Abstract
To explain the pattern of preserved and superior abilities found in autism spectrum disorders, a hypothesis has emerged, which assumes that there is a developmental bias towards the formation of short-range connections. This would result in excessive activity and overconnectivity within susceptible local networks. These networks might become partially isolated and acquire novel functional properties. In turn, this would affect the formation of long-range circuits and systems governing top-down control and integration. Despite many tantalizing clues, mechanisms relating pathogenesis and altered cell function to the ‘disconnection’ of integrative and focal activity remain obscure. However, recent post-mortem studies of brains of individuals with autism have shown characteristic differences in the morphometry of radial cell minicolumns, which add credence to the connectivity hypothesis.
Keywords: autistic disorder, cortical minicolumn, cytoarchitecture
1. Introduction
Autism spectrum disorders (ASDs) comprise a heterogeneous array of conditions with seemingly dissociable phenotypes. In this special issue, the focus is on the phenotype that is characterized by uneven and sometimes superior ability in specific skills. We believe that a recently emerging hypothesis regarding brain organization and its disruption can throw some admittedly speculative light on the development of this phenotype. In recent years, a new perspective has emerged postulating systematic disruption of the control or coordination of specialized networks distributed throughout the cortex (Buxhoeveden & Casanova 2002). This approach assumes that there are hierarchical or distributed circuit and network interactions. In the case of autism, these appear to be disrupted. Thus we might expect to see altered processes of self-organization and integration in cortical networks at multiple levels of function and phases of development. Current biological and genetic research speculatively suggests that autism involves disruptions of synapse development and function. But how is one to imagine the nature of such disruptions? One possibility is that mechanisms susceptible to genetic or epigenetic disruption are affected at early neuroproliferative stages. This disruption would have widely distributed and compounded effects on later development. The integration of affected neurons within local circuits and, in turn, the incorporation of local circuits into larger self-organizing cortical networks, would amplify the effects of perturbed mechanisms. One way to understand the mechanisms involved is to look at the structures that involve multiples of neurons working together in a concerted fashion, so-called minicolumns.
2. Autism as a ‘minicolumnopathy’
Minicolumns are radially oriented arrangements of cellular elements, which have a stereotypical morphometry and are distributed throughout the cortex. They share common input–output operations mediated by recurrent circuits linking translaminar columns of pyramidal neurons (Mountcastle 1997; Buxhoeveden & Casanova 2002; DeFelipe 2005). These modules have commonly been considered to represent a canonical microcircuit contained within a defined cylindrical volume, and linked by specified patterns of connections within uniform modular arrays distributed throughout all areas of the neocortex.
Recent studies have shown equivalent alignment and spacing in pairwise comparisons between pyramidal cell columns, their associated apical dendrites and myelinated axon bundles, and aligned radial processes of certain interneurons containing the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). These cell elements are grouped in identical arrangements corresponding to minicolumns, which in turn are distributed within arrays that vary by cortical area in their distribution of minicolumns and cell composition (Ballesteros Yáñez et al. 2005; DeFelipe 2005).
GABA interneurons are varied in their molecular content, electrophysiological properties, morphology and distribution patterns within cortex, and are organized within circuits that are adapted to dynamically regulate the output of their associated pyramidal cell columns. GABA neurons however can be classified as one of a group of common inhibitory cell types, each defined by a particular combination of these properties (Markram et al. 2004). For example, most GABA neurons are immunoreactive for one of three calcium-binding proteins: parvalbumin (PV); calbindin (CB); or calretinin (CR). CR neurons are widely distributed in supragranular layers, and have processes that may extend radially across several layers and are regularly spaced in a radial columnar cytoarchitecture distributed throughout all cortical areas in monkeys and humans (Gabbott & Bacon 1996; Peters & Sethares 1997; Gabbott 2003; Ballesteros Yáñez et al. 2005). PV-positive neurons, by contrast, have predominantly tangentially oriented axonal processes that synapse on cell bodies of projection neurons and whose synchronized fast-spiking activity serves to coordinate the output of local minicolumnar groupings (Galarreta & Hestrin 2001b).
The output of local circuits of pyramidal cell columns is regulated by the phasic activity of networks of GABA neurons within minicolumns and across minicolumn networks. GABA neurons are functionally linked within subtype-specific networks or by characteristic patterns of connection with other subtypes of neurons (Gibson et al. 1999; Galarreta & Hestrin 2001a). Certain GABA neurons may transition between the phases of oscillatory activity at different frequencies and entrain their activity with networks of other GABA neuron subtypes.
