Abstract
Aims/hypothesis
Insulin stimulates phosphorylation cascades, including phosphatidylinositol-3-kinase (PI3K), phosphatidylinositol-dependent kinase (PDK1), Akt, and protein kinase C (PKC). Myristoylated alanine-rich C-kinase substrate (MARCKS), a PKCβII substrate, could link the effects of insulin to insulin-stimulated glucose transport (ISGT) via phosphorylation of its effector domain since MARCKS has a role in cytoskeletal rearrangements.
Methods
We examined phosphoPKCβII after insulin treatment of L6 myocytes, and cytosolic and membrane phosphoMARCKS, MARCKS and phospholipase D1 in cells pretreated with LY294002 (PI3K inhibitor), CG53353 (PKCβII inhibitor) or W13 (calmodulin inhibitor), PI3K, PKCβII and calmodulin inhibitors, respectively, before insulin treatment, using western blots. ISGT was examined after cells had been treated with inhibitors, small inhibitory RNA (siRNA) for MARCKS, or transfection with MARCKS mutated at a PKC site. MARCKS, PKCβII, GLUT4 and insulin receptor were immunoblotted in subcellular fractions with F-actin antibody immunoprecipitates to demonstrate changes following insulin treatment. GLUT4 membrane insertion was followed after insulin with or without CG53353.
Results
Insulin increased phosphoPKCβII(Ser660 and Thr641); LY294002 blocked this, indicating its activation by PI3K. Insulin treatment increased cytosolic phosphoMARCKS, decreased membrane MARCKS and increased membrane phospholipase D1 (PLD1), a protein regulating glucose transporter vesicle fusion resulted. PhosphoMARCKS was attenuated by CG53353 or MARCKS siRNA. MARCKS siRNA blocked ISGT. Association of PKCβII and GLUT4 with membrane F-actin was enhanced by insulin, as was that of cytosolic and membrane MARCKS. ISGT was attenuated in myocytes transfected with mutated MARCKS (Ser152Ala), whereas overproduction of wild-type MARCKS enhanced ISGT. CG53353 blocked insertion of GLUT4 into membranes of insulin treated cells.
Conclusions/interpretation
The results suggest that PKCβII is involved in mediating downstream steps of ISGT through MARCKS phosphorylation and cytoskeletal remodelling.
Keywords: F-actin, Glucose transporter 4, Insulin-stimulated glucose uptake, L6 myocytes, MARCKS, Phospholipase D1, PKCβ
Introduction
Insulin is responsible for translocating glucose transporter 4 (GLUT4), a transmembrane protein, to the membrane of muscle and fat cells. Involvement of upstream kinases, such as Akt (also called protein kinases B), has been studied extensively. The molecular scenario nearer the membrane is less understood. One kinase known to support insulin’s role in skeletal muscle is protein kinase C (PKC) βII. Physiologically, there are reports of polymorphisms in the PKCβ promoter that reduce promoter activity in humans. One polymorphism is associated with the decreased expression of PKCβII and results in decreased peripheral insulin-dependent glucose uptake [1]. Other polymorphisms in the PKCβ promoter are associated with diabetic vascular complications and diabetic neuropathy in people with type 1 diabetes [2, 3]. Despite the finding that PKCβII modulates insulin action in human skeletal muscle [4] and rat skeletal muscle and cell lines (for review see [5]), little is known about its molecular mechanisms.
Isozymes of PKCβ are implicated as both signal transducers and modulators of insulin signalling [6, 7]. PKC comprises a family of 12 serine/threonine kinase isozymes and their splice variants, which exhibit differential cellular distributions and substrate specificities [8]. To be active kinases, the conventional PKC isozymes PKCα, PKCβI, PKCβII and PKCγ require a series of post-translational modifications. These include phosphorylation by phosphatidylinositol-dependent kinase (PDK) 1/2 and autophosphorylation, the binding of two or three Ca2+ ions, followed by interaction with membrane lipids: acidic phosphatidylserine and non-polar diacylglycerol [8]. The β isoforms, PKCβI and PKCβII, are encoded by the same gene and differ in the last exon as a product of alternative splicing of PKCβ pre-mRNA, producing proteins that differ by their carboxyl-terminal 50–52 amino acids [9].
Previous studies show that insulin stimulates alternative splicing of PKCβ pre-mRNA in favour of the PKCβII isoform in BC3H-1 myocytes, L6 skeletal muscle cells, rat hepatocytes, HepG2 cells, 3T3-L1 preadipocytes, vascular smooth muscle cells and embryonic fibroblasts [10–12]. This occurs through enhanced exon inclusion [11]. Using a dominant-negative-acting PKCβII construct expressed in L6 myotubes, PKCβII rather than PKCβI was shown to be the splice variant promoting insulin-stimulated glucose transport [13]. Insulin also stimulates the phosphorylation of myristoylated alanine-rich C-kinase substrate (MARCKS), a high-affinity conventional PKC substrate, in cells, consistent with the activation of calcium-dependent conventional PKCs [14]. MARCKS is an acidic protein with a central 25 amino acid basic region, termed the effector domain (ED), which is a site of interaction with Ca2+–calmodulin, F-actin and membrane phospholipids [15]. The ED contains three PKC phosphorylation sites (Ser-152, Ser-156 and Ser-163), and ED phosphorylation by PKC on Ser-152 or Ser-156 disrupts interactions with Ca2+–calmodulin and aids in releasing MARCKS from membranes as well as altering its interactions with F-actin [16]. Phosphorylation of MARCKS by PKC unmasks membrane phosphatidylinositol 4,5-bisphosphate (PIP2), thus initiating cytoskeletal reorganisation through PIP2-mediated recruitment of actin polymerisation and treadmilling or tethering of actin filament cross-linking proteins, and proteins required to attach F-actin to membranes [17, 18]. PKCβ phosphorylation of MARCKS plays a role in vesicular trafficking in neurons [19]. Thus, the potential molecular role for PKCβII in insulin-stimulated glucose transport is hypothesised to occur via phosphorylation of MARCKS. MARCKS movement may also be reciprocal to phospholipase D binding to PIP2 in the membrane. Phospholipase D1 (PLD1), an enzyme regulated by agonist stimulation, has been proposed to function at many steps in vesicle trafficking and fusion [20, 21], and is associated with GLUT4 trafficking to exocytic sites [22].
Here, we studied the phosphorylation states of MARCKS and PKCβII and the distribution of PLD1 during insulin action and mutated a crucial PKC phosphorylation site in the MARCKS ED to determine whether it blocked glucose transport in cells expressing the mutant.
Methods
Cell culture
L6 rat skeletal myoblasts, obtained from Dr Amira Klip (The Hospital for Sick Children, Toronto, ON, Canada), were grown in α-minimal essential medium (α-MEM) containing 10% FBS at 37°C in a humidified 5% CO2, 95% air atmosphere in 24-well, six-well, 100-mm or 245-mm plates. Cells were grown to 80% confluence and induced to differentiate into multinucleated myotubes by reducing the FBS concentration to 2% for 2–4 days prior to the experiment. Cells were then washed with PBS and incubated in serum-free α-MEM for 4–6 h prior to each experiment. Insulin (Sigma-Aldrich, St Louis, MO, USA) was used at a final concentration of 10 μIU/ml and was added to the cells 30 min prior to cell lysis.
PI3 kinase and PKCβII inhibitors
LY294002 (Sigma-Aldrich) is a phosphatidylinositol 3-kinase (PI3K) inhibitor. LY294002 inhibits purified PI3K with an IC50 of 1.4 μmol/l [23]. Inhibition of PI3K is commonly associated with the inhibition of Akt phosphorylation on Thr308 by PDK1 [24, 25].
