Abstract
In recent years, accumulating evidence has suggested that vascular risk factors contribute to Alzheimer disease (AD). Vascular dementia had been traditionally considered secondary to stroke and vascular disease. It has been traditionally distinguished from AD, considered to be a purely neurodegenerative form of dementia. However, in light of this more recent literature, it appears that there is a spectrum: ranging from patients with pure vascular dementia to patients with pure AD and including a large majority of patients with contributions from both Alzheimer and vascular pathologies. In this article, we discuss the impact of vascular risk factors on AD and its consequences at the individual level and at the population level by highlighting the concept of attributable risk. We then discuss the key questions and next steps involved in designing a therapeutic trial to control vascular risk factors for the prevention of dementia.
GLOSSARY
- AD
= Alzheimer disease;
- VaD
= vascular dementia;
- WMH
= white matter hyperintensities.
Over the past 10 years, a growing body of literature, including several large population-based clinicopathologic or clinicoradiologic studies, has highlighted the important contribution of vascular risk factors (hypertension and diabetes as primary examples) in Alzheimer disease (AD).1-4 In the Rotterdam study, one of the first large studies that called attention to this issue, dementia was associated with the presence of atherosclerosis and this association applied to subjects clinically diagnosed with vascular-type dementia as well as those with AD.2 An association between high blood pressure and the risk of AD was also reported in other cohort studies with a 15- to 21-year follow-up.1,3 Diabetes, a high level of cholesterol, tobacco smoking, as well as other vascular risk factors have also been associated with a higher risk of AD.5-7 Furthermore, atrial fibrillation, hypertension, and angina have been shown to be associated with a greater rate of decline in patients with AD.8
Apart from the occurrence of a clinical stroke, the mechanisms by which vascular factors increase the risk of AD or accelerate cognitive deterioration among patients with AD are not yet fully elucidated. Most of these factors have been shown to be associated with subcortical lesions seen on brain MRI: white matter hyperintensities (WMH),9-12 lacunar infarctions,13-15 or cerebral microhemorrhages.16 There is also evidence to suggest that lowering blood pressure may stop or delay the progression of WMH.17 The extent of WMH has been clearly linked both to cognitive impairment and the risk of incident dementia in several population-based studies.11,18-20 Further, small, clinically silent brain infarctions appear to be at least as strong a risk for subsequent dementia21 as larger, clinically evident strokes.22,23 It is, however, likely that these lesions do not fully explain the impact of vascular factors on the brain and that there exist other more subtle structural changes that may have consequences related to cognition and dementia.
The discovery of the association between vascular risk factors and AD does not question nor negate the presumed degenerative mechanisms thought to underlie pure AD. It can be hypothesized that vascular and degenerative mechanisms actually develop in parallel. A small burden of cerebral ischemic or hemorrhagic lesions caused by vascular factors may disclose the expression of plaques and tangles associated with AD in a patient with latent dementia. In other words, vascular brain injury could act additively or synergistically with concomitant AD pathology to produce more severe cognitive dysfunction than either process alone. This interpretation is supported by extensive clinical–pathologic data indicating that subjects with both vascular disease and AD pathology show either more severe cognitive impairment during life than those with pure AD24-26 or require less severe AD pathology to produce the same amount of cognitive impairment.27-30 Most of the vascular lesions described in these studies were lacunar infarcts or microinfarcts rather than large hemispheric infarctions, supporting the importance of small vascular lesions also noted in population-based clinical–radiographic studies.21,31
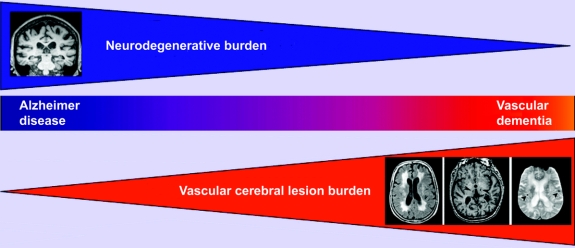
