1-deficient B cells via TNF-R2 was unable to cause TRAF2 or ASK1 degradation, thus resulting in prolonged p38 and JNK activation (Zhao et al., 2007). Signaling via TNF-R1 does not trigger c-IAP1 translocation or ubiquitination of either substrate. Therefore, c-IAP1 is responsible for the elimination of signaling intermediates downstream of TNF-R2, which might explain the paradoxical synergy between TNF-R2 (which does not activate caspase-8) and TNF-R1 for the induction of apoptosis (Chan and Lenardo, 2000; Duckett and Thompson, 1997; Erickson et al., 1994; Rothe et al., 1993). In this case, reductions in TRAF2, MAPKs, and perhaps other unknown targets remove biological barriers (such as NF-κB) to the pro-apoptotic activity of caspase-8 induced by TNF-R1 signaling.
Unlike TNF-R2, what physiologic role, if any, IAPs might play in TNF-R1 signaling is not clear. Data from several groups suggest that XIAP and c-IAPs might positively regulate TNF-R1-induced NF-κB activation. For example, silencing of XIAP expression in cell lines that do not constitutively express TNF-R2 decreased TNF-induced NF-κB reporter activity (Gaither et al., 2007; Hofer-Warbinek et al., 2000). Similarly, expression of c-IAP2 without its RING domain or siRNA-mediated reduction of c-IAP1 expression inhibited TNF-induced IκB degradation and thus NF-κB activation (Tang et al., 2003; Chu et al., 1997). Primary HUVECs (human umbilical vein endothelial cells) deficient in both c-IAP1 and c-IAP2 also showed limited NF-κB activation as assessed by reduced phosphorylation of IKKα, IKKβ, and RelA in response to TNF but not IL-1β (Santoro et al., 2007). One possible mechanism for c-IAP1’s involvement in this process has been suggested by a study in which its overexpression resulted in NEMO polyubiquitination and NF-κB activation (Tang et al., 2003). Because all of the data favoring a role for IAP function in TNF-R1 signaling are indirect, usually involving protein overexpression, these findings and their interpretations should be treated with caution, especially given that mice lacking either XIAP, c-IAP1, or c-IAP2 had no apparent changes in this pathway (Conte et al., 2006; Conze et al., 2005; Harlin et al., 2001).
IAPs also participate in signaling mediated by BMPs (bone morphogenetic proteins), proteins that regulate diverse activities throughout development in vertebrates and invertebrates (Shiozaki and Shi, 2004). The C-terminal portion of XIAP binds BMP receptors and the N-terminal BIR1 binds TAB1, thus recruiting the TAB1-interacting MAPK kinase kinase TAK1 (Yamaguchi et al., 1999). This oligomerization of the TAB1/TAK1 complex triggers proximity-induced TAK1activation and upregulation of NF-κB activity (Lu et al., 2007). The XIAP/TAB1 interaction is critical for XIAP-induced NF-κB activation, because mutations in XIAP that disrupt TAB1 binding or loss of TAB1 expression reduces the ability of XIAP to activate NF-κB (Lu et al., 2007). Furthermore, XIAP but not c-IAP1 acted synergistically with TAB1 and TAK1 in Xenopus embryos to affect neural differentiation (Yamaguchi et al., 1999). Thus, XIAP-mediated NF-κB activation is probably important for BMP signaling.
Innate immunity
IAPs play a role in defending organisms against microbial infection by regulating immune responses in both Drosophila and mammals. Unlike their mammalian counterparts, flies lack an adaptive immune system and depend on an innate immune response mediated by two distinct signaling pathways to ward off bacterial infection. Infiltrating Gram-negative bacteria trigger activation of the IMD (immune deficiency) pathway, which shares striking similarities with the mammalian TNF-R1 signaling cascade leading to NF-κB activation and expression of peptides with anti-microbial properties (AMPs). Expression of AMPs in response to Gram-negative bacteria was reduced in cell lines in which DIAP2 was knocked down, suggesting a role for this IAP in the IMD response (Gesellchen et al., 2005; Kleino et al., 2005). RNAi-mediated depletion of DIAP2 in the adult fat body, the insect analog of the mammalian liver and the major organ of AMP synthesis, also abrogated anti-peptide gene expression upon infection. Importantly, DIAP2-null flies exposed to Gram-negative bacteria failed to mount an IMD response and died (Huh et al., 2007; Leulier et al., 2006). The mutant flies were rescued by wild type, but not the E3-deficient RING-mutant, DIAP2. The role of DIAP2, however, is limited to IMD signaling because DIAP2-null flies showed no defects in immune responses triggered via the Toll pathway, a second innate signaling mechanism flies use to protect from Gram-positive infections. This ability of IAPs to protect from bacterial infection is conserved in mammals. By example, mutations in NAIP5, one of the polymorphic NAIP alleles, in mice, enhanced susceptibility to infection by Legionella pneumophila, a Gram-negative bacterium that replicates in macrophages (Wright et al., 2003). Interestingly, in contrast to DIAP2, NAIP contains NOD and LRR domains that can function as microbial-product sensors and possess no E3 activity. Thus, the ability of NAIP5 to confer resistance to microbial infection is likely to be mediated by a mechanism distinct from that of DIAP2. Such a notion is supported by the finding that in mouse macrophages, NAIP5 restricts replication of intracellular pathogens by promoting caspase-1 activation (Zamboni et al., 2006). Although the mechanistic details are sparse, it appears that the NAIP5 LRR domain detects invading L. pneumophila and triggers caspase-1 activation, restricting microbial growth either by cleavage of molecules that are necessary for pathogen replication or by promoting death of infected macrophages.
