Abstract
Neurogenesis persists in several regions of the adult mammalian brain. Although the hippocampus and olfactory bulb are most commonly studied in the context of adult neurogenesis, there is an increasing body of evidence in support of neurogenesis occurring outside of these two regions. The current study expands upon previous data by showing newborn neurons with a mature phenotype are located in several olfactory and limbic structures outside of the hippocampus and olfactory bulb, where we previously described DCX/BrdU immature neurons. Notably, newborn neurons with a mature neuronal phenotype are found in the olfactory tubercles, anterior olfactory nuclei, tenia tecta, islands of Calleja, amygdala and lateral entorhinal cortex. The appearance of newborn neurons with a mature phenotype in these regions suggests that these structures are destinations, and that newborn neurons are not simply passing through these structures. In light of the increasing body of evidence for neurogenesis in these, and other olfactory, limbic and striatal structures, we hypothesize that brain regions displaying adult neurogenesis are functionally linked.
Keywords: adult neurogenesis, rostral migratory stream, lesions, BrdU, NeuN, doublecortin
Introduction
There are two well-described neurogenic regions of the adult brain, the subventricular zone (SVZ) and the subgranular zone. Granule cells are generated in the subgranular zone of the dentate gyrus and migrate a short distance to the granule cell layer where they are integrated into existing hippocampal circuitry [1–4]. The SVZ is a much more robust and widespread neurogenic region in that newly generated neurons have been shown to migrate a much longer distance and in larger numbers to the olfactory bulb [5]. Functional studies of these newly generated neurons in the hippocampus and olfactory bulb have indicated that they are incorporated into the existing circuitry and have similar electrophysiological characteristics as the mature cells in these structures [4, 6–8]
Altman [9] provided initial evidence for adult neurogenesis in the SVZ using [3H] thymidine-labeling. Subsequently, numerous reports showed that there is a robust rostral migratory stream (RMS) from the SVZ to the olfactory bulb [10–14]. These previous studies have examined newly generated neurons emanating from the SVZ and along the RMS using neuron-specific molecular markers such as, Tuj1, TUC-4, PSA-NCAM, doublecortin (DCX) and Bcl-2 combined with the mitotic marker bromodeoxyuridine (BrdU) to confirm that these newborn cells are newly generated neurons [15–17]. Recent data have shown that neurons born in specific regions of the SVZ have an inherent program to migrate to specific areas of the olfactory bulb [18]. Thus, despite the popular belief that progenitor cells in the SVZ are “stem cells”, it appears as though the SVZ progenitor cells have a specific signal for where the cells they generate are intended to reside within specific regions of the olfactory bulb. Thus, cells that are migrating along the RMS appear to have a predetermined destination [18].
The fact that the newly generated neurons migrating along the RMS have a specific destination suggests that they have a specific function. Gheusi [19] and others have shown that newly generated neurons in the olfactory bulb are important for olfactory discrimination learning [19–21]. Moreover, Shapiro et al [22] confirmed that olfactory enrichment increases the number of newly generated neurons in the olfactory bulb [20] and also showed that olfactory enrichment enhances the differentiation of newborn neurons in the piriform cortex. In a separate paper, Shapiro et al [23] elucidated the migratory route of newly generated neurons originating from the most ventrocaudal portion of the SVZ, and showed their migration to the piriform cortex. It is interesting to note that Merkle et al [18] detected few if any cells in the olfactory bulb that originated from this most ventrocaudal portion of the lateral ventricle. Taken together, these data suggest that the final destination for the newly generated neurons arising from the progenitor cells located in this caudal portion of the SVZ is different than that for the other parts of the SVZ.
There are several lines of evidence showing that newly generated neurons derived from the SVZ migrate to numerous forebrain regions as reported for monkeys, rodents and rabbits. These include: the amygdala [24,25], striatum [26], piriform cortex [22–24,27,28] and the olfactory tubercles [23,24]. In addition, Yang et al. [17] examined newly generated neurons near the lateral ventricles in the adult mouse brain and found that a population of neurons also migrates dorsally to the corpus callosum and ventrally to the nucleus accumbens, ventromedial striatum, ventrolateral septum, and bed nucleus of the stria terminalis. Thus, there is accumulating evidence for the existence of newly generated neurons derived from the adult SVZ that migrate to other brain regions besides the olfactory bulb.