PKCβII was inhibited pharmacologically by preincubating cells for 30 min with 1 μmol/l CG53353 (also known as DAPH 2), a gift from Dr Dorianno Fabbro, Novartis, Basel, Switzerland. CG53353 is concentration-selective for PKCβII inhibition (IC50 for PKCα 1.9 μmol/l, PKCβI 3.8 μmol/l, PKCβII 0.41 μmol/l, PKCγ 22 μmol/l, PKCζ> 500 μmol/l [26]). CG53353 was used at a concentration shown not to affect the activation of the insulin receptor, the EGF receptor (HER-1), c-erbB2 (HER-2), the platelet-derived growth factor receptor or IGF-1 receptor [26]. W13, a calmodulin inhibitor, was from Sigma-Aldrich.
Western blot analysis
Protein extracts from cells grown on 100 mm plates were prepared by adding 500 μl lysis buffer per plate (20 mmol/l Tris, pH 7.5, 150 mmol/l NaCl, 1% Triton X-100, 50 mmol/l NaF, 0.2 mmol/l Na3VO4 and complete protease inhibitor cocktail (Roche, Basel, Switzerland), scraping cells and transferring them to a 1.5 ml tube. Between 10 and 20 μg of total protein from each sample was separated by SDS-PAGE (10% acrylamide) followed by electroblotting onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Membranes were probed in TBST buffer (20 mmol/l Tris, pH 7.5, 150 mmol/l NaCl, 0.05% Tween-20) using anti-PKCβII (Ser641) (Abcam, Cambridge, MA, USA). Anti-phosphoMARCKS antibody was from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Phospholipase D1 and anti-phosphoAkt (Thr308 and Ser473) antibodies were from Cell Signaling Technology, Danvers, MA, USA. Anti-phosphoPKCβII (Ser660) to NH2-EGF(pS) FVNSEFLKPEVKS-COOH (657–673) was raised in rabbits by Bio-Synthesis, Lewisville, TX, USA. This peptide epitope is unique to PKCβII and the antibody does not cross-react with other PKCs. MARCKS antibody has been described earlier [26]. Non-specific binding was blocked by the presence of 5% dried skimmed milk or porcine gelatine.
Membrane fractionation
Protein extracts from cells grown on 100 mm plates were prepared by adding 5 ml ice-cold PBS per plate, scraping the cells and transferring them to 15 ml tubes. Cells were pelleted (centrifugation at 700×g for 5 min), resuspended in 500 μl swelling buffer (20 mmol/l Tris, pH 7.5, 10 mmol/l NaCl, 50 mmol/l NaF, 0.2 mmol/l Na3VO4 and Complete Protease Inhibitor Cocktail) for 10 min before addition of Triton X-100 (to 1%), and disrupted by 20 passes in a Dounce homogeniser. The homogenate was then centrifuged (3000×g, 10 min) to remove nuclei and debris. The supernatant fraction was then centrifuged at 25,000×g for 30 min. The cytosolic fraction was present in the supernatant fraction and the pellet was resuspended in 40 μl lysis buffer to produce a plasma membrane fraction.
Immunoprecipitation with anti-actin antibody
L6 myotubes were treated for 30 min with or without 10 μIU/ml insulin (0.41 mg/l), washed with cold PBS then incubated with 2 mmol/l DSP (a cleavable cross-linker; Pierce, Rockford, IL, USA) at room temperature for 30 min, followed by 15 min with stop buffer (20 mmol/l Tris, 20 mmol/l glycine). Cells were scraped, homogenised in 500 μl lysis buffer and incubated at 4°C with agitation overnight with 20 μl (200 μg) protein-A magnetic beads prebound to anti-actin antibody from Santa Cruz Biotechnology (sc-10731) according to NEB protocol S1425S. The relevant proteins were then extracted by magnetic separation (magnetic separator from New England Biolabs, Ipswich, MA, USA), washed three times in lysis buffer and resuspended in 50 μl Laemmli buffer containing 5% β-mercaptoethanol. Lysates were subjected to western analysis as described above.
Measurement of insulin-stimulated glucose transport by 2-deoxyglucose uptake
L6 myoblasts were cultured as described above but in 24-well plates. Cells were rinsed with PBS and incubated in serum-free α-MEM for 4 h prior to experiments. Inhibitors were added 2 h prior to experiments. Cells were then washed and incubated in PBS with 1% BSA at 37°C with inhibitors and/or 10 μIU/ml insulin 30 min prior to addition of 10 nmol 2-deoxy[3H]glucose (50–150 μCi/μmol; Perkin Elmer, Boston, MA, USA) and incubation (6 min, 37°C). Cells were washed three times with cold PBS and lysed in 1% SDS. Radioactivity was determined by liquid scintillation counting.
Inhibition of PKCβII and MARCKS production with siRNA
Small inhibitory RNA (siRNA) was performed using the Silencer siRNA Cocktail Kit (Ambion/Applied Biosystems, Foster City, CA, USA). Two approaches were used for PKCβII siRNA to avoid ‘off-target’ involvement. For the first siRNA, the PKCβII-specific exon (exon 17–156 nucleotides) was amplified by PCR using the primers (forward) 5′-TAATACGACTCACTATAGGGTACTTG TGGGCGAAACGCTG-3′ and (reverse) 5′-TAATACGA CTCACT ATAGGGTACTTTAGCTCTTGACTTC-3′. These were purified, digested by RNase III and repurified. The digested exon (100 nmol/l) was then transfected into cells with Lipofectamine reagent (Invitrogen, Carlsbad, CA, USA) using the standard protocol. Next, siRNA to PKCβII (no. 103309), MARCKS (no. 59351) or Silencer negative control (no. 4611; Ambion) was transfected into cells. Silencer and scrambled siRNA were the controls for the two methods. Briefly, RNA and Lipofectamine were mixed before adding to 80% confluent myotubes in serum-free medium. Serum was added after 5 h and cells were incubated for 36–48 h at 37°C. Western analysis and ISGT assays were performed as described above.
Overproduction of MARCKS and the MARCKS effector domain mutant S152A cDNA
Rat MARCKS cDNA with the serine→alanine point mutation in Ser-152 was constructed as described [27]. Myotubes in 24-well plates were transiently transfected with 0.1 μg pcDNA3 plasmid containing wild-type or mutated MARCKS using Lipofectamine as described above. After transfection (48 h), cells were serum-starved for 6 h prior to determining 2-deoxy-[3H]glucose uptake. S152A MARCKS and wild type MARCKS overproduction and phosphorylation were verified by western blot analysis [27].
Assay for membrane-associated GLUT4
Membrane recycling of glucose transporters occurs in metabolically active cells. To inhibit this, cells were incubated with 2 mmol/l potassium cyanide [28], then L-1-tosylamido-2-phenylethyl chloromethyl ketone (TPCK)-treated trypsin (Sigma-Aldrich) was added to the cells at a final concentration of 1 mg/ml to cleave the exofacial loops of membrane-inserted GLUT4, and digestion proceeded for 30 min at 37°C. At the end of the digestion period, soybean trypsin inhibitor (Sigma-Aldrich) was added to a final concentration of 2 mg/ml and the cell monolayer was quickly washed twice with PBS containing trypsin inhibitor and 2% albumin. Cells were pelleted (3×g, 5 min) and homogenisation buffer was added prior to disrupting cells. Cells were centrifuged (100,000×g, 45 min) and the membrane pellet was resuspended in lysis buffer; proteins were resolved by SDS-PAGE. GLUT4 transporters inserted into the plasma membrane are detected as fragments at a lower, approximately 33 kDa, band on immunoblot probed with an anti-GLUT4 antibody (sc-7938; Santa Cruz Biotechnology) generated to the central domain of the protein (amino acids 230–290).