Output in beta (15–30 Hz) and lower frequency bands is associated with longer range interareal transmission, as the long cycle length allows for the integration of a variety of inputs with a broader range of signal timing variation (Kopell et al. 2000). CR+ interneurons were shown to form a coherent oscillatory network in the beta-frequency band, which transiently coupled to gamma-band frequency output of tangential FS basket cell networks in deep layer III/IV (Szabadics et al. 2001; Beierlein et al. 2003). Relevant to the discussion of autism, beta-band activity has been shown to coordinate functional activity across frontoparietal networks involved in top-down visual attention activity (Schnitzler & Gross 2005) as well as sensory gating (Kisley & Cornwell 2006; Hong et al. 2008).
3. Altered minicolumnar morphometry in autism
Minicolumnar cytoarchitecture shows systematic differences in the comparisons of post-mortem cortical tissue series of ASD and control cases (Casanova et al. 2002a,b, Casanova et al. 2006a,b). Minicolumns were found to be narrower in all areas examined with the greatest decrease found in BA 9. No difference was found in the core width of pyramidal cell columns, indicating that reduced minicolumnar width reflected primarily the loss of peripheral neuropil (figure 1). The number of minicolumns in each frame was increased. Cell dispersion was greater in the test series, indicating a decrease in the alignment of pyramidal cells along the core axis and possible disorganization of local circuits within the minicolumn core. In addition, the volume density of pyramidal cell bodies measured by the grey level index (GLI) showed an increase in the amplitude difference across axis-to-axis intervals. This suggested to us that there was an increased delimitation of the minicolumnar core periphery and loss of directly adjacent peripheral neuropil, including proximal dendrite segments.
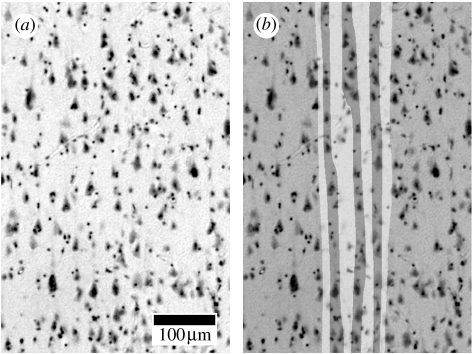
Figure 1.
The appearance of different compartments of the minicolumns under the microscope is shown. Here a tissue slice (a) taken from the inferior orbital gyrus in the human brain and (b) Nissl stained and processed with a method known as the grey level index, or GLI (Schlaug et al. 1995). Minicolumnar cores (dark bands) comprise mostly pyramidal cells. These are surrounded by peripheral neuropil (light bands), comprising interneurons and also neural processes not visible in Nissl stains.
As overall GLI data showed no interaction by group, increased numbers of minicolumns and equivalent cell spacing within minicolumnar cores implied that pyramidal cells within each column must be, in some combination, smaller or fewer in number. These results suggest possible developmental disruptions in the formation of minicolumnar microcircuits. It remains to be seen how these findings might relate to pathophysiological processes underlying ASD phenotypes. Perhaps the reduced volume of peripheral neuropil indicates reductions in the numbers of radially oriented GABA neurons or in the extent of their axonal and dendritic processes.
4. Development of area hierarchies
V1 is an area in the occipital part of the brain, which is vital to visual processing. Interposed minicolumn networks in V1 constitute stimulus-response maps, i.e. retinotopic location, frequency, orientation, contrast, direction of motion, etc. Ordered patterns of the activity of various stimulus maps are integrated in associated areas selective for higher order feature representations, including geometric motifs, facial or body features and spatial relationships. Feature extraction is modulated by the coordinate activity in other sense modalities and top-down input providing semantic or contextual, emotional and goal-related information. Interaction of local groups of minicolumns and their intrinsic interconnection by collaterals and interneurons have adapted to be responsive to invariant properties of the environment, which have significance for reproductive success. Thus, wiring patterns are highly constrained between V1 and extrastriate areas associated with face recognition (Batardière et al. 2002; Kennedy et al. 2007).
Disrupted patterns of coordinated oscillatory output in distributed minicolumn networks might be associated with cortical disconnectivity in autism. Likewise, altered oscillatory activity in developing cortical circuits may contribute to impaired development of intra-areal and transcortical connections. Oscillatory output of GABA neurons in the beta-frequency range guides early stage development of radial columnar circuits of pyramidal and radial interneurons. Synchronized GABAergic inhibitory output results in a brief period of increased sub-threshold depolarization during which correlated firing of presynaptic spikes may generate a postsynaptic action potential, thereby strengthening the weights of the participating synapses. This mechanism of spike timing-dependent plasticity results in the strengthening of synapses contributing to selected circuits and the elimination of weaker or discordant synapses. This activity serves to specify and refine both local and long-distance connections according to the oscillatory output frequency generated by specific GABA interneuron circuits.