The goal of this study was to further describe the distribution of newly generated cells derived from the SVZ in several olfactory and limbic structures and to determine whether they differentiate into neurons. BrdU-labeling combined with double-labeling for immature and mature neuronal markers was used to elucidate the phenotype and morphology of these newborn cells. Laser-scanning confocal microscopy was used to confirm that the BrdU-label was contained within neurons and not within satellite cells. Lesions were made to interrupt the migratory pathway to some of these destinations to provide additional evidence that the populations of newly generated cells observed in some of these structures were not born locally.
Methods
BrdU Injections
Single BrdU injections
At 4h prior to sacrifice, a single BrdU injection (i.p. 100 mg/kg) was given to adult C57/Bl6 mice (2 months old) mice (N=4). This short time point was examined to determine if cells might be generated locally within the examined olfactory and limbic structures.
Daily BrdU injections
Male C57/Bl6 mice (2 months old; N =20) were used for this study. The mice were allowed access to food and water ad libitum and were kept on a normal 12 hr light/dark cycle. BrdU was injected i.p. (Sigma, St. Louis, Missouri, USA; 100mg per kg), once daily, beginning on day 1 and continuing to day 4. The mice were perfused a week after the last BrdU injection by first anesthetizing with xylazine and ketamine, followed by transcardial perfusions with 50 ml of 0.9% NaCl followed by 4% paraformaldehyde. Brains were post-fixed overnight and cryoprotected with 30% sucrose in 0.1 M phosphate buffer (PB, pH 7.4) for 48 hr, embedded in Tissue-Tek OCT (Sakura Finetek, Torrance, California, USA), and sectioned on a cryostat in the coronal plane at 40 μm. Sections were placed on slides or collected free-floating into an antifreeze solution and stored at −80° C.
Lesions
Lesions (N=4) were made in the RMS of adult CD-1 mice to determine the effect on neurogenesis in the piriform cortex and adjacent brain structures by assessing the number of DCX-labeled cells. Briefly, animals were first anesthetized using xylazine/ketamine (10 mg/kg) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The scalp was excised above the cranium, and a drill was used to cut a hole in the cranium. Then, a sterile #11 scalpel blade was inserted into the hole 2.90 mm deep to produce a lesion between the lateral ventricle and the rostral piriform cortex at −1.80 mm to −1.00 mm from bregma [29]. After suturing their skin, the animals were placed back into their home cages and their weight was monitored twice daily to ensure that they were eating and drinking, and that no infections or other adverse effects of the lesion occurred. The animals were allowed to survive for 21 days post-lesion, to ensure that the only DCX-labeled cells would be the ones that were born after the lesion [15]. Age-matched controls (N=4) that had the hole drilled into their skull, but with no lesion, were used for comparison. The animals were perfused as described above. Analysis of DAB reacted DCX-labeled tissue was performed using a brightfield microscope (Carl Zeiss, Inc, Thornwood, NY, USA).
Immunohistochemistry
For DCX/BrdU- and NeuN/BrdU-double-labeling, every sixth 40 μm section was examined. The protocol for immunostaining was the same as that previously described [22]. This double labeling procedure was used to identify whether BrdU-labeled cells had an immature, or mature neuronal phenotype.
Laser-scanning confocal microscopy
Fluorescent-tagged secondary antibodies were used to allow simultaneous visualization of immunoreaction product using 2 separate laser channels of a confocal scanning microscope (Olympus, Tokyo, Japan). These channels were sequentially scanned to avoid cross-excitation. This method was employed to verify that the BrdU-labeled nucleus was contained within the NeuN-labeled or DCX-labeled perikaryal cytoplasm, and not within satellite cells, as previously suggested for cortical BrdU-labeled cells [30].
Results
The results from the current study using DCX/BrdU preparations support previous studies that newly born neurons are found in several olfactory/limbic forebrain regions besides the hippocampus, piriform cortex and olfactory bulb. In addition, the results from the NeuN/BrdU preparations extend upon previous findings and suggest that the newly born neurons in these brain regions develop a mature phenotype. The results from lesions ventral to the elbow of the RMS showing a decreased number of DCX-labeled cells in the piriform cortex ipsilateral to the lesion support the idea that the newborn neurons in this region are migrating from the SVZ.