Copper homeostasis
Copper is an essential trace metal that is a co-factor for enzymes involved in a variety of biological processes. A complex network of protein interactions regulates cellular uptake and efflux of copper and ensures proper homeostasis to avoid toxic effects of excessive copper levels (Stern et al., 2007). Among these proteins are ATP7B (a P type ATPase) and COMMD1 (copper metabolism gene MURR1 Domain containing 1), which are involved in the export of copper from cells. Point mutations in ATP7B cause Wilson’s disease, an autosomal recessive condition manifested by pathological accumulation of copper, particularly in brain and liver, thereby resulting in neurological damage to the basal ganglia and cirrohosis, respectively. Copper toxicosis syndrome resembling Wilson’s disease occurs in Bedlington terriers, and is caused by a deletion in COMMD1 that results in a non-functional truncated product. The first clue that IAPs might have an unexpected role in copper homeostasis came from yeast two hybrid studies which identified an association between XIAP and COMMD1 (Burstein et al., 2004). Subsequent studies showed that XIAP binds COMMD1 via its BIR3 domain, and that XIAP-mediated COMMD1 ubiquitination promotes its proteasomal degradation. Ecotopic expression of XIAP decreased COMMD1 levels and increased intracellular copper concentrations. Conversely, silencing of endogenous XIAP in 293 cells increased COMMD1 expression and lowered the intracellular concentration of copper. More importantly, liver tissue and fibroblasts from XIAP-deficient mice had increased COMMD1 and decreased copper levels compared to wild-type controls (Burstein et al., 2004). These observations provide an excellent example of how two functional motifs, a BIR and a RING domain, cooperate in an unpredictable manner to regulate a metabolic process unrelated to apoptosis.
If XIAP is a rate-limiting component in determining intracellular copper concentration, one might expect that there would be a feedback loop in which copper levels would modulates its function, and the recent discovery that XIAP itself binds copper suggests that this might indeed be the case (Mufti et al., 2006). In addition to zinc, XIAP has a strong binding affinity for copper, but little affinity for other divalent metal ions such as iron or calcium, both in cells and cell-free systems. XIAP-copper binding is apparently coordinated by cysteine residues in the BIR and RING domains, although it is not yet clear if these are the same cysteines that coordinate zinc. Given that increasing concentrations of zinc failed to reverse XIAP-copper binding it is possible that the metal ions bind to XIAP via distinct cysteines, or that copper has a greater affinity than zinc. Regardless, copper binding has profound and unusual effects on XIAP. First, it induces a conformational change that can be seen as a characteristic shift in electrophoretic mobility, with copper-bound XIAP from cells migrating more rapidly than unbound XIAP. Increasing amounts of copper trigger a graded shift in XIAP mobility, suggesting that copper can bind multiple regions of XIAP. This idea is in agreement with the finding that copper is capable of binding individual XIAP BIR and RING domains. Furthermore, the mobility shift was not due to cellular factors because it was recapitulated by incubating recombinant bacterially-expressed XIAP with copper, indicating that the shift results from an intrinsic change in the conformation of XIAP itself (Mufti et al., 2006). This finding was confirmed by limited proteolytic digestion that showed that free and copper-bound XIAP exhibited different mobility patterns. Second, copper-induced conformational changes make XIAP quite unstable in cells and susceptible for proteasomal degradation. Culturing of mammalian 293 cells with copper sulfate reduced XIAP levels and, more strikingly, liver from Wilson’s disease patients and Bedlington terriers had decreased XIAP levels compared to controls. XIAP from these samples also showed the mobility shift characteristic of copper-bound XIAP (Mufti et al., 2006).