Results
Insulin activation of PKCβII by PI3K pathway
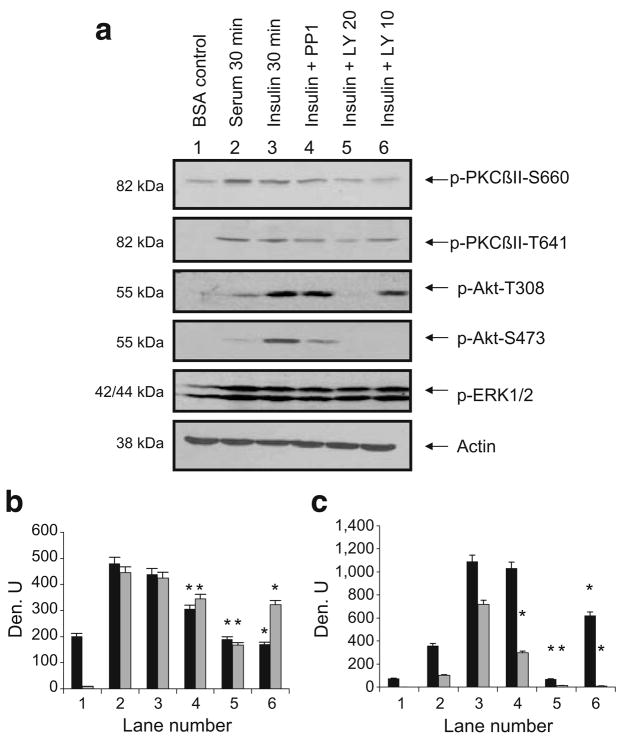
PI3K activates numerous kinases, including Akt and PKC isozymes, by its subsequent activation of PDK1 and its phosphorylation of serine/threonine residues in their activation loops [29]. However, since insulin activation of PKCβII by PI3K had not been demonstrated in L6 myocytes, we examined phosphoPKCβII (Thr641 and Ser660) in cells treated with serum, insulin and LY294002, a PI3K inhibitor, or the Src inhibitor PP1 (Fig. 1). When cells were serum-starved for 6 h and treated with insulin for 30 min, phosphoPKCβII (Thr641 and Ser660) levels more than doubled, and with Thr641 there was >40-fold stimulation over control (lane 3). Serum re-addition was also an activator of both phosphorylation sites (lane 2). PKCβII Ser660 phosphorylation was blocked by LY294002 at both concentrations, but Thr641 phosphorylation was blocked better by 20 μmol/l LY294002. Ser660, a residue in the alternatively spliced region, is autophosphorylated and thought to require the prior phosphorylation of activation loop residues. Ser660 phosphorylation was more sensitive to LY294002 inhibition. 4-Amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP1), a tyrosine kinase inhibitor, had a small inhibitory effect on both sites. Akt Thr308 and Ser473 phosphorylation was also stimulated by insulin, but serum was not a good activator in L6 myotubes. LY294002 blocked insulin-induced phosphorylation of both sites. PP1 inhibited insulin-induced Akt Ser473 phosphorylation, indicating the potential upstream regulation of this site by tyrosine kinases. The extracellular signal-regulated kinase or ERK1/2 pathway, activated equally by serum and insulin and not regulated by PI3K or Src kinases, is shown as a control. Thus, PKCβII Ser660 and Thr641 phosphorylation was activated in a PI3K-dependent manner by insulin.
Fig. 1.
a Insulin increased phosphoPKCβII. L6 myotubes were serum-starved for 6 h prior to pretreatment with PP1 (10 μmol/l), LY294002 (20 or 10 μmol/l) for 30 min followed by serum (10%) or insulin (10 μIU/ml) for 30 min. Whole-cell lysates were separated by SDS-PAGE (10%) and transferred to membranes for western blot analysis as indicated with anti-phosphoPKCβII, phosphoAkt, phosphoERK1/2 or actin. Blots were scanned and quantitated using UnScanIt gel digitising software (Silk Scientific, Orem, UT, USA). b, c Relative densitometric units (Den. U) for phosphoPKCβII S660 and T641 and for phosphoAkt T308 and S473. Black bars show units for pPKCβII-S660 (b) and pAkt-T308 (c) and grey bars show units for p-PKCβII-T641 (b) and pAkt-S473 (c). The experiment was repeated three times. Significant inhibition (*p<0.05) of insulin effects by inhibitors on pPKCβII and pAkt sites, as determined using Prism4 (GraphPad Software, La Jolla, CA, USA) by one-way ANOVA. p, phospho
Insulin induces PKCβ II and Ca2+–calmodulin-dependent phosphorylation of MARCKS
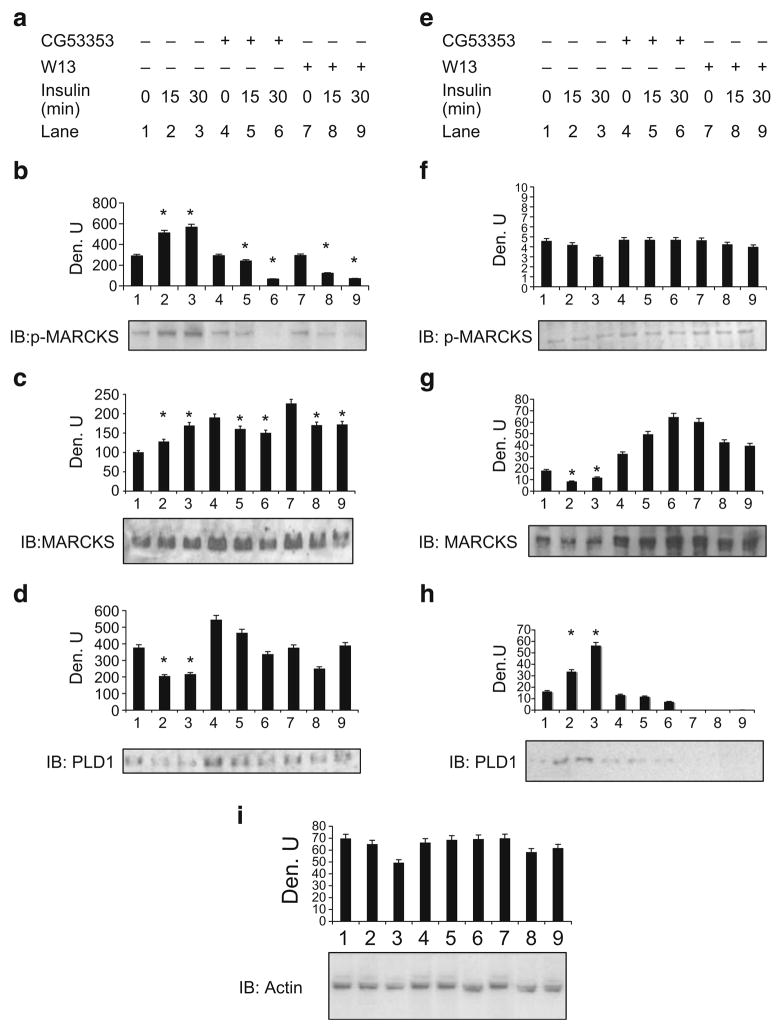
We hypothesised that MARCKS, a conventional PKC substrate, was the potential mediator of PKCβII action related to GLUT4-storage vesicle movement. We previously identified MARCKS as a target of insulin-stimulated PKC activity in L6 myotubes [14]; however, a molecular role for MARCKS was not hypothesised. To further investigate the effects of MARCKS in the context of PKC-mediated stimulation of insulin action, we examined phosphorylated MARCKS in cytosol and membrane fractions of L6 cells. Previous studies reported that the calmodulin antagonist W13 inhibits the effect of insulin on glucose transport in 3T3L1 adipocytes [30]. Both activated PKC and calcium-bound calmodulin (CaCaM) act on MARCKS [31]. When MARCKS is phosphorylated by PKCβII or bound by CaCaM, the ED is masked and MARCKS, released from the membrane, is detected as phosphoMARCKS in the cytosol [15, 32].
