Abstract
Increased caloric intake in dietary obesity could be driven by central mechanisms that regulate reward-seeking behavior. The mesolimbic dopamine system, and the nucleus accumbens in particular, underlies both food and drug reward. We investigated whether rat dietary obesity is linked to changes in dopaminergic neurotransmission in that region. Sprague–Dawley rats were placed on a cafeteria-style diet to induce obesity or a laboratory chow diet to maintain normal weight gain. Extracellular dopamine levels were measured by in vivo microdialysis. Electrically evoked dopamine release was measured ex vivo in coronal slices of the nucleus accumbens and the dorsal striatum using real-time carbon fiber amperometry. Over 15 weeks, cafeteria-diet fed rats became obese (>20% increase in body weight) and exhibited lower extracellular accumbens dopamine levels than normal weight rats (0.007±0.001 vs. 0.023±0.002 pmol/sample; P<0.05). Dopamine release in the nucleus accumbens of obese rats was stimulated by a cafeteria-diet challenge, but it remained unresponsive to a laboratory chow meal. Administration of d-amphetamine (1.5 mg/kg i.p.) also revealed an attenuated dopamine response in obese rats. Experiments measuring electrically evoked dopamine signal ex vivo in nucleus accumbens slices showed a much weaker response in obese animals (12 vs. 25 × 106 dopamine molecules per stimulation, P<0.05). The results demonstrate that deficits in mesolimbic dopamine neurotransmission are linked to dietary obesity. Depressed dopamine release may lead obese animals to compensate by eating palatable “comfort” food, a stimulus that released dopamine when laboratory chow failed.
Keywords: nucleus accumbens, striatum, feeding, body weight, amphetamine, hyperphagia
The rapid rise of dietary obesity in industrialized societies indicates that non-homeostatic signaling pathways that allow for chronic positive energy intake may be responsible. A crucial question is why laboratory animals and humans keep on eating energy-rich, palatable food to the degree that they become obese. From an evolutionary perspective, it is to be expected that the brain developed a system to respond to natural rewards, such as food. These central mechanisms are conserved across species in order to ensure survival (Kelley and Berridge, 2002) and could interact with or modulate the circuitry that regulates body weight. Therefore, availability of rewarding palatable food may lead to increased caloric intake and weight gain that homeostasis-driven mechanisms, originating primarily in the hypothalamus, may not overcome. This possibility may explain, at least in part, the epidemic proportions of dietary obesity.
Prominent among neural systems are the mesolimbic dopamine pathways, where the action of dopamine, particularly in the nucleus accumbens terminals, is known to mediate reinforcement mechanisms. The activation of this system includes elevation of dopamine levels and changes in dopamine turnover after natural rewarding behaviors like feeding (Hernandez and Hoebel, 1988; Radhakishun et al., 1988). In addition, dopamine in the nucleus accumbens (and the adjacent dorsal striatum) is known to increase with exposure to food-associated stimuli and motor activity related to the attainment of food (Mogenson and Wu, 1982; Bradberry et al., 1991; Salamone et al., 1991). It is, therefore, reasonable to expect that dietary obesity may be linked to the mesolimbic dopamine-releasing ability of palatable high-energy food.
In this study, we investigated whether chronic exposure (15 weeks) of rats to a high-energy, palatable cafeteria diet causes changes in nucleus accumbens dopamine. This highly palatable diet is successful in inducing dietary obesity in rats and is the most relevant to the development of human obesity (Sclafani and Springer, 1976). Furthermore, the cafeteria diet allowed us to distinguish between high-fat and high-carbohydrate preferences and whether such preferences impacted mesolimbic dopamine release. We found that Sprague–Dawley rats took the majority of their daily caloric intake from from high-carbohydrate sources and developed diet-induced obesity (DIO). Furthermore, they demonstrated depressed basal dopamine release in the nucleus accumbens and an attenuated dopamine response to a standard chow meal or systemic administration of d-amphetamine.
EXPERIMENTAL PROCEDURES
Animals
Female albino Sprague–Dawley rats (Taconic, Hudson, NY, USA), were matched for a body weight of 300 g each at the age of 3 months. Female animals were chosen because, in contrast to male rats, the body weight of laboratory-chow fed females is relatively stable over time. Animals were housed individually in the same room under a 12-h reverse light/dark cycle (lights on: 6 pm, lights off: 6 am). Under these conditions we observed no impact of the estrous cycle phase on mesolimbic dopamine release (Geiger et al., 2008). All animals were used in accordance to the published guidelines of the U.S. National Institutes of Health (NIH) and the Institutional Animal Care and Use Committee (IACUC) of Tufts University and the Tufts Medical Center. All efforts were made to limit the number of animals used in order to minimize animal use and suffering.