Olfactory/limbic forebrain regions have newly generated neurons with immature neuronal phenotype
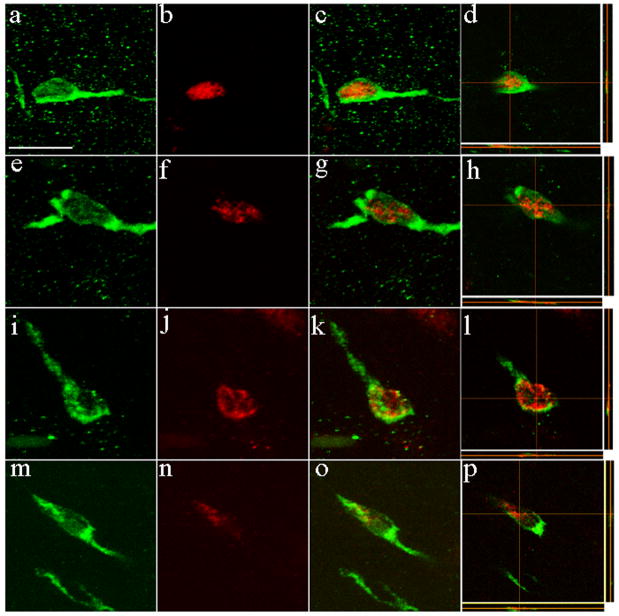
DCX/BrdU double-labeled cells were observed in other gray matter regions of the adult rodent forebrain besides the piriform cortex and olfactory bulb. These included: the striatum (Fig. 1i), the lateral entorhinal cortex (Fig. 1j), olfactory tubercle (Fig. 1k), amygdalapiriform area, amygdalohippocampal area, presubiculum, parasubiculum, subiculum, islands of Calleja, tenia tecta, anterior olfactory nucleus and the accessory olfactory bulb (some data not shown). DCX/BrdU double-labeled cells were also found in multiple (e.g., anterior, basolateral, basomedial) amygdaloid nuclei (Fig. 1h). These double-labeled cells were relatively small (6–10 μm), had a thin shell of perikaryal cytoplasm and were mostly bi-polar or multi-polar. Their processes were typically less than 20 μm in length and were not usually branched. The DCX/BrdU double-labeled cells observed in the striatum had nuclear sizes of 7–10 μm and had thin shells of perikaryal cytoplasm, consistent with previous data from Dayer et al. (26). Their processes were typically unbranched and less than 15 μm in length (Fig. 1j). The DCX/BrdU double-labeled cells found in the islands of Calleja, olfactory tubercle and anterior olfactory nucleus were typically unipolar or bipolar in appearance, and their processes were less than 20 μm in length. No such cells were found in any of the regions examined at 4 hours after the BrdU-injection.
Figure 1.
Confocal Z-stack images of DCX/BrdU double-labeled cells in several telencephalic structures in the adult mouse brain. The images are organized such that the DCX immunolabeling (green) is shown in the first column, the BrdU immunolabeling (red) is found in the second column, the merged image is in the third column and the last column shows a modified merged image with cross hairs to indicate where the orthogonal images (thin rectangular panels to the right and at the bottom) were obtained. (a–d) basolateral amygdaloid nucleus, (e–h) lateral entorhinal cortex, (i–l) striatum, (m–p) olfactory tubercle. Note that these cells have a relatively small (less than 5 μm) nucleus, a thin shell of perikaryal cytoplasm, and have only 1 or 2 rudimentary processes. Scale bar in a represents 6 μm for (a–d); 5μm for (e–h); 4 μm for (i–l); 6 μm for (m–p).
Lesion Study of the RMS and DCX-labeled cells in the piriform cortex
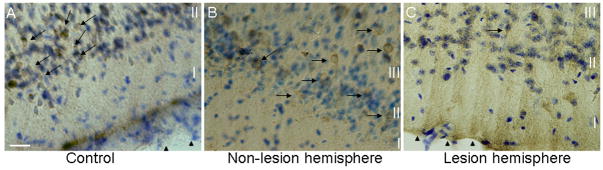
Data from mice with lesions of the RMS showed an almost complete absence of DCX-labeled cells in the rostral portion of the ipsilateral piriform cortex of lesioned mice, as compared to non-lesioned control mice (Fig. 2). Thus, intersecting the RMS from the rostral portion of the piriform cortex reduces the number of DCX-labeled cells in this region. In addition, the rostral piriform cortex contra-lateral to the lesioned hemisphere showed an increased number of DCX-labeled cells (Fig. 2).
Figure 2.
Bright-field micrographs of DCX-immunolabeled and Nissl counterstained sections in the piriform cortex from lesioned and non-lesioned mice. In A, DCX-labeled cells (arrows) in the piriform cortex of a control mouse are shown. Note the pial surface (arrowheads) in the lower right portion of the micrograph. In B, DCX-labeled cells (arrows) are shown from the piriform cortex non-lesioned hemisphere of a mouse that received a lesion of the RMS on the contra-lateral side. Note that DCX-labeled cells are abundant. In C, the piriform cortex is shown in the hemisphere ipsilateral to the lesion. There are reduced numbers of DCX-labeled cells (arrow) in the piriform cortex at 21 days after a lesion bisecting the RMS. Note the pial surface (arrowheads) in the lower left corner. Scale bar in A = 25 μm for all three panels.