Importantly, whereas zinc binding is required for E3 activity (Lorick et al., 1999), copper binding reduces the ability of XIAP to inhibit caspase activity (the effect on E3 activity has not been reported) (Mufti et al., 2006). It is therefore intriguing that the copper-induced conformational changes in XIAP are not permanent, and can be reversed by copper chelators including tetrathiomolybdate and bathocuproinedisulfonic acid (Mufti et al., 2006). This reversible nature suggests that XIAP might be at the center of a copper ion feedback loop in which increased copper levels alter XIAP conformation and inhibit function (in this case decreasing COMMD1ubiquitination and degradation and promoting cellular copper export). Conversely, low intracellular copper would enhance XIAP-mediated COMMD1 ubiquitination, resulting in a compensatory decrease in copper export. This possibility can be tested by determining the effect of copper-binding on XIAP E3 activity.
Cell migration and morphogenesis in Drosophila
Cell migration and morphogenesis are normal developmental processes that involve the reorganization of actin cytoskeleton and detachment of cells from their neighbors, features that were originally described in apoptotic cells. This striking similarity led to the speculation that, as in apoptosis, these developmental processes might also be controlled by IAPs. Subsequently, DIAP1 was shown to regulate cell migration, actin dynamics, and differentiation of sensory organ precursor cells apparently by controlling non-apoptotic caspase activity (Geisbrecht and Montell, 2004; Oshima et al., 2006; Kuranaga et al., 2006; Kanuka et al., 2005). During Drosophila oogenesis, specialized follicle cells migrate within the egg chamber in a manner that requires the activity of small GTPase Rac, and expression of dominant negative Rac (RacN17) inhibits cell migration. The surprising observation that DIAP1 has a role in promoting cell motility came from a screen for Drosophila genes that, when overexpressed, suppress the migration defect caused by RacN17 (Geisbrecht and Montell, 2004). DIAP1 suppressed cell migration mediated by Rac but not that mediated by an unrelated transcription factor, and did not rescue the defect in myoblast fusion caused by RacN17. These results coupled with the finding that DIAP1 binds Rac (both GTP and GDP bound forms) in cells suggested that its role is limited to Rac signaling pathways that mediate cell migration. Importantly, follicle cells in the egg chambers of flies with mutations in DIAP1 BIR1 or BIR2 exhibited defects in cell migration, indicating that both domains contribute to regulation of cell migration. DIAP1 also affects actin-dependent cellular organization. Expression of DIAP1 in Drosophila cells resulted in F-actin protrusions and a serrate/stellate morphology, which was greatly enhanced by co-expression of constitutively active Rac (RacV12) (Oshima et al., 2006). This finding suggests that a functional interaction between Rac and DIAP1 regulates cell motility by affecting the actin cytoskeleton. Such a conclusion is consistent with the observation of reduced F-actin staining of egg chambers from migration-defective DIAP1 BIR-mutant flies. The defects in cell motility and F-actin staining were found to result from increased DIAP1-inhibitable Drosophila caspase (Dronc) activity, not from increased apoptosis, because loss-of-function mutations in Dark (an activator of Dronc), or expression of dominant negative forms of Dronc, rescued RacN17-induced migration defects (Geisbrecht and Montell, 2004). Furthermore, reduction of DIAP1 levels increased caspase activity and destabilized F-actin-based structures without an increase in cell death (Oshima et al., 2006). Conversely, a decrease in Dronc or Dark levels affected morphogenesis by stabilizing F-actin-based structures. Thus, by negatively regulating the non-apoptotic function of Dronc, DIAP1 controls cell migration and actin cytoskeleton assembly/cell morphogenesis.
It is increasingly clear that in some cells the quantity of DIAP1 controls caspase-mediated non-apoptotic pathways that affect developmental processes, such as Drosophila sensory organ precursor (SOP) development in imaginal wing discs (Kanuka et al., 2005). Expression of DIAP1 or knockdown of a kinase (IKKε) that phosphorylates and causes the degradation of endogenous DIAP1 resulted in improper SOP development (Kuranaga et al., 2006). Unlike DIAP1, there is no evidence that mammalian IAPs control non-apoptotic caspase-mediated signaling. Interestingly, however, the function of IKKε in the degradation of IAPs is conserved in mammalian cells. The mammalian IKKε homolog, NAK (NF-κB activating kinase), directly phophorylates and promotes mammalian XIAP degradation (Kuranaga et al., 2006). As these defects are not caused by apoptosis, it is conceivable that mammalian XIAP, like DIAP1, might have a non-apoptotic role in certain development processes. One such example might be mammary gland lobuloalveolar development, which is abnormal in the late stages of pregnancy of XIAP-null mice (Olayioye et al., 2005).