To determine whether this occurred in L6 myotubes, cells were pretreated with CG53353 (a PKCβII-specific inhibitor) or W13 before insulin treatment and fractionated, and extracts were examined by western blotting. In this protocol, nuclei and other heavy organelles are removed first, and the remaining extract is divided into soluble (cytosol) and non-soluble (membrane-containing) fractions as described in the Methods. After 30 min of insulin treatment, cytosolic phosphoMARCKS was twofold more intense on the blot. The cytosolic increase was blocked when cells were treated with CG53353 or W-13 (Fig. 2b, lanes 4–9). Membrane phosphoMARCKS decreased somewhat with insulin treatment but was not significantly altered by inhibitor treatment (Fig. 2f). Cytosolic MARCKS pools were increased with insulin treatment but decreased with inhibitors compared with their controls in lanes 4 and 7 (Fig. 2c). Membrane MARCKS pools decreased following insulin treatment but not with inhibitors (Fig. 2g, lanes 4–9). Since PLD1 is thought to be targeted to MARCKS sites in the membrane following the release of phosphoMARCKS to the cytosol, PLD1 was immunoblotted; membrane PLD1 increased in the membrane following insulin treatment (Fig. 2h, lanes 1–3). Both inhibitors blocked this increase (lanes 5–9). A decrease in cytosolic PLD1 was demonstrated in insulin-treated control cells, and the trend was muted by the inhibitors (Fig. 2d). Actin levels did not significantly change, although a modest decrease in the presence of insulin alone was noted in the membrane (Fig. 2i). Prior studies indicate that the cytosolic translocation of MARCKS is increased both by PKC-mediated phosphorylation and by the binding of CaCaM (for review see [33]). PLD1 activity was also associated with GLUT4 vesicle trafficking to exocytic sites [22]. Here, insulin stimulated MARCKS phosphorylation and its release from the membrane was countered by an increase in cytosolic phosphoMARCKS. In addition, PKCβII appeared to phosphorylate MARCKS in response to insulin, as indicated by the inhibition of phosphoMARCKS by CG53353 (Fig. 2b).
Fig. 2.
Cytosolic MARCKS phosphorylation was blocked by PKCβII inhibition. L6 cells were grown as described in Methods. a–d Cytosol; e–i membrane. Subcellular fractions were separated by SDS-PAGE, transferred to nitrocellulose and probed using phosphoMARCKS (p-MARCKS), MARCKS, PLD1 or actin antibodies as indicated. The experiment is representative of three that showed similar results on immunoblots (IB). Densitometric scans (Den. U), determined as described in Fig. 1, are shown above each blot/panel. *p<0.05, unpaired t test
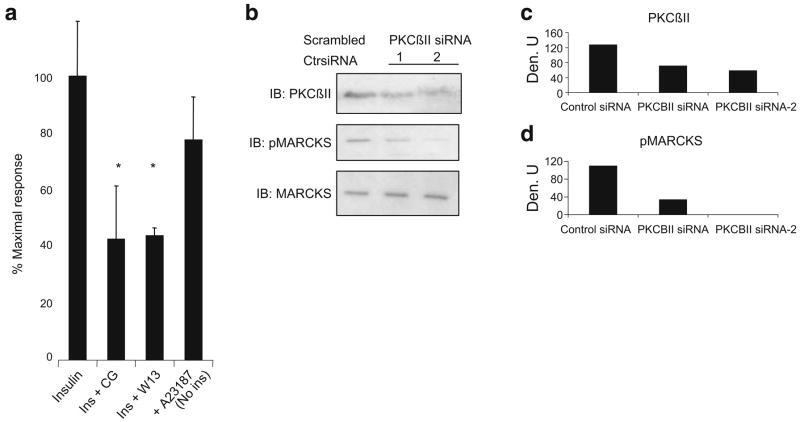
These inhibitors also blocked (>60%) ISGT (Fig. 3a). In order to mimic the activation of calmodulin, we increased intracellular calcium levels by addition of the ionophore A23187. This addition, in the absence of insulin, produced a moderate but significant stimulation of glucose uptake (Fig. 3a). To determine whether the inhibition of MARCKS phosphorylation by CG53353 noted in Fig. 2b was due to PKCβII inhibition, two different PKCβII siRNAs were transfected into L6 myotubes and phosphoMARCKS was examined (Fig. 3b–d). The siRNA reduced PKCβII levels by 50–60%, and MARCKS phosphorylation was reduced by more than 80–90% in insulin-treated cells.
Fig. 3.
Insulin-stimulated glucose uptake was reduced by inhibiting either PKCβII (CG, CG53353) or CaCaM (W13) and mimicked by addition of a calcium ionophore (A23187). a L6 myotubes were grown as described and 2-deoxy-D-[1,2-3H]glucose uptake was then determined as outlined in Methods. The experiment was performed in duplicate and repeated on two occasions with similar results. Data are expressed as percentage of maximal insulin response. *p<0.05 for inhibition of ISGT in the presence of inhibitors, Student’s t test. b Cells were transfected with two different PKCβII siRNAs or a scrambled control siRNA as described in Methods. Forty-eight hours later, cells were exposed to insulin (10 μIU/ml) for 30 min. Cell lysates were analysed by immunoblotting (IB) for PKCβII, pMARCKS and MARCKS as described in the Methods. The siRNA lanes represent two separate experiments with different siRNA preparations. Lanes were scanned as described in the legend of Fig. 1 and results are shown in the graphs (c, d). Ctr, control; Den. U, densitometric units; Ins, insulin
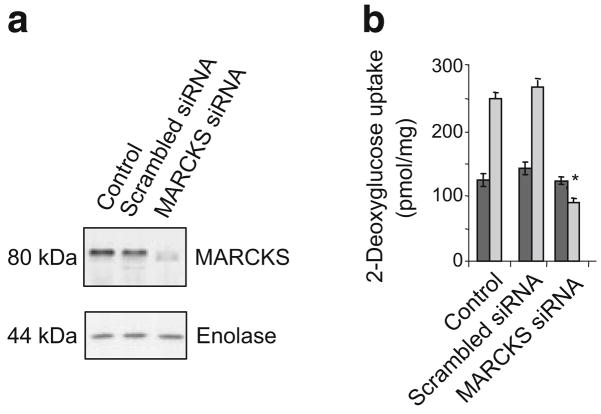
Next, a commercial siRNA (Ambion) to reduce MARCKS levels was transfected into the cells (Fig. 4a). Decreasing MARCKS production fully blocked the insulin-induced increase in 2-deoxy[3H]glucose uptake (p<0.05) (Fig. 4b).