Olfactory/limbic forebrain regions have newly generated neurons with mature neuronal phenotype
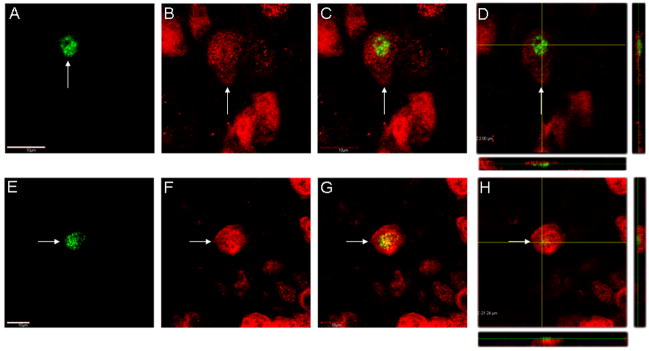
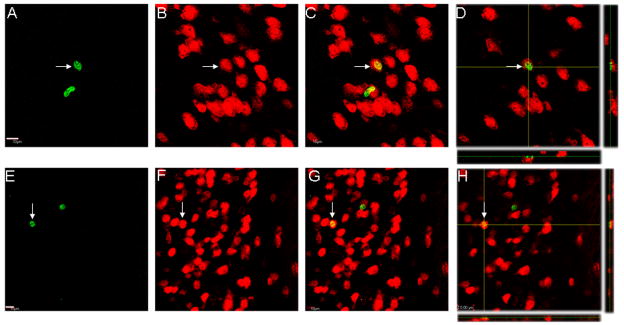
NeuN/BrdU double-labeled cells were found in brain regions outside the olfactory bulb and hippocampus and included several regions where DCX/BrdU-labeled cells were observed. These regions include: anterior olfactory nucleus (Fig. 3), lateral to the olfactory ventricle, the insula Calleja magna complex (Fig. 3) located along the most medial portion of the mouse brain, the ventral tenia tecta (not shown) the deep layers (layers V & VI) of the lateral entorhinal cortex (Fig. 4) and several amygdaloid nuclei, including the basolateral nucleus and the central amygdaloid nucleus (Fig. 4). Laser-scanning confocal microscopy confirmed that the BrdU-labeling was located within the NeuN-labeled cell body. Orthogonal views further confirmed these observations (Figs. 3D,H and 4D,H). It is pertinent to note that in the olfactory tubercles, of 57 BrdU-labeled cells examined, only 1 (< 2%) was confirmed to be double-labeled for NeuN. Alternatively, in the other structures examined, ~5–10% of the BrdU-labeled cells were double-labeled for NeuN (minimum of 25 BrdU-labeled cells examined per region). Thus, NeuN/BrdU-labeled cells are found throughout several regions of the rostral/caudal extent of the adult mouse brain at 11 days after the first of 4 daily BrdU-injections.
Figure 3.
Confocal Z-stack and orthogonal images of NeuN/BrdU double-labeled cells in the anterior olfactory nuclei and islands of Calleja. In A–D, a NeuN(red)/BrdU(green) double-labeled cell (arrow) is shown from the dorsal portion of the anterior olfactory nuclei. The merged view is shown in C and the orthogonal view through the middle of the cell is shown in D. In E–H, a NeuN(red)/BrdU(green) double-labeled cell(arrow) is shown from the most medial portion of the islands of Calleja, also known as the insula Calleja magna complex. The merged view is shown in G and the orthogonal view through a portion of the cell body is shown in H. Scale bar in A = 10 μm for A–D. Scale bar in E = 10 μm for E–H.
Figure 4.
Confocal Z-stack and orthogonal images of NeuN/BrdU double-labeled cells in the limbic system. In A–D, a NeuN(red)/BrdU(green) double-labeled cell(arrow) is shown from the central amygdaloid nuclei. The merged view is shown in C and the orthogonal view through the center of the cell is shown in D. Note that there is another BrdU-labeled cell that is not doubled-labeled for NeuN. In E–H, a NeuN(red)/BrdU(green) double-labeled cell (arrow) is shown from the lateral entorhinal cortex. The merged view is shown in G and the orthogonal view through this cell body is shown in H. Note that there is another BrdU-labeled cell that is not doubled-labeled for NeuN. Scale bar in A = 10 μm for A–D. Scale bar in E = 10 μm for E–H.