Cell division
Survivin plays essential role in cell division, its absence promotes abnormal mitosis and cell death (Altieri, 2006; Lens et al., 2006). Survivin functions as a subunit of the CPC that modulates multiple events during mitosis. During cell division, survivin directs CPC movement to different locations from the inner centromere during prometaphase to midbody during cytokenesis, and participates in the organization of the center spindle by associating with polymerized microtubles (Jeyaprakash et al., 2007; Lens et al., 2006; Uren et al., 2000). Reconstitution experiments in which survivin variants were expressed in survivin-depleted cells revealed that different domains coordinate CPC localization and function. Whereas the survivin BIR domain is essential for the localization of the CPC to centromeres, the survivin C-terminus is sufficient to target it to the central spindle and midbody. Conditional knockout of survivin in thymocytes results in multiple mitotic defects including formation of defective spindles without microtubules leading to cell death (Okada et al., 2004). Conversely, overexpression in cell lines stabilizes microtubules and suppresses spindle growth (Altieri, 2006; Lens et al., 2006). Furthermore, the cell division defects caused by survivin loss largely overlap with those caused by depletion of other CPC complex components indicating that it plays an indispensable role in the function of the CPC. This function of survivin is conserved throughout evolution, with survivin orthologs being found in yeast (Birp1) and C elegans (BIR1). The eventual death observed in cells upon survivin depletion likely results from perturbed cell division rather than the loss of any putative anti-apoptotic activity (Altieri, 2006; Lens et al., 2006).
Concluding remarks
The original discovery that viral IAP proteins intervene in the apoptotic process directed the focus of research on IAPs to their effects on cell death. This research led to a flurry of initial observations that virtually all BIR-containing proteins can inhibit apoptosis by directly binding and inhibiting caspases. It was subsequently realized that mammalian IAPs suppress apoptosis only when expressed at levels far in excess of their physiological concentrations, such as in experimental overexpression systems and some cancers. At physiologic concentrations, RING-containing mammalian IAPs appear to offer little resistance to apoptosis, perhaps in part because of their autoubiquitination and degradation. Therefore, the term applied to this family, IAP (“inhibitor of apoptosis”), might be considered a misnomer in that it does not reflect the true nature of normal IAP biological activities. An alternative nomenclature that names the family after its distinctive structural feature, the BIR, has been proposed for mammalian IAPs (Silke and Vaux, 2001). The BIRC (BIR-containing protein) terminology has the advantage that the names emphasize the only feature that all IAPs share and do not imply function, especially as new functions continue to be identified at a rapid pace. The IAP common names and the BIRC equivalents are shown in Fig. 1.
A key to understanding IAP function is its characteristic BIR motif, which is found in viruses, yeast, C. elegans, invertebrates, and mammals, as well as in plant homologs. BIRs, like many other zinc-coordinating domains, are protein-protein interaction modules, and various IAP BIRs (sometimes in conjunction with flanking sequences) bind such diverse proteins as caspases (apoptosis and signaling), borealin and aurora B kinase (cell division), TAB1 (BMP signaling), AIF (reactive oxygen species production), and TRAF2 (TNF receptor superfamily intracellular signaling). Many IAPs have acquired one or more different functional domains, the best-studied being RINGs, and in this case BIR-protein interactions bring targeted molecules into proximity with ubiquitin protein ligase activity. There are examples of other motifs in IAPs, such as CARD and UBC domains, whose functional significance in these contexts remains to be described. The findings that treatment of transformed cells with SMAC-mimetics decreases c-IAP1 levels thereby triggering NF-κB activation and TNF-autocrine signaling, and that the loss of XIAP in humans perturbs NKT cell homeostasis and leads to X-linked lymphoproliferative syndrome, are as yet poorly understood clues that indicate the complex nature of IAP biology. Further analyses of knockout mice, targeted substitutions of critical BIR and RING residues, and experiments of nature such as familial IAP mutations will no doubt greatly increase our appreciation of the pleomorphic functions of the IAP family.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute. We thank Remy Bosselut (NCI) for critical review of the manuscript.