Fig. 4.
Insulin-stimulated increase in 2-deoxyglucose uptake in L6 cells was blocked by MARCKS siRNA. a L6 cells were grown to 80% confluence and allowed to fuse into myotubes for 4 days. Cells were transfected with siRNA for MARCKS purchased from Ambion as described. Forty-eight hours later, cells were treated with insulin for 30 min. Half of the cells were lysed to perform western blot analysis (a), in which enolase was used to access loading of the gel and half were assayed for 2-deoxy-D-[1,2-3H]glucose uptake (b). The uptake assay was performed in triplicate on two occasions with similar results. Dark grey bars show basal uptake and light grey bars show insulin-stimulated uptake. Results are mean±SEM. *p<0.05 compared with scrambled siRNA for insulin response, Student’s t test
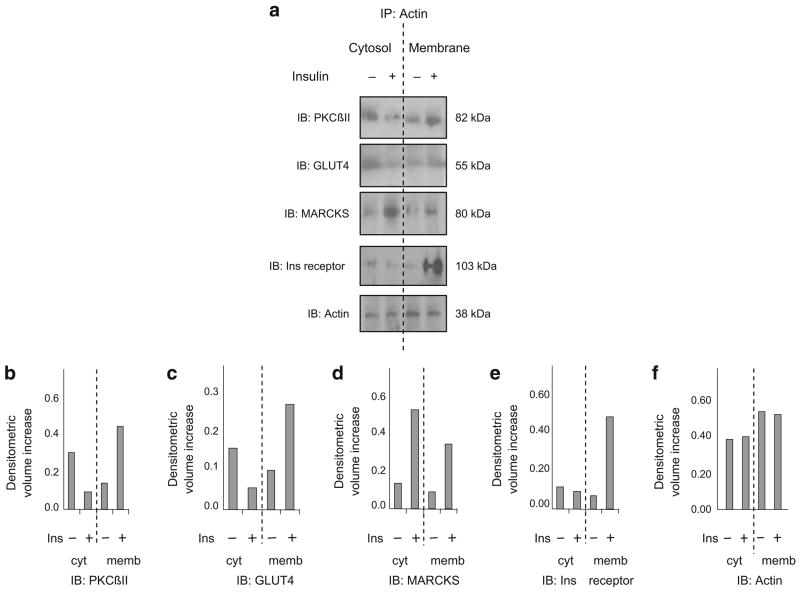
Immunoprecipitation demonstrated that PKCβII, GLUT4 and MARCKS were associated with actin in an insulin-dependent manner
Another indication of the interaction of PKCβII, MARCKS and GLUT4 following insulin treatment would be changes in their subcellular localisation and interactions. To approach this, cortical F-actin was used as a common cytoskeletal component and its antibody was used to immunoprecipitate cytosolic and membrane fractions of insulin-treated (30 min) and control cells. Proteins were separated by electrophoresis, and candidate proteins were probed by immunoblot analysis. In order to preserve the possible fragile nature of the interactions, cells were treated with the cleavable cross-linking agent dithiobis-succinimidyl propionate (DSP) before fractionation. After addition of insulin, PKCβII and GLUT4 decreased in the cytosolic fraction and increased in the particulate fraction (Fig. 5a–c). MARCKS increased in both cytosolic and particulate fractions after insulin treatment (Fig. 5d), indicating that insulin mobilised a pool of MARCKS that was previously unassociated with actin. Interestingly, the insulin receptor associated with actin, but only in the particulate fraction after insulin treatment (Fig. 5e). Levels of cytosolic and membrane actin changed only slightly (Fig. 5f). Thus, insulin stimulation resulted in a coordinated shift in the association of actin with PKCβII, GLUT4, MARCKS and the insulin receptor.
Fig. 5.
PKCβII, GLUT4 and MARCKS bound actin in an insulin-dependent manner. L6 myotubes were treated for 30 min with and without 10 μIU/ml insulin, treated with cross-linking agent and then immunoprecipitated (IP) with anti-actin antibody. The bound proteins were then extracted by magnetic separation and treated with dithiothreitol to break cross-links. a Proteins were separated by SDS-PAGE and subjected to immunoblotting (IB) for actin, PKCβII, GLUT4, MARCKS and the insulin receptor. b–f Densitometric analysis (as described in the legend of Fig. 1) for the relevant proteins in (a) are shown beneath the blots. The experiments were repeated on two occasions in separate experiments with similar results. Ins, insulin; cyt, cytosol fraction; memb, membrane fraction
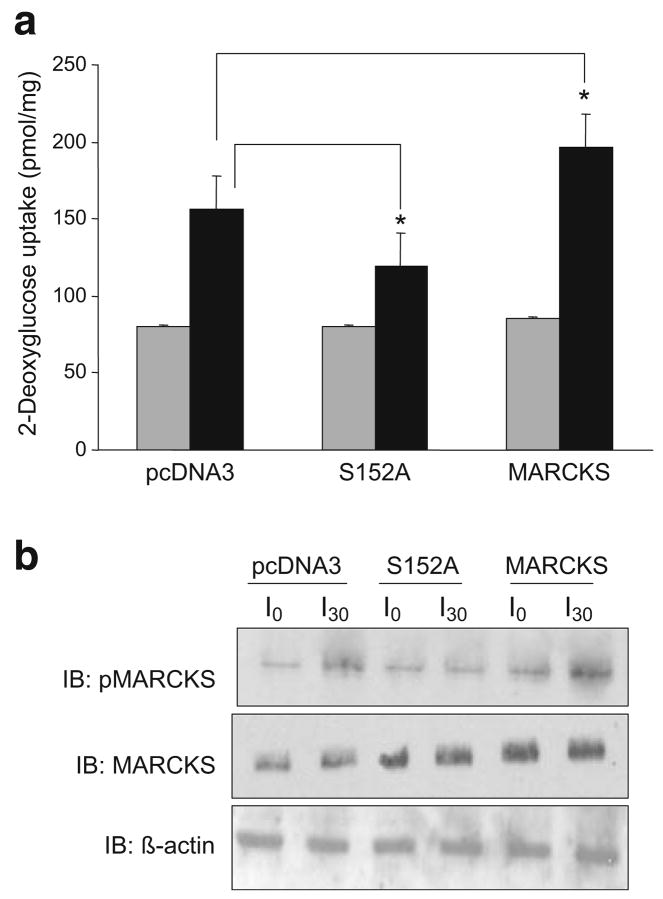
Overproduction of MARCKS mutated in the effector domain inhibited 2-deoxyglucose uptake
If MARCKS is necessary for GLUT4 transporter translocation to plasma membranes, it was hypothesised that phosphorylation of MARCKS ED by PKCβII was the putative site of action. Serine 152 and 156 are key phosphorylation sites for PKCβII in MARCKS; mutation of either site to alanine blocks the activation by MARCKS of phospholipase D [27], which has recently been implicated in GLUT4 docking [22]. The MARCKS S152A mutant was used here to investigate the potential involvement of MARCKS phosphorylation. Increasing MARCKS S152A to a twofold greater level than endogenous MARCKS blocked insulin-stimulated glucose transport by 50% (Fig. 6a). This was expected since blocking PKCβII sites would reduce the number of sites for GLUT4 transporters to insert. Overproduction of native MARCKS increased the effect of insulin on 2-deoxyglucose uptake by 40%. This suggested that by increasing available PKCβII phosphorylation sites in overproducing MARCKS cells, more transporters could potentially be inserted into the membrane. Figure 6b verifies the stimulation by insulin of MARCKS phosphorylation in pcDNA3 and wild-type MARCKS-transfected cells. Cells transfected with mutant S152A confirmed the increased amount of MARCKS, but increased phosphorylation was not detected (Fig. 6b [IB: pMARCKS]).