Cafeteria diet composition
Animals were divided into the cafeteria DIO group (also described as the dietary obese group below) and the laboratory chow-fed group (normal weight group). All groups were fed ad libitum. The cafeteria diet included high-fat components such as Crisco (33% vegetable shortening, 67% Purina powder), salami, cheddar cheese and peanut butter; and high-carbohydrate components such as sweetened condensed milk (Magnolia brand mixed with water, 1:1), chocolate chip cookies, milk chocolate, bananas, marshmallows and a 32% sucrose solution. This highly palatable diet has been shown to be very effective in inducing dietary obesity in rats and mimic the development of human obesity (Sclafani and Springer, 1976). Each of the components was available at all times and changed four times a week. The cafeteria DIO group, in addition to palatable food, was also given ad libitum access to Purina laboratory chow. To identify diet preferences, the intake of each of the components of the cafeteria diet was measured over two 48-h periods during the eleventh week of the diet. Body weights were recorded once each week.
Stereotaxic surgery
Stereotaxic surgery was performed during week 7 of the study (n=24 cafeteria DIO rats, n=32 laboratory chow rats). Animals were anesthetized with ketamine (60 mg/kg i.p.) and xylazine (10 mg/kg i.p.) for implantation of bilateral 10 mm, 21 gauge stainless-steel microdialysis guide cannulas aimed at the posterior nucleus accumbens shell region. The stereotaxic coordinates were 10 mm anterior to interaural zero, 1.2 mm lateral to the midsagittal sinus and 4 mm ventral to the level skull surface. The probe dialysis fiber extended another 4 mm ventral to reach the target site (Paxinos and Watson, 2007). Following surgery, all animals were returned to their cages and continued on their dietary regimen.
Microdialysis and high performance liquid chromatography with electrochemical detection (HPLC-EC) procedure
Microdialysis was performed during week 14 of the study to allow for adequate recovery from surgery. For each microdialysis session animals were placed individually in microdialysis cages and probes were placed in the microdialysis cannulas 12–15 h before the first sample was collected. The site of implantation (left versus right) was counterbalanced. Microdialysis probes were of the concentric type, made locally and have shown a 10% recovery of neurochemicals in in vitro tests as described earlier (Hernandez et al., 1986). The probes were perfused with a Ringer’s solution (142 mM NaCl, 3.9 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 1.4 mM Na2HPO4, 0.3 mM NaN2PO4) at a rate of 1° µl/min. The dialysate was collected in 40 µl vials containing 5 µl of preservative (0.1 M HCl and 100° µM EDTA) to slow the oxidation of monoamines. Collection of samples began in the middle of the dark cycle, and all food was removed 3 h prior to sampling for all animals. Samples were collected at 30-min intervals for at leas least 2 h of baseline, followed by a systemic injection of d-amphetamine (1.5 mg/kg i.p.; Sigma, St. Louis, MO, USA). From each sample, 25 µl of dialysate was injected into an amperometric Antec HPLC-EC system (GBC, Inc., Boston, MA, USA) with a 10 cm Rainin column and a phosphate mobile phase buffer, which separates and detects dopamine, and the dopamine metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). The resulting peaks were then measured and recorded. Placement of the microdialysis probe in the target site was verified at the end of the experiment by histological examination of the probe tract following fixation of the brain with paraformaldehyde.
For animals presented with a 30-min laboratory chow or cafeteria-diet meal challenge instead of d-amphetamine, all groups were food-deprived for 12 h prior to the microdialysis experiment to ensure adequate motivation to eat.
Slice electrophysiology
Rat brains were rapidly placed into ice-cold oxygenated artificial cerebrospinal fluid (aCSF) on a Leica VT1000S vibratome (Leica Microsystems, Wetzlar, Germany), and cut in 300 µm coronal slices. The slice bath contained aCSF (124 mM NaCl, 2.0 mM KCl, 1.25 mM KH2PO4, 2.0 mM MgSO4, 25 mM NaHCO3, 1.0 mM CaCl2, 11 mM glucose, pH=7.3). After 1 h in aCSF slices were transferred to the recording chamber with perfusion of oxygenated aCSF set to 1 ml/min at 37 °C. Carbon fiber electrodes, 5 µm in diameter, with a freshly cut surface were placed in the nucleus accumbens shell or dorsal striatum ~50 µm into the slice, with the reference electrode (Ag/AgCl wire) inserted into the aCSF bath and the voltage set to + 700 mV (Axopatch 200 B, Axon Instruments Inc., Union City, CA, USA). The bipolar, twisted wire, stimulating electrode (wire diameter 0.005 in: MS 303/3, Plastics One, Inc., Roanoke, VA, USA) was placed within 100–200 µm of the carbon fiber electrode. A constant monophasic current stimulus of 2 ms at +500 µA was delivered by an Isoflex stimulus isolator (AMPI, Inc., Jerusalem, Israel) triggered by a constant-current stimulator (Model S88; Grass Technologies, West Warwick, RI, USA). The response of the amperometric electrode (change in baseline) was monitored and quantified by Superscope software (GW Instruments, Inc., Somerville, MA, USA). Electrodes were calibrated before and after use with background-subtracted voltammograms (five waves applied and averaged, 300 V/s, −400 to +1000 mV, in recording medium and medium with 10 µM dopamine). Amperometric peaks were identified as events greater than 3.5×the rms noise of the baseline. The event width was the duration between (a) the baseline intercept of the maximal incline from the baseline to the first point that exceeded the cutoff and (b) the first data point following the maximal amplitude that registered a value of ≤0 pA. The maximum amplitude (imax) of the event was the highest value within the event. To determine the total number of molecules (N) released, the total charge of the event between the baseline intercepts was determined, and the number of molecules estimated by the relation N=Q/nF, where Q is the charge, n the number of electrons donated per molecule, and F is Faraday’s constant (96,485 C per equivalent). Estimates were based on an assumption of two electrons donated per oxidized molecule of dopamine (Ciolkowski et al., 1994).