Discussion
The results from this study show that newborn neurons with a mature phenotype are found in several olfactory and limbic regions that were previously reported to contain newly born neurons derived from the SVZ. This suggests that these brain regions where NeuN/BrdU-labeled cells are found represent destinations for these newly born neurons and not simply a reflection of their being in transit to another brain region. The functional significance for adult neurogenesis in these olfactory and limbic brain structures remains elusive.
Putative role of adult neurogenesis in the olfactory system
Data from the current study and others show that adult neurogenesis has been observed in key regions of the olfactory system. In rodents, lagomorphs and non-human primates, olfaction is still one of the predominant senses. The fact that the neural epithelium in the nasal mucosa consists of a local pool of constantly regenerating neurons [31] may provide a clue as to why neurogenesis occurs in brain structures that are part of the olfactory system. The axons from the continually regenerating olfactory receptor cells project to the olfactory bulb, where a robust pool of newly generated neurons occurs that is derived from the SVZ via the RMS [5]. It should be noted that evidence is mounting for the existence of this pathway in humans [32–34]. In addition, Rochefort et al. [20] have shown in adult mice that olfactory enrichment enhances the survival and differentiation of newborn neurons in the olfactory bulb, and it has been shown that newborn neurons in the olfactory bulb are important for olfactory discrimination learning [19]. Thus, newborn neurons in the olfactory bulb are functionally integrated into the existing olfactory circuitry indicating that they contribute to the modulation of the olfactory bulb’s output, the lateral olfactory tract.
The axons of the mitral cells of the olfactory bulb make up the lateral olfactory tract and their major projection is to the olfactory cortex, comprised mainly of the anterior olfactory nuclei, olfactory tubercle, tenia tecta and piriform cortex [35]. The fact that neurogenesis has been detected in these regions, albeit at relatively lower levels than that found in the olfactory bulb [22–24,36], suggests that newborn neurons in these regions might be involved in processing the signals encoded by the recently generated neurons in the olfactory bulb. It should be noted that neurons with hilar basal dendrites born after seizures in the dentate gyrus, have recently been modeled to show that just a few neurons integrated into the dentate circuitry can have profound effects on the excitability of the hippocampus (37). Thus, newly generated mature neurons observed in several olfactory structures receiving lateral olfactory tract input might play a role in the dynamic plasticity of the olfactory system.
Putative relationship between olfactory and limbic system neurogenesis
Similar to olfaction, the limbic system is phylogenetically one of the oldest systems in mammals. Interestingly, the lateral olfactory tract also sends projections to two key components of the limbic system, the amygdala and the entorhinal cortex [38,39]. The latter structure has a direct projection to the hippocampus, a structure with newly generated neurons that mature to become functional granule cells [6]. The fact that the data from the present study show newly generated mature neurons in the lateral entorhinal cortex and amygdala demonstrates a neurogenic link between the olfactory and limbic systems.
We propose that neurogenesis in each of these limbic structures plays a role in processing olfactory data. First, the hippocampus is anatomically situated to consolidate olfactory and emotional information, and functional data support this idea [35,40–46]. Second, the amygdala is known to be involved in fear response [47], and this structure may need to remain highly plastic in order to attach the appropriate emotional response to continuously varying stimuli, including olfactory stimuli. Indeed, fear conditioning has been shown to be dependent on newborn neurons in the hippocampus [48,49]. Considering the reliance that lower mammals, such as rodents, have on olfaction, it is obligatory that olfactory information is encoded with an emotional component. This is supported by the idea that the amygdala receives extensive projections from the olfactory cortex [50]. Third, the entorhinal cortex is another limbic structure that is anatomically linked to the olfactory system [39,51–53] in that it shares reciprocal connections with the piriform cortex [54–56]. Moreover, the entorhinal cortex sends a major projection to the dentate gyrus and amygdala [43,53,57]. Thus, the common thread amongst the structures where newly generated mature neurons are found in the adult brain is that they populate mainly limbic and olfactory structures. Therefore, we hypothesize that newborn neurons in olfactory and limbic structures are involved in synchronizing novel olfactory and emotional information.