Fig. 6.
Transient transfection of MARCKS serine-152-alanine mutant blocked insulin-stimulated 2-deoxyglucose uptake. L6 myotubes at 80% confluence were transiently transfected with pcDNA3, S152A MARCKS or wild-type MARCKS as described in the Methods for 48 h. 2-Deoxyglucose uptake was measured after 10 μIU/ml insulin treatment for 30 min. Duplicate wells were harvested for western blot analysis of phosphoMARCKS, MARCKS and actin (b). Transfection was performed in three separate experiments run in duplicate for 2-deoxyglucose uptake. Light grey shaded bars show basal uptake and dark grey bars show insulin-stimulated uptake. Results are mean± SEM. *p<0.05 compared with pcDNA3 for insulin response, Student’s t test
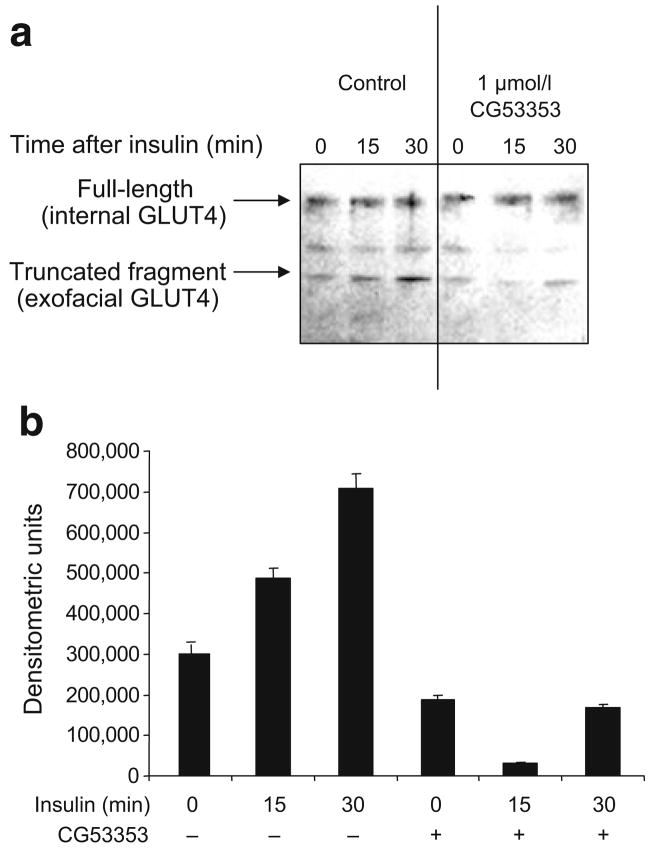
GLUT4 translocation is reduced by inhibiting PKCβII activity
Our previous studies and others indicate that specific inhibition of PKCβII activity blocks ISGT in myotubes [34]. The transporters GLUT4 and GLUT1 are both translocated to cell membranes following insulin stimulation; however, since the insulin regulation of GLUT4 has been more widely studied, we focused on it. Evidence of the involvement of PKCβII activity in GLUT4 trafficking was demonstrated when GLUT4 membrane insertion was examined using insulin and CG53353. A trypsin digestion assay that cleaves GLUT4 protein at specific exofacial portions was used. The result is a 33 kDa tryptic fragment that can be detected by western analysis using an antibody recognising the internal domain of GLUT4 [28]. Insulin treatment increased membrane-fused GLUT4 (33 kDa) and this was markedly inhibited by 1 μmol/l CG53353, a specific PKCβ inhibitor used at a concentration approximately tenfold more selective for PKCβII than for PKCβI (Fig. 7). Another GLUT4 fragment resulting from incomplete trypsin cleavage of exofacial loops was noted at a slightly higher molecular weight, and it was also blocked by CG53353.
Fig. 7.
a GLUT4 in the plasma membrane was reduced by inhibiting PKCβII activity. L6 cells were grown as described in the Methods. Prior to insulin treatment (10 μIU/ml for 30 min), cells were treated for 30 min with or without 1 μmol/l CG53353 or DMSO. Cells were then treated with KCN to halt metabolic activity and trypsin to cleave exofacial proteins. GLUT4 transporters inserted into the plasma membrane would be susceptible to proteolysis of the exofacial loops by trypsin. Cells were then lysed, membranes isolated and proteins were separated by SDS-PAGE, transferred to nitrocellulose and probed using anti-GLUT4 antibodies recognising the intracellular region of the protein. This detects the full-length (near membrane) and the cleaved (inserted in plasma membrane) protein. The experiment was performed on three occasions with similar results. b The change in the lower molecular mass, fully cleaved fragment determined by scanning as described in the legend of Fig. 1
Discussion
Whereas a number of proteins are identified as being associated with the insulin receptor and others as co-localising to the GLUT4 compartment, the linkage between the insulin signalling cascade and the GLUT4 compartment is not well defined. This study addressed the role of PKCβII as a transducer between these systems. As part of the transducer pathway, the roles of PI3K and Akt are established (reviewed in [35]). We found that PKCβII was activated in a PI3K-dependent manner, since Ser660 and Thr641 phosphorylation was blocked by the PI3K inhibitor LY294002. PKCβII is a known substrate of PDK1 [36] and PI3K is a known activator of PDK1 [25]; however, placement of phosphoPKCβII in the insulin signalling cascade had not previously been demonstrated in skeletal muscle cells.
The involvement of various PKCs in insulin signalling is widely reported; however, there appears to be conflicting evidence regarding which isoforms are involved and whether they transduce or modulate insulin action [5]. Wright et al. [37] presented evidence that PKCs may have opposing effects in different muscle fibre types. They suggested that PKCβII may act as a transducer of insulin signalling preferentially in fast-twitch muscle fibres, which coincides with the production of other established insulin-signalling molecules [38].
The observation by Blobe et al. [39] that activated PKCβII (but not PKCβI) associates with F-actin and the finding of others that F-actin is involved in the movement of glucose transporters [35] led us to hypothesise that PKCβII may facilitate GLUT4 translocation into the plasma membrane via MARCKS and F-actin tethering. Here we observed that insulin stimulated the phosphorylation of MARCKS in a PKCβII-dependent manner. MARCKS proteins are substrates for most isoforms of PKC (with the exception of atypical PKCs [40]). There is a possibility that phosphorylation of MARCKS by PKCβII altered the cortical F-actin mesh mediating GLUT4 translocation and allowed GLUT4 movement to the plasma membrane.
GLUT4 translocation in insulin-responsive cells has been compared to that of neurotransmitter vesicles in neuronal dendrites [41]. In neuronal cells, phorbol esters induce vesicle movement [42], a redistribution of the actin cytoskeleton [43] and phosphorylation of MARCKS (for review see [15]). Results of antisense treatment indicated that vesicular trafficking mediated by MARCKS was related to PKCβ in neurons [19]. Our studies using siRNA and CG53353 showed inhibition of MARCKS phosphorylation and GLUT4 membrane insertion. Insulin treatment of L6 cells altered the subcellular distribution of PKCβII, GLUT4, insulin receptor and MARCKS with F-actin. Moreover, mutation of a serine PKC phosphorylation site to alanine within the MARCKS ED caused it to act as a dominant negative and attenuate ISGT. This mutation blocks PKC-mediated activation of PLD1 [27]. In the plasma membrane, MARCKS associates with acidic phospholipids (mainly PIP2), which are also associated with the actin cytoskeleton [35].