Conversely, the loss of peripheral neuropil inhibition mediated by GABA neurons that are responsive to CR and somatostatin might alter local as well as long-distance anatomical and functional connectivity (Courchesne & Pierce 2005). Collateral excitation of neighbouring minicolumns would be increased, introducing additional noise into each discrete minicolumnar circuit and degrading the specificity and resolution of activity within differentially activated networks of minicolumns. Excitatory cross-activation among local networks of minicolumns would also sustain and grow intrinsic collateral connections between distributed segments of a feature map as well as between minicolumns in adjoining feature maps. This might possibly provide an enhanced or novel mechanism for local feature binding.
Lack of refinement of feedback connections could be the basis for synaesthesia (Hubbard & Ramachandran 2005). Synaesthesia is relevant to considerations of the savant syndrome. For example, Daniel Tammet relates in his autobiography (Tammet 2006) that his synaesthesia is vital to his ability to remember numbers and to carry out calculations. In an attempt to explain savant talent, Mottron and colleagues suggested that there may be increased local activity in specialized local or grapheme areas owing to environmental access/interest (Mottron et al. 2006). This could perhaps be explained by assuming that critical periods of synaptic plasticity affect adjoining areas (i.e. V4/V8) as a consequence of bias towards the activity of short-range connections. Furthermore, in visual cortex, feedback projections from layer V of visual association areas subtending grapheme processing to isomodal areas such as V4 are shorter than the corresponding feed-forward projections originating from layer II/III (Bannister 2005). It is therefore only to be expected that tones also activate lexical representations (i.e. simple sound to associative lexeme). These cross-modal connections are pre-existing (Pascual-Leone & Hamilton 2001) and could be facilitated by increased activation within adjacent areas. It is plausible that the activity is increased in association areas processing low-level perceptual information including grapheme/lexical information, phonemic, or visual object or feature representations, because access to such information is available during the window of synaptic development of those areas post-natally. Increased functionality of those areas is reinforced through positive feedback from the environment (Mottron et al. 2006). Increased connectivity within minicolumnar microcircuits, and their incorporation into specialized subnetworks within the cortical area, would allow for rapid efficient and discriminative processing of low-level perceptual/semantic representations, i.e. within word categories such as proper names. These subnetworks would be strengthened by increased connections to areas processing elemental features such as phonemes, morphemes or low-level visual features.
In summary, the pathological processes that lead to autism have distributed effects. We speculate that they may reflect a disruption of multiple fundamental processes during the patterning and organization of cortical cytoarchitecture. The effects of these disrupted processes may be manifested widely, yet altered connectivity/structure in early maturing regions will compound developmental disruptions in subsequently developing areas. Atypical or adaptive behaviours associated with these changes may well provide a basis for overtraining of compensatory or developmentally enhanced functions.
Footnotes
One contribution of 18 to a Discussion Meeting Issue ‘Autism and talent’.
References
- Ballesteros Yáñez I., Muñoz A., Contreras J., Gonzalez J., Rodriguez-Veiga E., DeFelipe J. Double bouquet cell in the human cerebral cortex and a comparison with other mammals. J. Comp. Neurol. 2005;486:344–360. doi: 10.1002/cne.20533. doi:10.1002/cne.20533 [DOI] [PubMed] [Google Scholar]
- Bannister A.P. Inter- and intra-laminar connections of pyramidal cells in the neocortex. Neurosci. Res. 2005;53:95–103. doi: 10.1016/j.neures.2005.06.019. doi:10.1016/j.neures.2005.06.019 [DOI] [PubMed] [Google Scholar]
- Batardière A., Barone P., Knoblauch K., Giroud P., Berland M., Dumas A.-M., Kennedy H. Early specification of the hierarchical organization of visual cortical areas in the macaque monkey. Cereb. Cortex. 2002;12:453–465. doi: 10.1093/cercor/12.5.453. doi:10.1093/cercor/12.5.453 [DOI] [PubMed] [Google Scholar]
- Beierlein M., Gibson J.R., Connors B.W. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. doi:10.1152/jn.00283.2003 [DOI] [PubMed] [Google Scholar]
- Buxhoeveden D.P., Casanova M.F. The minicolumn hypothesis in neuroscience. Brain. 2002;125:935–951. doi: 10.1093/brain/awf110. doi:10.1093/brain/awf110 [DOI] [PubMed] [Google Scholar]