Striatal adult neurogenesis and its putative function
An obvious exception to our proposed hypotheses is the presence of adult neurogenesis in the striatum [26,58]. It is pertinent to note that the islands of Calleja are considered to be a part of the striatal system [59–61]. The islands of Calleja are in the rostral portion of the rodent forebrain, situated very close to many olfactory structures. Moreover, it has been shown that the striatum forms reciprocal connections with several olfactory and limbic structures [62–64]. Considering the basic biological drives of feeding and reproduction, mammals have an inherent need to process an adequate response to novel olfactory and emotional stimuli. Therefore, the newborn neurons in the striatum and islands of Calleja might be involved in encoding the appropriate reward component to novel olfactory and emotional information, which is then consolidated into memories by the hippocampus, thus providing a link between the neurogenic regions of the adult brain.
Conclusion
The results from this study support previous data showing newborn neurons in multiple regions throughout the adult brain. This study also extends these findings by showing that mature newborn neurons are also found in these regions as indicated by double-labeling for NeuN and BrdU. Hypotheses are posed in an attempt to consolidate the existing literature on adult neurogenesis into a cogent theory for the putative role of neurogenesis in many regions of the adult brain. Future studies will need to simultaneously examine several neurogenic regions following olfactory sensory manipulations to obtain functional data for newborn neurons in the adult brain.
Supplementary Material
Supplemental movie 1 – The basal lateral nucleus of the amygdala. Two BrdU-labeled nuclei (green) are shown in the upper left panel. The upper right panel depicts a field of NeuN-labeled cells (Red). The lower left panel is a merged view of the BrdU and NeuN panels. Note that the upper BrdU-labeled cell is a satellite cell, but the lower BrdU cell is clearly contained within the NeuN-labeling.
Supplemental movie 2 – The ventral anterior olfactory nucleus. A brightly (top) and faintly- (Bottom) labeled BrdU-nuclei (green) are shown in the upper left panel. The upper right panel depicts a field of NeuN-labeled cells (Red). The lower left panel is a merged view of the BrdU and NeuN panels. Note that the brightly-labeled BrdU cell on the top of the image is contained within the NeuN-label.
Supplemental movie 3 – Islands of Calleja. A BrdU-labeled nuclei (green) is shown in the upper left panel. The upper right panel depicts NeuN-labeled cells (Red). The lower left panel is a merged view of the BrdU and NeuN panels. Note that this cell was cut in half by the sectioning, thus allowing for the visualization of the BrdU-labeled nuclei within the NeuN-labeled cell. Note that if the movie is paused at about 90 degrees rotation, the indentations inside of the NeuN-labeled cell where the BrdU-labeled nuclei is positioned can be visualized.
Acknowledgments
We acknowledge the technical support of Dr. Mathew Blurton-Jones for the doublecortin confocal images.
Role of the funding source The authors wish to acknowledge funding from NIH grant R01-NS38331 (to C.E.R.). This funding source had role or influence in the study design, collection, analysis, interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Conflict of interest No conflict of interest of any kind exists for any of the authors of this manuscript and the author’s, or the author’s institution has no financial or other relationship with other people or organizations that may inappropriately influence this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shapiro LA, Korn MJ, Shan Z, Ribak CE. GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res. 2005;1040:81–91. doi: 10.1016/j.brainres.2005.01.098. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro LA, Upadhyaya P, Ribak CE. Spatiotemporal profile of dendritic outgrowth from newly born granule cells in the adult rat dentate gyrus. Brain Res. 2007;1149:30–37. doi: 10.1016/j.brainres.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–34. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 4.Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–07. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–86. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–34. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laplagne DA, Kamienkowski JE, Espósito MS, Piatti VC, Zhao C, Gage FH, Schinder AF. Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci. 2007;25:2973–81. doi: 10.1111/j.1460-9568.2007.05549.x. [DOI] [PubMed] [Google Scholar]
- 8.Grubb MS, Nissant A, Murray K, Lledo PM. Functional maturation of the first synapse in olfaction: development and adult neurogenesis. J Neurosci. 2008;28:2919–32. doi: 10.1523/JNEUROSCI.5550-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb . J Comp Neurol. 1969;137:433–58. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 10.Bayer SA. 3H-Thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–40. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- 11.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 12.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–48. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 13.Luskin MB, Boone MS. Rate and pattern of migration of lineally-related olfactory bulb interneurons generated postnatally in the subventricular zone of the rat. Chem Senses. 1994;19:695–714. doi: 10.1093/chemse/19.6.695. [DOI] [PubMed] [Google Scholar]