Tissue micropunches
Cafeteria DIO or laboratory chow-fed rats (n=11/group) were euthanized as in the previous experiment and 1 mm diameter punches of the dorsal striatum and nucleus accumbens were taken from 300 µm brain slices. The punches were then exposed to 40 mM KCl solution for 3 min to stimulate dopamine release. Extracellular dopamine levels were then measured using the HPLC method described above.
Data analysis
Two-way ANOVA (group×time) with repeated measures and Fisher post hoc analysis as appropriate was used for the analysis of the microdialysis data. One-way ANOVA was used for all other assays. For the slice experiments, results from five different stimulations on the same slice were averaged per slice before the ANOVA was run. Results are expressed as mean±standard error of the mean (SEM).
RESULTS
Dietary obese rats have a strong preference for highly palatable food
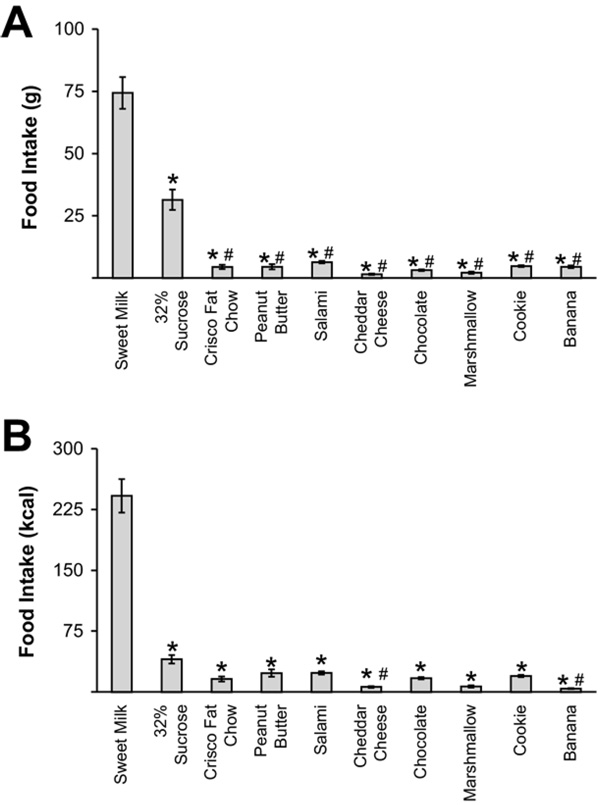
Cafeteria DIO rats showed a strong preference for sweet milk (74.4±6.4 g; 241±21 kcal) and the 32% sucrose solution (31.4±4.1 g; 40±5 kcal) (Fig. 1A, B, F(9,127)=116.9854, P<0.01). In addition, these animals ate significantly less of the Purina chow (5.66±1.02 g) compared to the laboratory chow fed animals (54.7 ±2.3 g; F(1,27)=419.681, P<0.01). After 14 weeks on the cafeteria diet, rats gained 53.7% of their initial body weight to a final weight of 444.9±19.0 g. After the same period, rats on laboratory chow reached a final weight of 344.0±10.8 (Fig. 2A).
Fig. 1.
Cafeteria diet component preferences in obese rats. Average consumption of cafeteria diet components in grams (A) and kcal (B) over two 48-h periods during week 11 of dietary regimen shows a preference for sweet milk and sucrose solution (mean±SEM; * P<0.01 relative to sweet milk; # P<0.05 relative to 32% sucrose solution).