To summarize, the important question is no longer whether vascular factors contribute to dementia, but to determine their relative weight in contribution to all types of dementia in the general population. There is strong evidence to suggest that there is a spectrum: on one end, those with relatively pure dementia of vascular type, on the other end, those with relatively pure AD, and in-between there is a contribution from both AD and vascular pathologies, most likely representing the largest group (figure).32-34
Figure Reconsidering the classification of dementia
Schematic diagram of common dementias depicts proposed alternative classification of dementia. Alzheimer disease (AD) and vascular dementia (VaD) fall on a continuous spectrum of disease, composed of a gradient of features of both AD and VaD (center panel; see text for detailed discussion). Neurodegenerative mechanisms play a greater role on the left side of the spectrum (upper panel). The impact of subcortical lesions of cerebral small-vessel disease increases to the right of the spectrum (lower panel). Note white matter hyperintensities on FLAIR sequences (left), lacunar lesions on T1-weighted sequences (arrowheads, center), and cerebral microhemorrhages on gradient-echo sequences (arrowheads, right). These silent brain lesions may, in part, mediate the risk of dementia associated with vascular risk factors.
RE-EXAMINATION OF THE CLASSIFICATION OF DEMENTIAS
Since the 1970s, the concept of vascular dementia (VaD), a type of dementia secondary to stroke and vascular disease, has been distinguished from the purely neurodegenerative form of dementia (AD). Since then, many clinical, neuropsychological, radiologic, and pathologic criteria have been proposed in an attempt to distinguish these entities35-38 in order to identify a homogenous group of patients who supposedly all share a common specific underlying mechanism of dementia. Thus, in principle, mechanism-specific therapies can then be designed for this homogenous group of patients, thereby maximizing potential therapeutic benefit. Unfortunately, no classification method to date has achieved this theoretical goal. Despite continuing attempts, there is no consensus on how to separate these two entities. As a consequence, the relative frequency of VaD has been estimated in a range going from 0 to 85% of all patients with dementia.32,39 Further, medications for the symptomatic treatment of AD have also been shown to be beneficial in cases of VaD.40-44
Recent data on the role of vascular risk factors in AD discussed above provide an explanation for our failure so far to achieve this goal: if indeed there exists a spectrum of patients ranging from those with pure AD to those with pure VaD, distinguishing a subgroup of patients within this spectrum relies highly on where the threshold is set. In other words, apart from the ends of the spectrum where pure VaD or pure AD lie, the majority of patients cannot be easily classified as being in one group or the other.
Because in most patients, clinical dementia is a mixture of neurodegenerative and vascular features which may be impossible to untangle, it seems urgent to reconsider our current classification scheme. The existing classification strategy imposes reductionist thinking that could be misleading for understanding the underlying mechanisms of various types of dementia, for treating individual patients, or for devising strategies of prevention at the population level (figure).
IMPACT OF VASCULAR FACTORS IN DEMENTIA AT THE POPULATION LEVEL
The main consequence of this uncertainty concerning this reappraisal of the classification of dementias at the population level is a dramatic reevaluation of the impact of vascular risk factors on the risk of dementia. This reevaluation depends on the key difference between relative risk and population attributable risk. Elevated blood pressure or diabetes does not spectacularly increase the risk of AD in individual patients with either condition (relative risk).45 By contrast, as hypertension and diabetes are common conditions with a relatively high prevalence, this modest increase in risk translates into an elevated attributable risk at the population level. For example, based on results from the Honolulu-Asia Aging Study,46 the population attributable risk of dementia for untreated hypertension can be estimated at approximately 40%. It would therefore be higher than the estimated 20% population attributable risk of the ɛ4 allele of the APOE genotype, a potent genetic risk factor for dementia with a high relative risk of about 2 to 3 for ɛ3/4 genotype and 11 to 12 for ɛ4/4 genotype.47,48 In other words, the impact of a risk factor can be considerable on a population if it is common, even though the increased risk of disease at the individual level is relatively low.