- 14.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–81. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 15.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 17.Yang HK, Sundholm-Peters NL, Goings GE, Walker AS, Hyland K, Szele FG. Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. J Neurosci Res. 2004;76:282–95. doi: 10.1002/jnr.20071. [DOI] [PubMed] [Google Scholar]
- 18.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–84. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 19.Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA. 2000;97:1823–28. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–89. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–54. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro LA, Ng KL, Zhou QY, Ribak CE. Olfactory enrichment enhances the survival of newly born cortical neurons in adult mice. NeuroReport. 2007;18:981–85. doi: 10.1097/WNR.0b013e3281532bc1. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro LA, Ng KL, Kinyamu R, Whitaker-Azmitia P, Geisert EE, Blurton-Jones M, Zhou QY, Ribak CE. Origin, migration and fate of newly generated neurons in the adult rodent piriform cortex. Brain Struct Funct. 2007;212:133–48. doi: 10.1007/s00429-007-0151-3. [DOI] [PubMed] [Google Scholar]
- 24.Bedard A, Levesque M, Bernier PJ, Parent A. The rostral migratory stream in adult squirrel monkeys: contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2. Eur J Neurosci. 2002;16:1917–24. doi: 10.1046/j.1460-9568.2002.02263.x. [DOI] [PubMed] [Google Scholar]
- 25.Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA. 2002;99:11464–69. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–27. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luzzati F, Peretto P, Aimar P, Ponti G, Fasolo A, Bonfanti L. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc Natl Acad Sci USA. 2003;100:13036–41. doi: 10.1073/pnas.1735482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Marchis S, Fasolo A, Puche AC. Subventricular zone-derived neuronal progenitors migrate into the subcortical forebrain of postnatal mice. J Comp Neurol. 2004;476:290–300. doi: 10.1002/cne.20217. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- 30.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–73. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calof AL, Hagiwara N, Holcomb JD, Mumm JS, Shou J. Neurogenesis and cell death in olfactory epithelium. J Neurobiol. 1996;30:67–81. doi: 10.1002/(SICI)1097-4695(199605)30:1<67::AID-NEU7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 32.Bedard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Dev Brain Res. 2004;151:159–68. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Sanai N, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science. 2007:318–93. doi: 10.1126/science.318.5849.393a. [DOI] [PubMed] [Google Scholar]
- 34.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtås S, van Roon-Mom WM, Björk-Eriksson T, Nordborg C, Frisén J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–49. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter MB. Human neuroanatomy. 7. Baltimore: Williams & Wilkins; 1976. [Google Scholar]
- 36.Pekcec A, Loscher W, Potschka H. Neurogenesis in the adult rat piriform cortex. NeuroReport. 2006;17:571–74. doi: 10.1097/00001756-200604240-00003. [DOI] [PubMed] [Google Scholar]
- 37.Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci USA. 2008;105:6179–84. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Olmos JS, Beltramino CA, Alheid G. Amygdala and extended amygdala of the rat: A cytoarchitecttonical, fibrotectonical, and chemotectonical survey. In: Paxinos G, editor. The rat nervous system. London: Elsevier Academic Press; 2004. pp. 509–603. [Google Scholar]
- 39.Shipley MT, Ennis M, Puche AC. Olfactory system. In: Paxinos G, editor. The rat nervous system. London: Elsevier Academic Press; 2004. pp. 923–64. [Google Scholar]
- 40.Schwerdtfeger WK, Buhl EH, Germroth P. Disynaptic olfactory input to the hippocampus mediated by stellate cells in the entorhinal cortex. J Comp Neurol. 1990;292:163–77. doi: 10.1002/cne.902920202. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez P, Lipton PA, Melrose R, Eichenbaum H. Differential effects of damage within the hippocampal region on memory for a natural, nonspatial Odor-Odor Association. Learn Mem. 2001;8:79–86. doi: 10.1101/lm.38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaut KP, Bunsey MD, Riccio DC. Olfactory learning and memory impairments following lesions to the hippocampus and perirhinal-entorhinal cortex. Behav Neurosci. 2003;117:304–19. doi: 10.1037/0735-7044.117.2.304. [DOI] [PubMed] [Google Scholar]