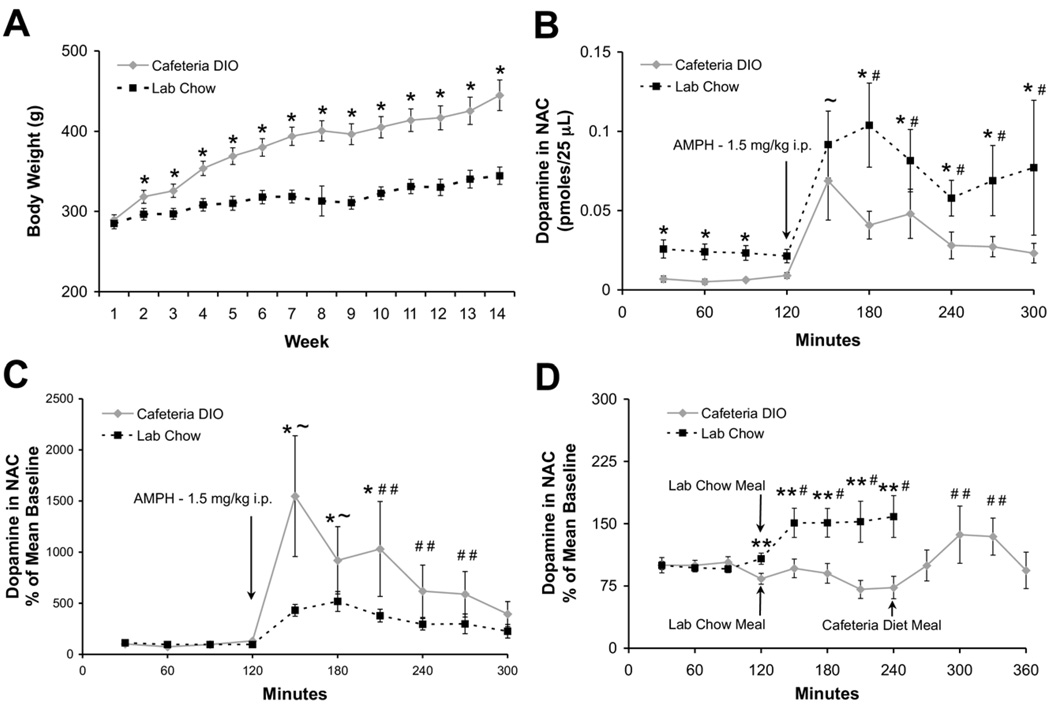
Fig. 2.
Basal, amphetamine- and laboratory chow meal–challenged nucleus accumbens dopamine levels are decreased in dietary obese rats. (A) Body weight of cafeteria DIO rats during a 14-week period was significantly more than the laboratory chow–fed group beginning at week 2 of the dietary regimen (* P<0.01 by one-way ANOVA). (B) Basal and amphetamine-challenged extracellular dopamine levels during week 14 in the nucleus accumbens of cafeteria DIO rats (n=9) was significantly lower than in chow-fed rats (n=13). (C) The percent increase from baseline after amphetamine was higher in the cafeteria DIO rats than in the chow-fed rats (* P<0.01 between groups, ~ P<0.05 within both groups, # P<0.05 within laboratory chow fed group only, # # P<0.05 within DIO group only relative to baseline prior to the amphetamine injection). (D) A plain chow meal was presented to cafeteria DIO (n=18) and chow-fed groups (n=22) after four baseline samples. Only the chow-fed group showed significant increases in dopamine after the meal. The cafeteria meal that was presented to a subset (n=8) of the cafeteria DIO group 2.5 h after the regular chow meal resulted in a significant increase in dopamine release (** P<0.05 between groups, # P<0.05 within the laboratory chow fed group only, # # P<0.05 within the cafeteria DIO group only relative to baseline prior to the laboratory chow or cafeteria meal).
Dietary obese rats have low basal dopamine and reduced amphetamine-stimulated dopamine release
At week 14 of the study, cafeteria DIO rats exhibited lower extracellular dopamine levels in the nucleus accumbens, compared to laboratory chow-fed rats (0.007±0.001 pmols/25 µL sample vs. 0.023±0.002 pmols/25 µL sample; respectively, Fig. 2B, F(1,19)=11.205; P<0.01), as measured by in vivo microdialysis. Baseline levels of the dopamine metabolites, DOPAC and HVA, were also found to be significantly lower in the cafeteria DIO rats. DOPAC levels in cafeteria DIO rats were 3.13±0.42 vs. 8.53±0.56 pmol in laboratory chow-fed rats (F(1,10)=14.727, P<0.01). HVA levels were 1.0±0.28 vs. 4.28±0.33 pmol respectively (F(1,20)=6.931, P<0.05). After the establishment of a steady baseline of dopamine, rats were given a 1.5 mg/kg i.p. injection of amphetamine. The total release of stimulated dopamine levels was less in cafeteria DIO rats compared to laboratory chow-fed animals (Fig. 2B, F(9,162)=2.659, P<0.01).