The importance of vascular risk factors should therefore not be uniquely estimated in the context of dementias that are strongly associated with these factors (such as subcortical ischemic vascular dementia or poststroke dementia) but for the spectrum of all dementias including AD. Indeed this perspective would be reasonable from a population point of view: what is important in the broadest public health sense is not the relative proportion of one type of dementia or another, but the global clinical impact and morbidity associated with all dementias. In this context of uncertain nosologic frontiers between different types of diseases, we should aim to have an overall estimation of the role of risk factors independently of classifications. Not doing so may cause a large underestimation of the impact of shared risk factors, and may result in a missed opportunity for the prevention of the most common forms of dementia.
Defining the exact role of these vascular risk factors on all dementias including AD is particularly important from the perspective of defining a robust strategy for prevention. Because the most common forms of dementia affect the old or very old, even a modest delay in the appearance or worsening of cognitive deterioration could translate into a large reduction of the incidence of disease as these subjects would die from other causes before entering an overt stage of dementia. Indeed it is estimated that among the 106 million cases of AD expected globally by the year 2050, about 23 million could be avoided completely if it was possible to delay the start of disease by 2 years starting in the year 2010.49
A DIFFERENT VISION OF THE CLASSIFICATION OF DEMENTIA FOR BETTER PATIENT CARE
The concept of attributable risk and its consequences should also be considered at the individual patient level. Given the considerable amount of evidence, we feel that clinicians should abandon the overly simplistic dichotomous classification of these disorders and accept the possibility of including multiple etiologies when taking care of patients with dementia. This is with the understanding that each patient lies somewhere along the spectrum of dementias mentioned above. In practice, this means that a clinician treating a patient with AD should acknowledge that the patient's symptoms could be due in part or could be precipitated by vascular risk factors. In a similar fashion, in patients with vascular-related dementia, accompanying neurodegenerative pathology could, at least in part, explain the clinical presentation. We suggest that clinicians should take into account the respective influences of both vascular risk factors and neurodegenerative pathologies. For example, if vascular risk factors are present in a patient with probable AD, clinicians should identify these factors to patients and caregivers and emphasize their relative contribution to the patient's cognitive impairment.
The advantages of this alternative conception are self-evident. Rather than placing patients in one diagnostic category or another, which implicitly assigns a putative and unique mechanism of disease causation, this method would permit the clinician to consider other factors which could play a role, albeit modest, in the initial manifestation or worsening of the symptoms. In the absence of an etiology-based therapy for dementia, an important therapeutic goal is to maximize the cognitive capacity of the patient and increase the period of dementia-free living. Aggressively treating vascular risk factors such as elevated blood pressure or poorly controlled diabetes could potentially avoid cognitive deterioration and its major consequences for patient autonomy, dignity, as well as caregiver and societal burden.
This goal, although apparently modest, should not be underestimated. A delay of several months of the onset of more severe stages of dementia is precious for the patient and his or her loved ones. Furthermore, the individual variability on the impact of these vascular lesions is likely to be important and, in certain patients, treatment of these factors could potentially have a large effect and help to slow down or halt cognitive deterioration for several years.
However, although available observational data from numerous epidemiologic studies are concordant, they do not allow one to evaluate the efficacy of treatment of vascular risk factors on the prevention of dementia. Neither do they allow one to determine which patients would potentially best respond to such treatments. In order to initiate preventive strategies for dementia, physicians must rely on the results from randomized trials.