- 43.Gnatkovsky V, Uva L, de Curtis M. Topographic distribution of direct and hippocampus-mediated entorhinal cortex activity evoked by olfactory tract stimulation. Eur J Neurosci. 2004;20:1897–1905. doi: 10.1111/j.1460-9568.2004.03627.x. [DOI] [PubMed] [Google Scholar]
- 44.Hjorth-Simonsen A. Projection of the lateral part of the entorhinal area to the hippocampus and fascia dentata. J Comp Neurol. 1972;146:219–32. doi: 10.1002/cne.901460206. [DOI] [PubMed] [Google Scholar]
- 45.Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- 46.Stäubli U, Le TT, Lynch G. Variants of olfactory memory and their dependencies on the hippocampal formation. J Neurosci. 1995;15:1162–71. doi: 10.1523/JNEUROSCI.15-02-01162.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 49.Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- 50.Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- 51.Room P, Groenewegen HJ, Lohman AH. Inputs from the olfactory bulb and olfactory cortex to the entorhinal cortex in the cat. I. Anatomical observations. Exp Brain Res. 1984;56:488–96. doi: 10.1007/BF00237989. [DOI] [PubMed] [Google Scholar]
- 52.Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: III. Subcortical afferents. J Comp Neurol. 1987;264:396–408. doi: 10.1002/cne.902640307. [DOI] [PubMed] [Google Scholar]
- 53.Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. London: Elsevier Academic Press; 2004. pp. 635–704. [Google Scholar]
- 54.Chapman A, Racine RJ. Piriform cortex efferents to the entorhinal cortex in vivo: kindling-induced potentiation and the enhancement of long-term potentiation by low-frequency piriform cortex or medial septal stimulation. Hippocampus. 1997;7:257–70. doi: 10.1002/(SICI)1098-1063(1997)7:3<257::AID-HIPO2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 55.Insauti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: Cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus. 1997;7:146–83. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 56.Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 57.Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review Ann N Y Acad Sci. 2000;911:369–91. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 58.Fallon J, Reid S, Kinyamu R, Opole I, Opole R, Baratta J, Korc M, Endo TL, Duong A, Nguyen G, Karkehabadhi M, Twardzik D, Patel S, Loughlin S. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci USA. 2000;97:14686–91. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ribak CE, Fallon JH. The island of Calleja complex of rat basal forebrain. I. Light and electron microscopic observations. J Comp Neurol. 1982;205:207–18. doi: 10.1002/cne.902050302. [DOI] [PubMed] [Google Scholar]
- 60.Fallon JH, Loughlin SE, Ribak CE. The islands of Calleja complex of rat basal forebrain. III. Histochemical evidence for a striatopallidal system. J Comp Neurol. 1983;218:91–120. doi: 10.1002/cne.902180106. [DOI] [PubMed] [Google Scholar]
- 61.Heimer L, Zaborszky L, Zahm DS, Alheid GF. The ventral striatopallidothalamic projection: I. The striatopallidal link originating in the striatal parts of the olfactory tubercle. J Comp Neurol. 1987;255(4):571–91. doi: 10.1002/cne.902550409. [DOI] [PubMed] [Google Scholar]
- 62.Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–38. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- 63.Santiago AC, Shammah-Lagnado SJ. Efferent connections of the nucleus of the lateral olfactory tract in the rat. J Comp Neurol. 2004;471:314–32. doi: 10.1002/cne.20028. [DOI] [PubMed] [Google Scholar]
- 64.Ubeda-Bañon I, Novejarque A, Mohedano-Moriano A, Pro-Sistiaga P, de la Rosa-Prieto C, Insausti R, Martinez-Garcia F, Lanuza E, Martinez-Marcos A. Projections from the posterolateral olfactory amygdala to the ventral striatum: neural basis for reinforcing properties of chemical stimuli. BMC Neurosci. 2007;8:103. doi: 10.1186/1471-2202-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental movie 1 – The basal lateral nucleus of the amygdala. Two BrdU-labeled nuclei (green) are shown in the upper left panel. The upper right panel depicts a field of NeuN-labeled cells (Red). The lower left panel is a merged view of the BrdU and NeuN panels. Note that the upper BrdU-labeled cell is a satellite cell, but the lower BrdU cell is clearly contained within the NeuN-labeling.
Supplemental movie 2 – The ventral anterior olfactory nucleus. A brightly (top) and faintly- (Bottom) labeled BrdU-nuclei (green) are shown in the upper left panel. The upper right panel depicts a field of NeuN-labeled cells (Red). The lower left panel is a merged view of the BrdU and NeuN panels. Note that the brightly-labeled BrdU cell on the top of the image is contained within the NeuN-label.
Supplemental movie 3 – Islands of Calleja. A BrdU-labeled nuclei (green) is shown in the upper left panel. The upper right panel depicts NeuN-labeled cells (Red). The lower left panel is a merged view of the BrdU and NeuN panels. Note that this cell was cut in half by the sectioning, thus allowing for the visualization of the BrdU-labeled nuclei within the NeuN-labeled cell. Note that if the movie is paused at about 90 degrees rotation, the indentations inside of the NeuN-labeled cell where the BrdU-labeled nuclei is positioned can be visualized.