Dietary obese rats release dopamine in the nucleus accumbens when eating highly palatable food, not plain laboratory chow
Fig. 2D shows that the levels of extracellular dopamine in the cafeteria DIO rats did not increase detectably in response to a meal of laboratory chow. Animals ate on average 1.3±0.4 g of chow over 30 min. However, when a subset of these animals (n=8) was then fed the cafeteria diet for 30 min, dopamine increased 19.3% from 0.027±0.003 to 0.033±0.004 pmols/25 µL sample (F(11,187)=8.757, P<0.05). DOPAC levels also increased by 17.13%±6.14%. In contrast, dopamine levels in the laboratory chow-fed animals increased by 51.10%±17.31% (F(7,119)=3.902, P<0.05) 1 h after the chow meal (animals ate on average 5.7±0.8 g, significantly more than the DIO animals; F(1,33)=26.459, P<0.01). However, we do not expect that the lower food intake by the DIO animals is the direct cause for the lack of dopamine release in these animals since food intake as low as 0.6 g has been reported to stimulate dopamine release in the nucleus accumbens of rats (Martel and Fantino, 1996). Furthermore, other studies have shown that differences in the amount of dopamine released are not necessarily directly correlated to the amount of food present, but may also be affected by other stimuli such as satiety level of the animal, palatability and novelty effects of the food presented (Hoebel et al., 2007). A cafeteria diet was not given as a challenge to laboratory chow-fed animals because it was expected to induce novelty effects that would confound any comparisons with the cafeteria DIO animals.
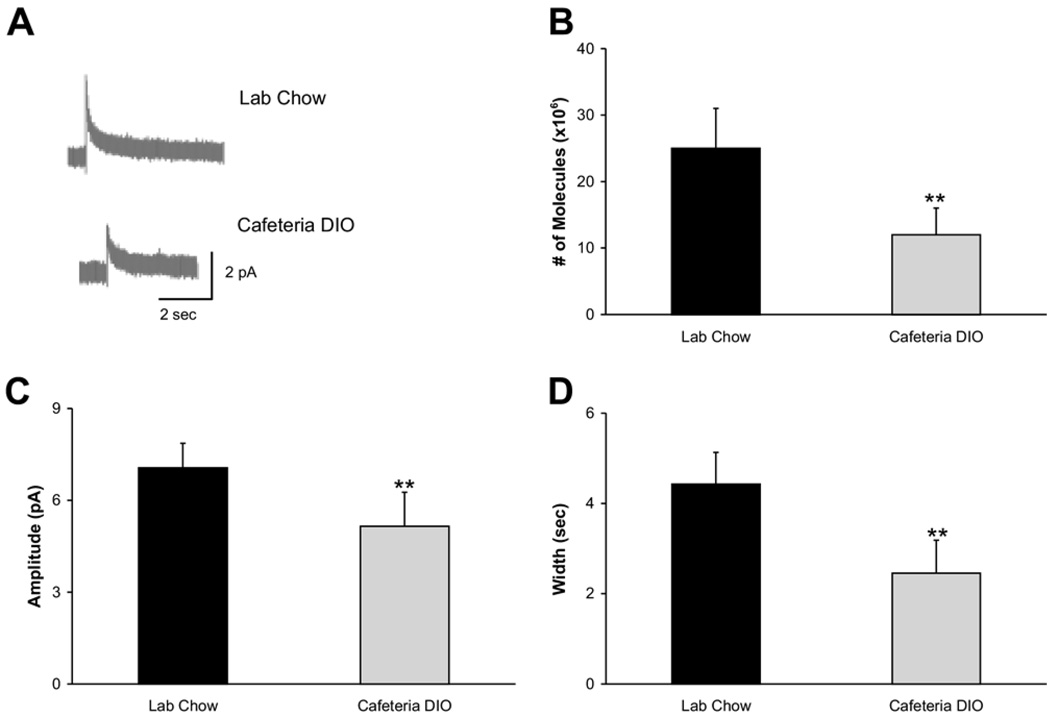
Electrically stimulated dopamine release is attenuated in acute coronal brain slices from dietary obese rats
Fig. 3A shows representative amperometric traces from nucleus accumbens shell slices of normal vs. dietary obese rats (n=30 stimulations in seven slices vs. 24 stimulations in five slices respectively). Cafeteria DIO rats had lower electrically evoked dopamine release than laboratory chow-fed rats (12×106±4×106 vs. 25×106±6×106 molecules; Fig. 3B, F(1,52)=2.1428, P<0.05). This difference in evoked dopamine release reflects both a decrease in event amplitude (5.16±1.10 pA in cafeteria DIO rats vs. 7.06±0.80 pA in laboratory chow-fed rats; Fig. 3C, F(1,52)=2.4472, P<0.05) and width (2.45±0.73 s in cafeteria DIO rats vs. 4.43±0.70 s in laboratory chow-fed rats, Fig. 3D, F(1,52)=3.851, P<0.05).
Fig. 3.
Evoked dopamine release from the nucleus accumbens in brain slices (A) Representative traces from acute coronal nucleus accumbens slices of chow-fed animals (top; n=30 stimulations in seven slices) and cafeteria DIO animals (bottom; n=24 stimulations in five slices). The average number of molecules released (B), event amplitude (C), and width (D) from the dietary obese animals was significantly lower than in chow-fed animals (** P<0.05).