References
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Arora V, Cheung HH, Plenchette S, Micali OC, Liston P, Korneluk RG. Degradation of survivin by the X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem. 2007;282:26202–26209. doi: 10.1074/jbc.M700776200. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ, Clem RJ, Miller LK. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol. 1994;68:2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Wilson G. Apoptosis genes and resistance to cancer therapy: what does the experimental and clinical data tell us? Cancer Biol Ther. 2003;2:477–490. doi: 10.4161/cbt.2.5.450. [DOI] [PubMed] [Google Scholar]
- Burstein E, Ganesh L, Dick RD, van De Sluis B, Wilkinson JC, Klomp LW, Wijmenga C, Brewer GJ, Nabel GJ, Duckett CS. A novel role for XIAP in copper homeostasis through regulation of MURR1. EMBO J. 2004;23:244–254. doi: 10.1038/sj.emboj.7600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RJ, Fechheimer M, Miller LK. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- Conte D, Holcik M, Lefebvre CA, Lacasse E, Picketts DJ, Wright KE, Korneluk RG. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol. 2006;26:699–708. doi: 10.1128/MCB.26.2.699-708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte D, Liston P, Wong JW, Wright KE, Korneluk RG. Thymocyte-targeted overexpression of xiap transgene disrupts T lymphoid apoptosis and maturation. Proc Natl Acad Sci U S A. 2001;98:5049–5054. doi: 10.1073/pnas.081547998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze DB, Albert L, Ferrick DA, Goeddel DV, Yeh WC, Mak T, Ashwell JD. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Ranson M, Lacasse E, Ganganagari JR, St-Jean M, Jayson G, Durkin J, Dive C. Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound ( AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2006;95:42–48. doi: 10.1038/sj.bjc.6603220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi J, Lin L, Kelly J, Liston P, MacKenzie AE. Neuronal apoptosis-inhibitory protein does not interact with Smac and requires ATP to bind caspase-9. J Biol Chem. 2004;279:40622–40628. doi: 10.1074/jbc.M405963200. [DOI] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, Salvesen GS, Reed JC, Altieri DC. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- Duckett CS, Thompson CB. CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 1997;11:2810–2821. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MJ, O’Donovan N, Brennan DJ, Gallagher WM, Ryan BM. Survivin: a promising tumor biomarker. Cancer Lett. 2007;249:49–60. doi: 10.1016/j.canlet.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Duiker EW, Mom CH, de Jong S, Willemse PH, Gietema JA, van der Zee AG, de Vries EG. The clinical trail of TRAIL. Eur J Cancer. 2006;42:2233–2240. doi: 10.1016/j.ejca.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhasid R, Sahar D, Merling A, Zivony Y, Rotem A, Ben-Arush M, Izraeli S, Bercovich D, Larisch S. Mitochondrial pro-apoptotic ARTS protein is lost in the majority of acute lymphoblastic leukemia patients. Oncogene. 2004;23:5468–5475. doi: 10.1038/sj.onc.1207725. [DOI] [PubMed] [Google Scholar]
- Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KC, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- Fulda S. Inhibitor of apoptosis proteins as targets for anticancer therapy. Expert Rev Anticancer Ther. 2007;7:1255–1264. doi: 10.1586/14737140.7.9.1255. [DOI] [PubMed] [Google Scholar]
- Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J, Sage D, Slisz J, Tran M, Straub C, Ramsey T, Iourgenko V, Huang A, Chen Y, Schlegel R, Labow M, Fawell S, Sellers WR, Zawel L. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- Geisbrecht ER, Montell DJ. A role for Drosophila IAP1-mediated caspase inhibition in Rac-dependent cell migration. Cell. 2004;118:111–125. doi: 10.1016/j.cell.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Gesellchen V, Kuttenkeuler D, Steckel M, Pelte N, Boutros M. An RNA interference screen identifies Inhibitor of Apoptosis Protein 2 as a regulator of innate immune signalling in Drosophila. EMBO Rep. 2005;6:979–984. doi: 10.1038/sj.embor.7400530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GJ, Appasani K, Parcells JP, Mukhopadhyay NK, Jaklitsch MT, Richards WG, Sugarbaker DJ, Bueno R. Inhibitor of apoptosis protein-1 promotes tumor cell survival in mesothelioma. Carcinogenesis. 2002;23:1017–1024. doi: 10.1093/carcin/23.6.1017. [DOI] [PubMed] [Google Scholar]
- Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004;23:1627–1635. doi: 10.1038/sj.emboj.7600155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Sekine K, Kawabata A, Nakamura H, Ishioka T, Ohata H, Katayama R, Hashimoto C, Zhang X, Noda T, Tsuruo T, Naito M. Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nat Cell Biol. 2004;6:849–860. doi: 10.1038/ncb1159. [DOI] [PubMed] [Google Scholar]
- Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi K, Takasawa R, Yoshimori A, Goh T, Tanuma S, Kuchitsu K. Identification of a novel gene family, paralogs of inhibitor of apoptosis proteins present in plants, fungi, and animals. Apoptosis. 2005;10:471–480. doi: 10.1007/s10495-005-1876-1. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Norton RS, Vaux DL, Day CL. Solution structure of a baculoviral inhibitor of apoptosis (IAP) repeat. Nat Struct Biol. 1999;6:648–651. doi: 10.1038/10701. [DOI] [PubMed] [Google Scholar]
- Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, de Martin R. Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem. 2000;275:22064–22068. doi: 10.1074/jbc.M910346199. [DOI] [PubMed] [Google Scholar]
- Holley CL, Olson MR, Colon-Ramos DA, Kornbluth S. Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nat Cell Biol. 2002;4:439–444. doi: 10.1038/ncb798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Rich RL, Myszka DG, Wu H. Requirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J Biol Chem. 2003;278:49517–49522. doi: 10.1074/jbc.M310061200. [DOI] [PubMed] [Google Scholar]
- Huh JR, Foe I, Muro I, Chen CH, Seol JH, Yoo SJ, Guo M, Park JM, Hay BA. The Drosophila inhibitor of apoptosis (IAP) DIAP2 is dispensable for cell survival, required for the innate immune response to gram-negative bacterial infection, and can be negatively regulated by the reaper/hid/grim family of IAP-binding apoptosis inducers. J Biol Chem. 2007;282:2056–2068. doi: 10.1074/jbc.M608051200. [DOI] [PubMed] [Google Scholar]
- Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- Inagaki H. Mucosa-associated lymphoid tissue lymphoma: molecular pathogenesis and clinicopathological significance. Pathol Int. 2007;57:474–484. doi: 10.1111/j.1440-1827.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Kanuka H, Kuranaga E, Takemoto K, Hiratou T, Okano H, Miura M. Drosophila caspase transduces Shaggy/GSK-3beta kinase activity in neural precursor development. EMBO J. 2005;24:3793–3806. doi: 10.1038/sj.emboj.7600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Braggio E, Henry T, Zhu YX, Fogle H, Price-Troska T, Ahmann G, Mancini C, Brents LA, Kumar S, Greipp P, Dispenzieri A, Bryant B, Mulligan G, Bruhn L, Barrett M, Valdez R, Trent J, Stewart AK, Carpten J, Bergsagel PL. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleino A, Valanne S, Ulvila J, Kallio J, Myllymaki H, Enwald H, Stoven S, Poidevin M, Ueda R, Hultmark D, Lemaitre B, Ramet M. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 2005;24:3423–3434. doi: 10.1038/sj.emboj.7600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranaga E, Kanuka H, Tonoki A, Takemoto K, Tomioka T, Kobayashi M, Hayashi S, Miura M. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell. 2006;126:583–596. doi: 10.1016/j.cell.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Lacasse EC, Kandimalla ER, Winocour P, Sullivan T, Agrawal S, Gillard JW, Durkin J. Application of XIAP antisense to cancer and other proliferative disorders: development of AEG35156/ GEM640. Ann N Y Acad Sci. 2005;1058:215–234. doi: 10.1196/annals.1359.032. [DOI] [PubMed] [Google Scholar]
- Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Leulier F, Lhocine N, Lemaitre B, Meier P. The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol Cell Biol. 2006;26:7821–7831. doi: 10.1128/MCB.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW, Korneluk RG. Identification of XAF1 as an antagonist of XIAP anti-Caspase activity. Nat Cell Biol. 2001;3:128–133. doi: 10.1038/35055027. [DOI] [PubMed] [Google Scholar]
- Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci U S A. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Lin SC, Huang Y, Kang YJ, Rich R, Lo YC, Myszka D, Han J, Wu H. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- Martin SJ. Destabilizing influences in apoptosis: sowing the seeds of IAP destruction. Cell. 2002;109:793–796. doi: 10.1016/s0092-8674(02)00802-4. [DOI] [PubMed] [Google Scholar]
- McEleny K, Coffey R, Morrissey C, Williamson K, Zangemeister-Wittke U, Fitzpatrick JM, Watson RW. An antisense oligonucleotide to cIAP-1 sensitizes prostate cancer cells to fas and TNFalpha mediated apoptosis. Prostate. 2004;59:419–425. doi: 10.1002/pros.10371. [DOI] [PubMed] [Google Scholar]