TOWARD THE DESIGN OF A DEMENTIA PREVENTION STUDY
It has not yet been convincingly shown in a therapeutic trial that controlling vascular factors reduces the risk of AD. The main reason is that, to date, no trial has been specifically designed with the prevention of dementia as the primary endpoint. Several trials using blood pressure lowering drugs in hypertensive subjects50-52 or in patients with a history of stroke53 have included dementia as a secondary endpoint. Their results remain inconclusive but they usually have poorly addressed the central issues of type of cognitive testing, duration of follow-up, calculation of study power based on cognitive events, type of patients included, and inclusion of neuroimaging criteria. The Syst-Eur study, the only trial to date that has shown that a blood pressure lowering treatment reduces the risk of AD, was based on only 32 cases of dementia in a cohort of 2,418 patients.51 An open extension of the follow-up of patients enrolled in that trial confirmed the results of the main study with a doubling of the number of cases but with the limitations of open studies.54
One of the most important questions in designing a prevention trial is the role of age on the relationship between vascular factors and the risk of dementia. Observational studies suggest that the relative risk of dementia associated with elevated blood pressure diminishes with age,55 a pattern that is also seen for stroke. Although this observed risk attenuation does not have an unequivocal explanation, it raises the issue of the efficacy of lowering blood pressure on the risk of dementia in older subjects. An alternative strategy would be to conduct a therapeutic trial on slightly younger subjects, between ages 65 and 75, for example. The major limitation of this type of trial is that the risk of dementia is relatively low in this age group. Thus, to demonstrate a clinically significant effect of blood pressure lowering, one would have to increase the number of subjects or the duration of follow-up (perhaps 10 years or more), which could be challenging in terms of the infrastructural and financial resources required to conduct such a trial.
The question of optimal age range is part of broader reflections on the definition of the optimal target population for therapeutic intervention. Cardiovascular studies published to date have recruited patients with certain vascular risk factors, such as hypertension or previous vascular event. A therapeutic prevention study of dementia should be done in patients with cognitive deterioration at risk for but who do not meet the criteria for dementia. The identification of this group of patients is more complicated than identifying subjects with vascular risk factors—this could be done by recruiting patients with cognitive complaints and a confirmed deficit on validated neuropsychological tests. As vascular pathologies may have greater impact on non-memory cognitive domains such as executive dysfunction and processing speed,56 inclusion criteria should not be restricted to only those with memory impairment but be broadened to individuals with deficits in any defined cognitive domain. The possibility of selecting subjects at high vascular risk within this group (patients with clinical risk factors or lesions due to small-vessel disease on neuroimaging) also becomes an important consideration.
Another major question concerns the type of workup required and the endpoint to be measured. MR imaging seems essential for selecting patients for the trial, for validating the impact of vascular factor reduction on the brain, and for understanding the variability of this effect across categories of patients. However, this type of evaluation may be difficult to achieve for large numbers of patients due to logistic considerations and the inherent high costs. Furthermore, the clinical endpoint in such a study also needs to be carefully defined. Using a continuous measure of cognitive function in addition to a specific threshold (which may only represent an arbitrary cutpoint) would provide a clinically meaningful and complementary quantitative measure of the effect of treatment.
Even a fundamental question such as the type of therapeutic intervention remains open. In the literature, there is no definitive argument to choose one type of intervention over another or to prefer one type of drug. Should one treat only one risk factor, or should a trial attempt an intervention on multiple risk factors? Should one treat subjects based on a threshold of risk, or should the intervention be given to all patients regardless of their estimated vascular risk? In subjects with high vascular risk, should the intervention replace or be added to their current treatment? Should one limit interventions to medication therapies or should non-medication intervention also be considered (e.g., exercise or diet)?
These set of questions, by no means exhaustive, demonstrate the complexities of designing such a trial. Options should be considered after careful reflection among experts as definitive scientific evidence for these options is lacking. However, one cannot be certain these choices will necessarily avoid reaching an inconclusive result, even in a carefully designed trial. Before launching a large therapeutic trial of long duration, it may be a reasonable option to conduct several smaller proof of concept studies on a limited number of precisely defined subjects such as those individuals at highest risk for dementia associated with vascular risk factors. This was done, for example, in a recently published trial of patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, a model of subcortical vascular dementia.41 Similar trials could potentially be designed in high risk populations such as these which then could be then tested in broader populations if initial results provide promise. Such potential approaches represent the important initial steps that must be taken in order to prevent the common forms of dementia in years to come.