One model proposed for MARCKS action in the unstimulated state is that it binds to and masks membrane PIP2 groups [44]. When phosphorylated by PKCβII, MARCKS is released, allowing PIP2 to interact with actin scaffold proteins [15] and/or to activate PLD1 [27]. We found that insulin treatment increased membrane PLD1 levels and that CG53353 blocked this increase. Thus, insulin-stimulated MARCKS phosphorylation could provide a crucial link to the observation that PLD1 regulates the fusion of GLUT4 vesicles with plasma membranes [22]. Phosphorylation of MARCKS by PKCβII would allow it to become cytosolic, as we observed here, and promote the interaction of PLD1 with PIP2, as reported for GLUT4 translocation [22].
The stimulatory effects of A23187 on glucose uptake in adipocytes suggest a role for free Ca2+ in glucose uptake [45], while chelation of intracellular Ca2+ inhibited the effects of insulin on glucose uptake [46]. In skeletal muscle insulin increases near-membrane but not global Ca2+ levels [47]. It is likely that the Ca2+-dependent proteins PKCβII and calmodulin would operate downstream of this event. The inhibition of ISGT by W13 here is similar to that reported in 3T3-L1 cells [46] and consistent with CaCaM being involved in vesicular movement.
Stimulation of L6 myotubes by insulin correlated with changes in the distribution of PKCβII, GLUT4, insulin receptor and MARCKS bound to membrane actin. Moreover, the reduction in the amount of MARCKS by siRNA resulted in decreased ISGT, and overproduction of the MARCKS ED mutant Ser-152-Ala blocked ISGT. This suggests that PKCβII acts on MARCKS to modulate glucose transporter movement since inhibition of PKCβII reduced membrane insertion of GLUT4. This scenario provides a molecular mechanism for PKCβII via MARCKS involvement in insulin action.
Acknowledgments
L6 cells were provided by A. Klip, Hospital for Sick Children, Toronto, Canada. CG53353 was provided by D. Fabbro, Novartis, Basel, Switzerland. We thank T. Butler (University of South Florida, Tampa, FL, USA) for critical reading of the manuscript, and D. Mancu (University of South Florida, Tampa, FL, USA) for technical assistance. This work was supported by the Department of Veterans Affairs Merit Review (D. R. Cooper) and a Merit Review Entry Program (N. A. Patel), by American Heart Association Florida Affiliate Research, Postdoctoral Research Fellowship Award 0020484B (D. S. Chappell) and National Institutes of Health 2RO1-DK054393 (D. R. Cooper).
Abbreviations
- CaCaM
Calcium-bound calmodulin
- ED
Effector domain
- ISGT
Insulin-stimulated glucose transport
- MARCKS
Myristoylated alanine-rich C kinase substrate
- MEM
Minimal essential medium
- PDK1
Phosphatidylinositol-dependent kinase
- PI3K
Phosphatidylinositol-3-kinase
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PKC
Protein kinase C
- PLD1
Phospholipase D1
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contributor Information
D. S. Chappell, Department of Molecular Medicine, University of South Florida, 12901 Bruce B. Downs Blvd, Tampa, FL 33612, USA
N. A. Patel, Department of Molecular Medicine, University of South Florida, 12901 Bruce B. Downs Blvd, Tampa, FL 33612, USA The Research Service, James A. Haley Veterans Hospital, Tampa, FL, USA.
K. Jiang, Department of Molecular Medicine, University of South Florida, 12901 Bruce B. Downs Blvd, Tampa, FL 33612, USA
P. Li, Department of Molecular Medicine, University of South Florida, 12901 Bruce B. Downs Blvd, Tampa, FL 33612, USA
J. E. Watson, The Research Service, James A. Haley Veterans Hospital, Tampa, FL, USA
D. M. Byers, Atlantic Research Centre, Departments of Pediatrics and Biochemistry and Molecular Biology, Dalhousie University, Halifax, NS, Canada
D. R. Cooper, Department of Molecular Medicine, University of South Florida, 12901 Bruce B. Downs Blvd, Tampa, FL 33612, USA, e-mail: dcooper@health.usf.edu The Research Service, James A. Haley Veterans Hospital, Tampa, FL, USA.
References
- 1.Osterhoff MA, Heuer S, Pfeiffer M, et al. Identification of a functional protein kinase C beta promoter polymorphism in humans related to insulin-resistance. Mol Genet Metab. 2008;93:210–215. doi: 10.1016/j.ymgme.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda Y, Suehiro T, Osaki F, Tsuzura S, Kumon Y, Hashimoto K. Polymorphisms in the 5′-upstream region of the PKCbeta gene in Japanese patients with Type 2 diabetes. Diabet Med. 2004;21:1113–1120. doi: 10.1111/j.1464-5491.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- 3.Araki S, Ng DP, Krolewski B, et al. Identification of a common risk haplotype for diabetic nephropathy at the protein kinase C-beta1 (PRKCB1) gene locus. J Am Soc Nephrol. 2003;14:2015–2024. doi: 10.1097/01.asn.0000077347.27669.5c. [DOI] [PubMed] [Google Scholar]
- 4.Cortright RN, Azevedo JL, Jr, Zhou Q, et al. Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E553–562. doi: 10.1152/ajpendo.2000.278.3.E553. [DOI] [PubMed] [Google Scholar]
- 5.Sampson SR, Cooper DR. Specific protein kinase C isoforms as transducers and modulators of insulin signaling. Mol Genet Metab. 2006;89:32–47. doi: 10.1016/j.ymgme.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillay TS, Xiao S, Keranen L, Olefsky JM. Regulation of the insulin receptor by protein kinase C isoenzymes: preferential interaction with beta isoenzymes and interaction with the catalytic domain of betaII. Cell Signal. 2004;16:97–104. doi: 10.1016/s0898-6568(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 7.Formisano P, Beguinot F. The role of protein kinase C isoforms in insulin action. J Endocrinol Invest. 2001;24:460–467. doi: 10.1007/BF03351048. [DOI] [PubMed] [Google Scholar]
- 8.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 9.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 10.Chalfant CE, Mischak H, Watson JE, et al. Regulation of alternative splicing of protein kinase Cbeta by insulin. J Biol Chem. 1995;270:13326–13332. doi: 10.1074/jbc.270.22.13326. [DOI] [PubMed] [Google Scholar]
- 11.Chalfant CE, Watson JE, Bisnauth LD, et al. Insulin regulates protein kinase CbetaII expression through enhanced exon inclusion in L6 skeletal muscle cells. A novel mechanism of insulin- and insulin-like growth factor-i-induced 5′ splice site selection. J Biol Chem. 1998;273:910–916. doi: 10.1074/jbc.273.2.910. [DOI] [PubMed] [Google Scholar]
- 12.Patel NA, Apostolatos HS, Mebert K, et al. Insulin regulates protein kinase CbetaII alternative splicing in multiple target tissues: development of a hormonally responsive heterologous minigene. Mol Endocrinol. 2004;18:899–911. doi: 10.1210/me.2003-0391. [DOI] [PubMed] [Google Scholar]
- 13.Chalfant CE, Ohno S, Konno Y, et al. A carboxy-terminal deletion mutant of protein kinase C beta II inhibits insulin-stimulated 2-deoxyglucose uptake in L6 rat skeletal muscle cells. Mol Endocrinol. 1996;10:1273–1281. doi: 10.1210/mend.10.10.9121494. [DOI] [PubMed] [Google Scholar]