- Mufti AR, Burstein E, Csomos RA, Graf PC, Wilkinson JC, Dick RD, Challa M, Son JK, Bratton SB, Su GL, Brewer GJ, Jakob U, Duckett CS. XIAP Is a copper binding protein deregulated in Wilson’s disease and other copper toxicosis disorders. Mol Cell. 2006;21:775–785. doi: 10.1016/j.molcel.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–199. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Nikolovska-Coleska Z, Meagher JL, Jiang S, Kawamoto SA, Gao W, Yi H, Qin D, Roller PP, Stuckey JA, Wang S. Design and characterization of bivalent Smac-based peptides as antagonists of XIAP and development and validation of a fluorescence polarization assay for XIAP containing both BIR2 and BIR3 domains. Anal Biochem. 2007 doi: 10.1016/j.ab.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A, Wegener E, Welteke V, Ferch U, Arslan SC, Ruland J, Scheidereit C, Krappmann D. Malt1 ubiquitination triggers NF-kappaB signaling upon T-cell activation. EMBO J. 2007;26:4634–4645. doi: 10.1038/sj.emboj.7601897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Bakal C, Shahinian A, Elia A, Wakeham A, Suh WK, Duncan GS, Ciofani M, Rottapel R, Zuniga-Pflucker JC, Mak TW. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med. 2004;199:399–410. doi: 10.1084/jem.20032092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye MA, Kaufmann H, Pakusch M, Vaux DL, Lindeman GJ, Visvader JE. XIAP-deficiency leads to delayed lobuloalveolar development in the mammary gland. Cell Death Differ. 2005;12:87–90. doi: 10.1038/sj.cdd.4401524. [DOI] [PubMed] [Google Scholar]
- Oshima K, Takeda M, Kuranaga E, Ueda R, Aigaki T, Miura M, Hayashi S. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Curr Biol. 2006;16:1531–1537. doi: 10.1016/j.cub.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Partheniou F, Kelsey SM, Srinivasula SM, Newland AC, Alnemri ES, Jia L. c-IAP1 blocks TNFalpha-mediated cytotoxicity upstream of caspase-dependent and -independent mitochondrial events in human leukemic cells. Biochem Biophys Res Commun. 2001;287:181–189. doi: 10.1006/bbrc.2001.5582. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenchette S, Cheung HH, Fong WG, LaCasse EC, Korneluk RG. The role of XAF1 in cancer. Curr Opin Investig Drugs. 2007;8:469–476. [PubMed] [Google Scholar]
- Potts MB, Vaughn AE, McDonough H, Patterson C, Deshmukh M. Reduced Apaf-1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. J Cell Biol. 2005;171:925–930. doi: 10.1083/jcb.200504082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts PR, Singh S, Knezek M, Thompson CB, Deshmukh M. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J Cell Biol. 2003;163:789–799. doi: 10.1083/jcb.200307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Shi M, Liu R, Yang QH, Johnson T, Skarnes WC, Du C. The Birc6 (Bruce) gene regulates p53 and the mitochondrial pathway of apoptosis and is essential for mouse embryonic development. Proc Natl Acad Sci U S A. 2005;102:565–570. doi: 10.1073/pnas.0408744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, de Saint Basile G, Latour S. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Samuel T, Okada K, Hyer M, Welsh K, Zapata JM, Reed JC. cIAP1 Localizes to the nuclear compartment and modulates the cell cycle. Cancer Res. 2005;65:210–218. [PubMed] [Google Scholar]
- Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281:1080–1090. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- Santoro MM, Samuel T, Mitchell T, Reed JC, Stainier DY. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet. 2007;39:1397–1402. doi: 10.1038/ng.2007.8. [DOI] [PubMed] [Google Scholar]
- Shi Y. Caspase activation, inhibition, and reactivation: a mechanistic view. Protein Sci. 2004;13:1979–1987. doi: 10.1110/ps.04789804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem Sci. 2004;29:486–494. doi: 10.1016/j.tibs.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci U S A. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silke J, Kratina T, Chu D, Ekert PG, Day CL, Pakusch M, Huang DC, Vaux DL. Determination of cell survival by RING-mediated regulation of inhibitor of apoptosis (IAP) protein abundance. Proc Natl Acad Sci U S A. 2005;102:16182–16187. doi: 10.1073/pnas.0502828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silke J, Vaux DL. Two kinds of BIR-containing protein - inhibitors of apoptosis, or required for mitosis. J Cell Sci. 2001;114:1821–1827. doi: 10.1242/jcs.114.10.1821. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z, Alnemri ES. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J Biol Chem. 2000;275:36152–36157. doi: 10.1074/jbc.C000533200. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- Stern BR, Solioz M, Krewski D, Aggett P, Aw TC, Baker S, Crump K, Dourson M, Haber L, Hertzberg R, Keen C, Meek B, Rudenko L, Schoeny R, Slob W, Starr T. Copper and human health: biochemistry, genetics, and strategies for modeling dose-response relationships. J Toxicol Environ Health B Crit Rev. 2007;10:157–222. doi: 10.1080/10937400600755911. [DOI] [PubMed] [Google Scholar]