CONTRIBUTORS
C.T. planned the outline of the paper; A.V. and C.T. wrote the first draft; all authors contributed to the final version.
ACKNOWLEDGMENT
The authors thank Drs. Annick Alpérovitch, Steven M. Greenberg, and Joël Ménard for helpful discussions during the preparation of this manuscript.
Address correspondence and reprint requests to Dr. Christophe Tzourio, INSERM, Unit 708, Hôpital de la Salpêtrière, 47, boulevard de l'Hopital, 75651 Paris Cedex 13, France tzourio@chups.jussieu.fr or Dr. Anand Viswanathan, Stroke Service and Memory Disorders Unit, Neurology Clinical Trial Unit, 175 Cambridge Street, Suite 300, Boston, MA 02114 aviswanathan1@partners.org.
Supported by ARNEVA (Association de Recherche en Neurologie Vasculaire), Hôpital Lariboisière, Paris, France.
Disclosure: The authors report no disclosures.
Received July 1, 2008. Accepted in final form October 13, 2008.
REFERENCES
- 1.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001;322:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofman A, Ott A, Breteler M, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet 1997;349:151–154. [DOI] [PubMed] [Google Scholar]
- 3.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 4.Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol 2006;60:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol 2008;7:184–190. [DOI] [PubMed] [Google Scholar]
- 6.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 2001;154:635–641. [DOI] [PubMed] [Google Scholar]
- 7.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005;65:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mielke MM, Rosenberg PB, Tschanz J, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology 2007;69:1850–1858. [DOI] [PubMed] [Google Scholar]
- 9.de Leeuw FE, deGroot JC, Oudkerk M, et al. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol 1999;46:827–833. [DOI] [PubMed] [Google Scholar]
- 10.Liao DP, Cooper L, Cai JW, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: The ARIC study. Neuroepidemiology 1997;16:149–162. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: The cardiovascular health study. Stroke 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 12.Veldink JH, Scheltens P, Jonker C, Launer LJ. Progression of cerebral white matter hyperintensities on MRI is related to diastolic blood pressure. Neurology 1998;51:319–320. [DOI] [PubMed] [Google Scholar]
- 13.Bernick C, Kuller L, Dulberg C, et al. Silent MRI infarcts and the risk of future stroke: The cardiovascular health study. Neurology 2001;57:1222–1229. [DOI] [PubMed] [Google Scholar]
- 14.Fisher CM. Lacunar strokes and infarcts: a review. Neurology 1982;32:871–876. [DOI] [PubMed] [Google Scholar]
- 15.Kazui S, Levi CR, Jones EF, Quang L, Calafiore P, Donnan GA. Risk factors for lacunar stroke: a case-control transesophageal echocardiographic study. Neurology 2000;54:1385–1387. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke 2006;37:550–555. [DOI] [PubMed] [Google Scholar]
- 17.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005;112:1644–1650. [DOI] [PubMed] [Google Scholar]
- 18.Burton EJ, Kenny RA, O'Brien J, et al. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke 2004;35:1270–1275. [DOI] [PubMed] [Google Scholar]
- 19.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol 2000;47:145–151. [DOI] [PubMed] [Google Scholar]
- 20.Swan GE, Decarli C, Miller BL, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology 1998;51:986–993. [DOI] [PubMed] [Google Scholar]
- 21.Vermeer SE, Prins ND, denHeijer T, Hofman A, Koudstaal PJ, Breteler M. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 22.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Arch Neurol 2003;60:1707–1712. [DOI] [PubMed] [Google Scholar]
- 23.Ivan CS, Seshadri S, Beiser A, et al. Dementia after stroke: The Framingham Study. Stroke 2004;35:1264–1268. [DOI] [PubMed] [Google Scholar]
- 24.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet 1999;354:919–920. [DOI] [PubMed] [Google Scholar]