- Casanova M.F., Buxhoeveden D.P., Switala A.E., Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casanova M.F., Buxhoeveden D.P., Switala A.E., Roy E. Neuronal density and architecture (gray level index) in the brains of autistic patients. J. Child Neurol. 2002;17:515–521. doi: 10.1177/088307380201700708. doi:10.1177/088307380201700708 [DOI] [PubMed] [Google Scholar]
- Casanova M.F., Van Kooten I.A.J., Switala A.E., Van Engeland H., Heinsen H., Steinbusch H.W.M., Hof P.R., Schmitz C. Abnormalities of cortical minicolumnar organization in the prefrontal lobes of autistic patients. Clin. Neurosci. Res. 2006;6:127–133. doi:10.1016/j.cnr.2006.06.003 [Google Scholar]
- Casanova M.F., et al. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. doi:10.1007/s00401-006-0085-5 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. doi:10.1016/j.conb.2005.03.001 [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Reflections on the structure of the cortical minicolumn. In: Casanova M.F., editor. Neocortical modularity and the cell minicolumn. Nova Biomedical; New York, NY: 2005. pp. 57–92. [Google Scholar]
- Gabbott P.L.A. Radial organisation of neurons and dendrites in human cortical areas 25. Brain Res. 2003;992:298–304. doi: 10.1016/j.brainres.2003.08.054. doi:10.1016/j.brainres.2003.08.054 [DOI] [PubMed] [Google Scholar]
- Gabbott P.L.A., Bacon S.J. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey, I: cell morphology and morphometrics. J. Comp. Neurol. 1996;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. doi:10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- Galarreta M., Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat. Rev. Neurosci. 2001;2:425–433. doi: 10.1038/35077566. doi:10.1038/35077566 [DOI] [PubMed] [Google Scholar]
- Galarreta M., Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. doi:10.1126/science.1061395 [DOI] [PubMed] [Google Scholar]
- Gibson J.R., Beierlein M., Connors B.W. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. doi:10.1038/47035 [DOI] [PubMed] [Google Scholar]
- Hong L.E., Summerfelt A., Mitchell B.D., McMahon R.P., Wonodi I., Buchanan R.W., Thaker G.K. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch. Gen. Psychiatry. 2008;65:1008–1016. doi: 10.1001/archpsyc.65.9.1008. doi:10.1001/archpsyc.65.9.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard E.M., Ramachandran V.S. Neurocognitive mechanisms of synesthesia. Neuron. 2005;48:509–520. doi: 10.1016/j.neuron.2005.10.012. doi:10.1016/j.neuron.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Kennedy H., Douglas R.J., Knoblauch K., Dehay C. Self-organization and pattern formation in primate cortical networks. In: Bock G., Goode J., editors. Cortical development: genes and genetic abnormalities. Wiley; Chichester, UK: 2007. pp. 178–198. [DOI] [PubMed] [Google Scholar]
- Kisley M.A., Cornwell Z.M. Gamma and beta neural activity evoked during a sensory gating paradigm: effects of auditory, somatosensory and cross-modal stimulation. Clin. Neurophysiol. 2006;117:2549–2563. doi: 10.1016/j.clinph.2006.08.003. doi:10.1016/j.clinph.2006.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N.J., Ermentrout G.B., Whittington M.A., Traub R.D. Gamma rhythms and beta rhythms have different synchronization properties. Proc. Natl Acad. Sci. USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. doi:10.1073/pnas.97.4.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Toledo-Rodriguez M., Wang Y., Gupta A., Silberberg G., Wu C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. doi:10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- Mottron L., Dawson M., Soulières I., Hubert B., Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. doi:10.1007/s10803-005-0040-7 [DOI] [PubMed] [Google Scholar]
- Mountcastle V.B. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. doi:10.1093/brain/120.4.701 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A., Hamilton R. The metamodal organization of the brain. In: Casanova C., Ptito M., editors. Vision: from neurons to cognition. Elsevier; Amsterdam, UK: 2001. [DOI] [PubMed] [Google Scholar]
- Peters A., Sethares C. The organization of double bouquet cells in monkey striate cortex. J. Neurocytol. 1997;26:779–797. doi: 10.1023/a:1018518515982. doi:10.1023/A:1018518515982 [DOI] [PubMed] [Google Scholar]
- Schlaug G., Schleicher A., Zilles K. Quantitative analysis of the columnar arrangement of neurons in the human cingulate cortex. J. Comp. Neurol. 1995;351:441–452. doi: 10.1002/cne.903510310. doi:10.1002/cne.903510310 [DOI] [PubMed] [Google Scholar]
- Schnitzler A., Gross J. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. doi:10.1038/nrn1650 [DOI] [PubMed] [Google Scholar]
- Szabadics J., Lorincz A., Tamás G. β and γ frequency synchronization by dendritic gabaergic synapses and gap junctions in a network of cortical interneurons. J. Neurosci. 2001;21:5824–5831. doi: 10.1523/JNEUROSCI.21-15-05824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammet D. Hodder & Stoughton; London, UK: 2006. Born on a blue day: a memoir of Aspergers and an extraordinary mind. [Google Scholar]