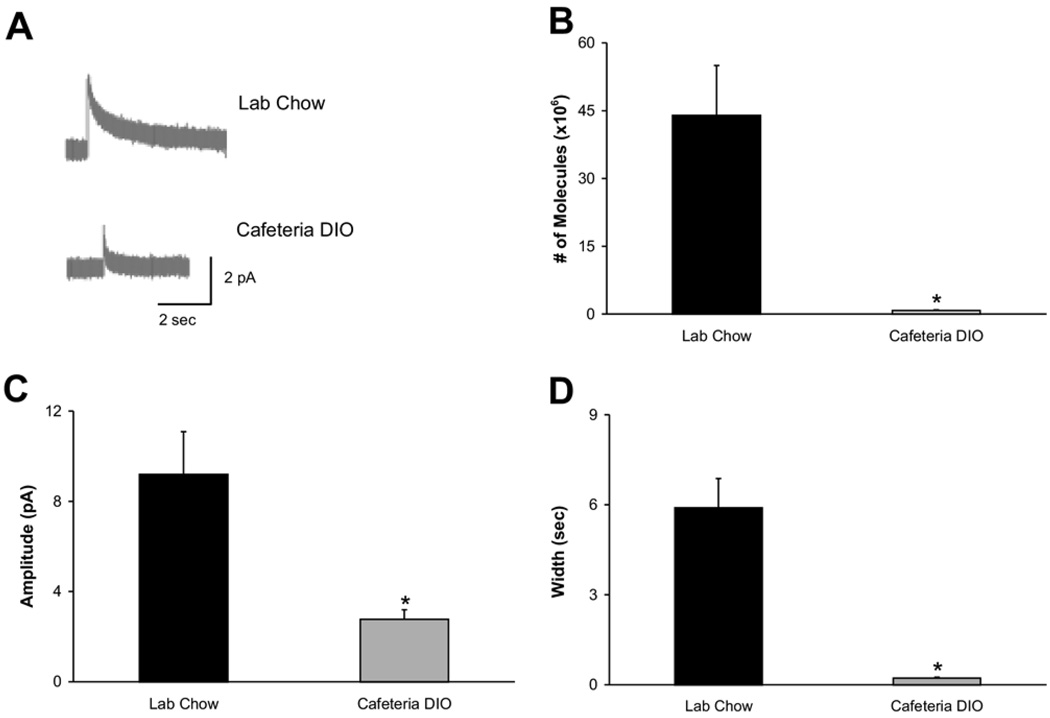
Fig. 4 shows that the same trends were present in dorsal striatal slices of the dietary obese rats. Representative traces from the laboratory chow-fed (n=31 stimulations in seven slices) and cafeteria DIO (n=15 stimulations in four slices) groups are shown in Fig. 4A. The electrically evoked dopamine release from the striatum was 0.8×106±0.1×106 in cafeteria DIO rats vs. 44×106±11×106 molecules (Fig. 4B, F(1,45)=6.0546, P<0.01) in the laboratory chow-fed animals. Again this reflects a decrease in both the event amplitude (2.77±0.42 vs. 9.20±1.88 pA; F(1,45)=7.8468, P<0.01) and width (0.22±0.03 vs. 5.90±0.98 s; F(1,45)=17.2823, P<=0.01) in the cafeteria DIO group (Fig. 4C, 4D).
Fig. 4.
Evoked dopamine release from the dorsal striatum in brain slices. (A) Representative traces from acute coronal dorsal striatum slices of chow-fed animals (top; n=31 stimulations in seven slices) and cafeteria DIO animals (bottom; n=15 stimulations in four slices). The average number of molecules released (B), event amplitude (C), and width (D) from the dietary obese animals were significantly lower than in chow-fed animals (* P<0.01).