- Sun C, Cai M, Gunasekera AH, Meadows RP, Wang H, Chen J, Zhang H, Wu W, Xu N, Ng SC, Fesik SW. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature. 1999;401:818–822. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- Sun C, Cai M, Meadows RP, Xu N, Gunasekera AH, Herrmann J, Wu JC, Fesik SW. NMR structure and mutagenesis of the third Bir domain of the inhibitor of apoptosis protein XIAP. J Biol Chem. 2000;275:33777–33781. doi: 10.1074/jbc.M006226200. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- Tang ED, Wang CY, Xiong Y, Guan KL. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J Biol Chem. 2003;278:37297–37305. doi: 10.1074/jbc.M303389200. [DOI] [PubMed] [Google Scholar]
- Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KH. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Wayson SM, Dixit VM, Fairbrother WJ, Vucic D. The inhibitor of apoptosis protein fusion c-IAP2.MALT1 stimulates NF-kappaB activation independently of TRAF1 AND TRAF2. J Biol Chem. 2006;281:29022–29029. doi: 10.1074/jbc.M605116200. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol. 2005;6:287–297. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2000;7:602–608. doi: 10.1038/76838. [DOI] [PubMed] [Google Scholar]
- Vernooy SY, Chow V, Su J, Verbrugghe K, Yang J, Cole S, Olson MR, Hay BA. Drosophila Bruce can potently suppress Rpr- and Grim-dependent but not Hid-dependent cell death. Curr Biol. 2002;12:1164–1168. doi: 10.1016/s0960-9822(02)00935-1. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin ASJ. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- Wilkinson JC, Wilkinson AS, Galban S, Csomos RA, Duckett CS. Apoptosis-inducing factor is a target for ubiquitination through interaction with XIAP. Mol Cell Biol. 2008;28:237–247. doi: 10.1128/MCB.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CW, Duckett CS. Reawakening the cellular death program in neoplasia through the therapeutic blockade of IAP function. J Clin Invest. 2005;115:2673–2678. doi: 10.1172/JCI26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, Long EM, Sadigh K, Abney AL, Bernstein-Hanley I, Dietrich WF. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol. 2003;13:27–36. doi: 10.1016/s0960-9822(02)01359-3. [DOI] [PubMed] [Google Scholar]
- Wu CJ, Conze DB, Li X, Ying SX, Hanover JA, Ashwell JD. TNF-alpha induced c-IAP1/TRAF2 complex translocation to a Ubc6-containing compartment and TRAF2 ubiquitination. EMBO J. 2005;24:1886–1898. doi: 10.1038/sj.emboj.7600649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhu J, Hu X, Zhu H, Kim HT, LaBaer J, Goldberg A, Yuan J. c-IAP1 cooperates with Myc by acting as a ubiquitin ligase for Mad1. Mol Cell. 2007;28:914–922. doi: 10.1016/j.molcel.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H, Matsumoto K. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QH, Du C. Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J Biol Chem. 2004;279:16963–16970. doi: 10.1074/jbc.M401253200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- Yoo SJ, Huh JR, Muro I, Yu H, Wang L, Wang SL, Feldman RM, Clem RJ, Muller HA, Hay BA. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat Cell Biol. 2002;4:416–424. doi: 10.1038/ncb793. [DOI] [PubMed] [Google Scholar]
- Yuan J. Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol Cell. 2006;23:1–12. doi: 10.1016/j.molcel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Conze DB, Hanover JA, Ashwell JD. Tumor necrosis factor receptor 2 signaling induces selective c-IAP1-dependent ASK1 ubiquitination and terminates mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:7777–7782. doi: 10.1074/jbc.M609146200. [DOI] [PubMed] [Google Scholar]
- Zhou H, Du MQ, Dixit VM. Constitutive NF-kappaB activation by the t(11;18)(q21;q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell. 2005;7:425–431. doi: 10.1016/j.ccr.2005.04.012. [DOI] [PubMed] [Google Scholar]