- 14.Arnold TP, Standaert ML, Hernandez H, et al. Effects of insulin and phorbol esters on MARCKS (myristoylated alanine-rich C-kinase substrate) phosphorylation (and other parameters of protein kinase C activation) in rat adipocytes, rat soleus muscle and BC3H-1 myocytes. Biochem J. 1993;295(Pt 1):155–164. doi: 10.1042/bj2950155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarmola EG, Edison AS, Lenox RH, Bubb MR. Actin filament cross-linking by MARCKS: characterization of two actin-binding sites within the phosphorylation site domain. J Biol Chem. 2001;276:22351–22358. doi: 10.1074/jbc.M101457200. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 18.Sundaram M, Cook HW, Byers DM. The MARCKS family of phospholipid binding proteins: regulation of phospholipase D and other cellular components. Biochem Cell Biol. 2004;82:191–200. doi: 10.1139/o03-087. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Wang X, Sumners C, Raizada MK. Obligatory role of protein kinase Cbeta and MARCKS in vesicular trafficking in living neurons. Hypertension. 2002;39:567–572. doi: 10.1161/hy0202.103052. [DOI] [PubMed] [Google Scholar]
- 20.Frohman MA, Morris AJ. Phospholipase D structure and regulation. Chem Phys Lipids. 1999;98:127–140. doi: 10.1016/s0009-3084(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 21.McDermott M, Wakelam MJ, Morris AJ. Phospholipase D. Biochem Cell Biol. 2004;82:225–253. doi: 10.1139/o03-079. [DOI] [PubMed] [Google Scholar]
- 22.Huang P, Altshuller YM, Hou JC, Pessin JE, Frohman MA. Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1. Mol Biol Cell. 2005;16:2614–2623. doi: 10.1091/mbc.E04-12-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 24.Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 2000;275:40400–40406. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- 25.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289:E187–196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 26.Buchdunger E, Mett H, Trinks U, et al. 4,5-bis(4-fluoroanilino)phthalimide: a selective inhibitor of the epidermal growth factor receptor signal transduction pathway with potent in vivo antitumor activity. Clin Cancer Res. 1995;1:813–821. [PubMed] [Google Scholar]
- 27.Morash SC, Douglas D, McMaster CR, Cook HW, Byers DM. Expression of MARCKS effector domain mutants alters phospholipase D activity and cytoskeletal morphology of SK-N-MC neuroblastoma cells. Neurochem Res. 2005;30:1353–1364. doi: 10.1007/s11064-005-8220-6. [DOI] [PubMed] [Google Scholar]
- 28.Czech MP, Buxton JM. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J Biol Chem. 1993;268:9187–9190. [PubMed] [Google Scholar]
- 29.Toker A, Meyer M, Reddy KK, et al. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- 30.Yang C, Watson RT, Elmendorf JS, Sacks DB, Pessin JE. Calmodulin antagonists inhibit insulin-stimulated GLUT4 (glucose transporter 4) translocation by preventing the formation of phosphatidylinositol 3,4,5-trisphosphate in 3T3L1 adipocytes. Mol Endocrinol. 2000;14:317–326. doi: 10.1210/mend.14.2.0425. [DOI] [PubMed] [Google Scholar]
- 31.Arbuzova A, Murray D, McLaughlin S. MARCKS, membranes, and calmodulin: kinetics of their interaction. Biochim Biophys Acta. 1998;1376:369–379. doi: 10.1016/s0304-4157(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 32.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 33.Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993;268:1501–1504. [PubMed] [Google Scholar]
- 34.Braiman L, Sheffi-Friedman L, Bak A, Tennenbaum T, Sampson SR. Tyrosine phosphorylation of specific protein kinase C isoenzymes participates in insulin stimulation of glucose transport in primary cultures of rat skeletal muscle. Diabetes. 1999;48:1922–1929. doi: 10.2337/diabetes.48.10.1922. [DOI] [PubMed] [Google Scholar]
- 35.Brozinick JT, Jr, Berkemeier BA, Elmendorf JS. Actin”g on GLUT4: membrane & cytoskeletal components of insulin action. Curr Diabetes Rev. 2007;3:111–122. doi: 10.2174/157339907780598199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toker A, Newton AC. Cellular signaling: pivoting around PDK-1. Cell. 2000;103:185–188. doi: 10.1016/s0092-8674(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 37.Wright DC, Geiger PC, Rheinheimer MJ, Han DH, Holloszy JO. Phorbol esters affect skeletal muscle glucose transport in a fiber type-specific manner. Am J Physiol Endocrinol Metab. 2004;287:E305–309. doi: 10.1152/ajpendo.00082.2004. [DOI] [PubMed] [Google Scholar]
- 38.Song XM, Ryder JW, Kawano Y, Chibalin AV, Krook A, Zierath JR. Muscle fiber type specificity in insulin signal transduction. Am J Physiol. 1999;277:R1690–1696. doi: 10.1152/ajpregu.1999.277.6.R1690. [DOI] [PubMed] [Google Scholar]
- 39.Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA. Protein kinase C beta II specifically binds to and is activated by F-actin. J Biol Chem. 1996;271:15823–15830. doi: 10.1074/jbc.271.26.15823. [DOI] [PubMed] [Google Scholar]
- 40.Uberall F, Giselbrecht S, Hellbert K, et al. Conventional PKC-alpha, novel PKC-epsilon and PKC-theta, but not atypical PKC-lambda are MARCKS kinases in intact NIH 3T3 fibroblasts. J Biol Chem. 1997;272:4072–4078. doi: 10.1074/jbc.272.7.4072. [DOI] [PubMed] [Google Scholar]
- 41.Simpson F, Whitehead JP, James DE. GLUT4-at the cross roads between membrane trafficking and signal transduction. Traffic. 2001;2:2–11. doi: 10.1034/j.1600-0854.2001.020102.x. [DOI] [PubMed] [Google Scholar]
- 42.Iannazzo L. Involvement of B-50 (GAP-43) phosphorylation in the modulation of transmitter release by protein kinase C. Clin Exp Pharmacol Physiol. 2001;28:901–904. doi: 10.1046/j.1440-1681.2001.03545.x. [DOI] [PubMed] [Google Scholar]
- 43.Baron W, de Vries EJ, de Vries H, Hoekstra D. Protein kinase C prevents oligodendrocyte differentiation: modulation of actin cytoskeleton and cognate polarized membrane traffic. J Neurobiol. 1999;41:385–398. doi: 10.1002/(sici)1097-4695(19991115)41:3<385::aid-neu7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Arbuzova A, Hangyas-Mihalyne G, McLaughlin S. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2001;276:5012–5019. doi: 10.1074/jbc.M008355200. [DOI] [PubMed] [Google Scholar]
- 45.Pershadsingh HA, Shade DL, Delfert DM, McDonald JM. Chelation of intracellular calcium blocks insulin action in the adipocyte. Proc Natl Acad Sci USA. 1987;84:1025–1029. doi: 10.1073/pnas.84.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem. 2001;276:27816–27824. doi: 10.1074/jbc.M011590200. [DOI] [PubMed] [Google Scholar]
- 47.Bruton JD, Katz A, Westerblad H. Insulin increases near-membrane but not global Ca2+ in isolated skeletal muscle. Proc Natl Acad Sci USA. 1999;96:3281–3286. doi: 10.1073/pnas.96.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]