- 25.Heyman A, Fillenbaum GG, Welsh-Bohmer KA, et al. Cerebral infarcts in patients with autopsy-proven Alzheimer's disease: CERAD, part XVIII. Consortium to Establish a Registry for Alzheimer's Disease. Neurology 1998;51:159–162. [DOI] [PubMed] [Google Scholar]
- 26.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: The nun study. JAMA 1997;277:813–817. [PubMed] [Google Scholar]
- 27.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet 1999;354:919–920. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Olichney JM, Hansen LA, Hofstetter CR, Thal LJ. Small concomitant vascular lesions do not influence rates of cognitive decline in patients with Alzheimer disease. Arch Neurol 2000;57:1474–1479. [DOI] [PubMed] [Google Scholar]
- 29.Zekry D, Duyckaerts C, Moulias R, et al. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol (Berl) 2002;103:481–487. [DOI] [PubMed] [Google Scholar]
- 30.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol 2007;62:59–66. [DOI] [PubMed] [Google Scholar]
- 31.Longstreth WT, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly- The Cardiovascular Health Study. Stroke 2002;33:2376–2382. [DOI] [PubMed] [Google Scholar]
- 32.Jellinger KA. Understanding the pathology of vascular cognitive impairment. J Neurol Sci 2005;229–230:57–63. [DOI] [PubMed] [Google Scholar]
- 33.Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA 2004;292:2901–2908. [DOI] [PubMed] [Google Scholar]
- 34.Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc 2006;81:223–230. [DOI] [PubMed] [Google Scholar]
- 35.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 1992;42:473–480. [DOI] [PubMed] [Google Scholar]
- 36.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 37.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 38.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 39.Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med 2007;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black S, Roman GC, Geldmacher DS, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke 2003;34:2323–2330. [DOI] [PubMed] [Google Scholar]
- 41.Dichgans M, Markus HS, Salloway S, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol 2008;7:310–318. [DOI] [PubMed] [Google Scholar]
- 42.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet 2002;359:1283–1290. [DOI] [PubMed] [Google Scholar]
- 43.Orgogozo JM, Rigaud AS, Stoffler A, Mobius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300). Stroke 2002;33:1834–1839. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology 2003;61:479–486. [DOI] [PubMed] [Google Scholar]
- 45.Qiu CX, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499. [DOI] [PubMed] [Google Scholar]
- 46.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000;21:49–55. [DOI] [PubMed] [Google Scholar]
- 47.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA 1997;278:1349–1356. [PubMed] [Google Scholar]
- 48.Slooter AJ, Cruts M, Kalmijn S, et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: The Rotterdam study. Arch Neurol 1998;55:964–968. [DOI] [PubMed] [Google Scholar]
- 49.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimer's and Dementia 2007;3:186–191. [DOI] [PubMed] [Google Scholar]
- 50.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255–3264. [PubMed] [Google Scholar]
- 51.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998;352:1347–1351. [DOI] [PubMed] [Google Scholar]
- 52.Lithell H, Hansson L, Skoog I, et al. The Study on cognition and prognosis in the elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003;21:875–886. [DOI] [PubMed] [Google Scholar]
- 53.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Int Med 2003;163:1069–1075. [DOI] [PubMed] [Google Scholar]
- 54.Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) Study. Arch Int Med 2002;162:2046–2052. [DOI] [PubMed] [Google Scholar]
- 55.Qiu CX, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499. [DOI] [PubMed] [Google Scholar]
- 56.Staessen JA, Richart T, Birkenhager WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension 2007;49:389–400. [DOI] [PubMed] [Google Scholar]