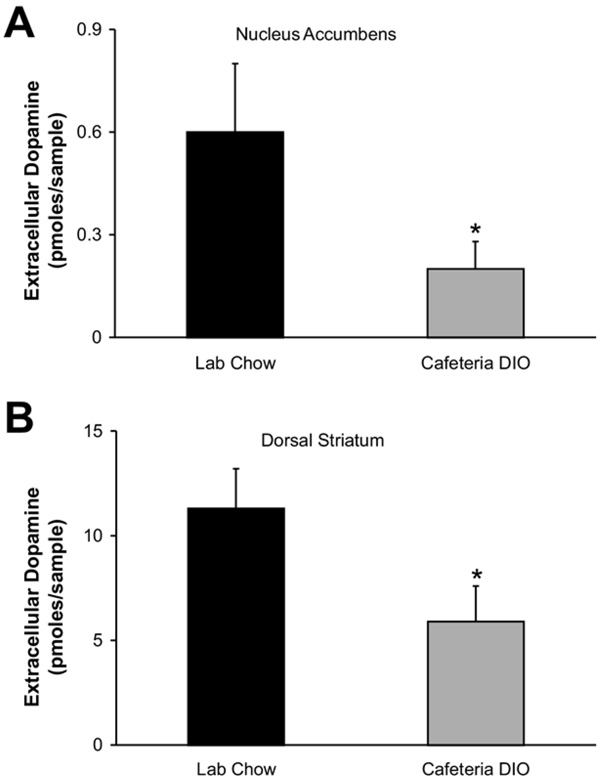
Potassium-stimulated dopamine release in tissue micropunches is reduced in the nucleus accumbens and striatum of dietary obese rats
Extracellular dopamine levels following KCl stimulation were measured by HPLC-EC and are shown in Fig. 5. Extracellular dopamine levels were 0.16±0.08 pmol/sample in the accumbens micropunches of obese animals (n=10 micropunches) compared to 0.65±0.23 pmol/sample in the micropunches from the control animals (n=11 micropunches; Fig. 5A; F(1,19)=4.1911, P<0.01). Extracellular dopamine levels were 5.9±1.7 pmol/sample in the striatal micropunches from obese (n=8 micropunches) rats and 11.3±1.9 pmol/sample in the same site from control (n=11 micropunches) rats (Fig. 5B; F(1,17)=7.5064, P<0.01).
Fig. 5.
Extracellular dopamine levels from potassium-stimulated tissue micropunches. Amount of dopamine released from (A) nucleus accumbens (n=11 micropunches from each group) and (B) dorsal striatum (n=8 micropunches from obese and n=11 micropunches from controls) was significantly lower in cafeteria DIO animals than in chow-fed animals (* P<0.01).
DISCUSSION
In this study, rats became overweight from eating a cafeteria diet with a preference for high-carbohydrate foods. In their overweight state, they had lower basal extracellular dopamine as well as chow-stimulated or amphetamine-stimulated dopamine in the nucleus accumbens. In studies using drugs of abuse, animals will work to keep dopamine levels in the nucleus accumbens above a certain level (Wise et al., 1995a,b; Ranaldi et al., 1999). In the present study, the abused “substance” is palatable food, so the low extracellular dopamine in the accumbens leads to increased consumption of palatable food.
Obese rats also showed attenuated levels of electrically stimulated dopamine in brain slices and potassium-stimulated stimulated dopamine in tissue micropunches from the nucleus accumbens and dorsal striatum. A central presynaptic deficit in dopamine exocytosis is, therefore, evident in dietary obesity since depression of evoked dopamine release is present in vivo, in acute striatal and accumbal brain slices and in tissue micropunches from dietary obese animals. We have seen a similar effect in a genetic model of obesity predisposition. In this model, mRNA and protein expression of regulators of dopamine synthesis and exocytosis including tyrosine hydroxylase and the neuronal vesicular monoamine transporter (VMAT2) are decreased in ventral tegmental area (VTA) dopamine neurons of obesity-prone animals (Geiger et al., 2008). Another potential site of pre-synaptic alteration is the plasma membrane dopamine reuptake transporter, DAT. The slice electrophysiology studies allow us to distinguish between differences in dopamine release versus reuptake kinetics. The difference in spike width suggests in principle that dietary obese animals animals may have not only less evoked release but also changes in reuptake due to differences in active DAT transporter sites on the plasma membrane. In Zucker fatty (fa/fa) rats, increased mRNA levels of the DAT transporter have been reported in the VTA (Figlewicz et al., 1998). The possibility of increased dopamine clearance is compatible with the decreased evoked dopamine signal in DIO rats in the present study.
We should note that amphetamine’s dopamine releasing ability was not attenuated in the obese animals (in terms of percent change from baseline) and this may “conspire” along with the lower dopamine absolute levels to drive the motivation of obese animals to obtain dopamine releasing stimuli. Amphetamine is a weak base that displaces dopamine from the vesicles to the cytosol and leads to increase of extracellular dopamine via reverse transport (Sulzer and Rayport, 1990). In cases of severe deficits in dopamine vesicular pools, as for example in the case of the vesicular transporter VMAT2 deficient mice, injection of amphetamine transiently stimulates new dopamine synthesis in the cytosol (Fon et al., 1997). An amphetamine-induced transient increase in cytosolic dopamine may explain the temporary increase in percent change of accumbens dopamine in the obese animals over that observed in normal weight animals and may contribute to the obese animals’ susceptibility to dopamine releasing stimuli along with the lower absolute extracellular dopamine levels in the accumbens.
What would be the mechanisms likely to mediate the presynaptic dopamine deficit in obese animals and drive their dietary preferences? The link between food preference and nucleus accumbens dopamine is clearly shown in the blunted response of the dietary obese animals to chow, but not to a palatable diet. Our findings complement recent work showing that a dopamine D1-type receptor (D1) receptor agonist enhanced the preference of rats for highly palatable food (Cooper and Al-Naser, 2006). In addition, nucleus accumbens dopamine is activated in rats trained to binge on sucrose (Avena et al., 2008), further supporting the involvement of central dopamine in the preference for palatable food rich in carbohydrates. We have demonstrated the central dopamine deficit reported in the present study in additional models of obesity, including the ob/ob leptin deficient mouse and the inbred obesity-prone rat (Fulton et al., 2006; Geiger et al., 2008). Thus, one possible signal linking palatable food consumption and accumbens dopamine release might be leptin. In humans with a congenital leptin deficiency, replacement of leptin reduces their hyperphagia and changes the activation of their ventral striatum with respect to visualization of palatable food (Farooqi et al., 2007). In rats it has also been shown that leptin will decrease self-administration of sucrose (Figlewicz et al., 2006, 2007). Other orexigenic inputs such as ghrelin and orexin have also been shown to be involved in the activation of the midbrain dopamine system (Rada et al., 1998; Helm et al., 2003; Abizaid et al., 2006; Narita et al., 2006). It would be interesting to further examine whether switching dietary obese animals to a normal laboratory chow on a chronic basis would maintain their preference for palatable food and the associated accumbens dopamine response to it independent of the expected changes in leptin, ghrelin or orexin and other signals related to appetite regulation.
CONCLUSION
In conclusion, the findings in this study show that the mesolimbic dopamine system plays a critical role in preference for high-energy diets, hyperphagia and the resulting dietary obesity. The nucleus accumbens and dorsal striatum dopaminergic neurotransmission are depressed in dietary obese rats. The animals can temporarily restore dopamine levels by eating highly palatable, high-energy food. These results suggest that selective targeting of presynaptic regulators of the mesolimbic dopamine system constitutes a promising approach for the treatment of dietary obesity.
Acknowledgments
This work was supported by DK065872 (ENP), F31 DA023760 (BMG, ENP), a Smith Family Foundation Award of Excellence in Biomedical Research (ENP) and P30 NS047243 (Tufts Center for Neuroscience Research).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- DAT
dopamine plasma membrane transporter
- DIO
diet-induced obesity
- DOPAC
dihydroxyphenylacetic acid
- HPLC-EC
high performance liquid chromatography with electrochemical detection
- HVA
homovanillic acid
- VMAT2
neuronal vesicular monoamine transporter
- VTA
ventral tegmental area
REFERENCES
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Gruen RJ, Berridge CW, Roth RH. Individual differences in behavioral measures: correlations with nucleus accumbens dopamine measured by microdialysis. Pharmacol Biochem Behav. 1991;39:877–882. doi: 10.1016/0091-3057(91)90047-6. [DOI] [PubMed] [Google Scholar]
- Ciolkowski EL, Maness KM, Cahill PS, Wightman RM, Evans DH, Fosset B, Amatore C. Disproportionation during electrooxidation of catecholamines at carbon-fiber microelectrodes. Anal Chem. 1994;66:3611–3617. [Google Scholar]
- Cooper SJ, Al-Naser HA. Dopaminergic control of food choice: contrasting effects of SKF 38,393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50:953–963. doi: 10.1016/j.neuropharm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Patterson TA, Johnson LB, Zavosh A, Israel PA, Szot P. Dopamine transporter mRNA is increased in the CNS of Zucker fatty (fa/fa) rats. Brain Res Bull. 1998;46:199–202. doi: 10.1016/s0361-9230(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KA, Rada P, Hoebel BG. Cholecystokinin combined with serotonin in the hypothalamus limits accumbens dopamine release while increasing acetylcholine: a possible satiation mechanism. Brain Res. 2003;963:290–297. doi: 10.1016/s0006-8993(02)04051-9. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Stanley BG, Hoebel BG. A small removable microdialysis probe. Life Sci. 1986;39:2629–2637. doi: 10.1016/0024-3205(86)90119-0. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7:617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel P, Fantino M. Influence of the amount of food ingested on mesolimbic dopaminergic system activity: a microdialysis study. Pharmacol Biochem Behav. 1996;55:297–302. doi: 10.1016/s0091-3057(96)00087-1. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Wu M. Neuropharmacological and electrophysiological evidence implicating the mesolimbic dopamine system in feeding responses elicited by electrical stimulation of the medial forebrain bundle. Brain Res. 1982;253:243–251. doi: 10.1016/0006-8993(82)90691-6. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam: Academic Press; 2007. [Google Scholar]
- Rada P, Mark GP, Hoebel BG. Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Res. 1998;798:1–6. doi: 10.1016/s0006-8993(98)00315-1. [DOI] [PubMed] [Google Scholar]
- Radhakishun FS, van-Ree JM, Westerink BH. Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci Lett. 1988;85:351–356. doi: 10.1016/0304-3940(88)90591-5. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neurosci. 1999;19:4102–4109. doi: 10.1523/JNEUROSCI.19-10-04102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976;17:461–471. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995a;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995b;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]