Abstract
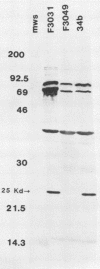
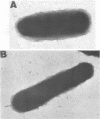
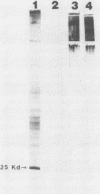
Brazilian purpuric fever (BPF) is a recently described fatal pediatric disease caused by systemic infection with Haemophilus influenzae biogroup aegyptius. Previous studies have shown that all H. influenzae biogroup aegyptius strains isolated from BPF cases and case contacts share several unique phenotypic and genotypic characteristics that differentiate them from other H. influenzae biogroup aegyptius strains isolated from conjunctivitis cases in Brazil. One key characteristic of this BPF clone is reactivity in a BPF-specific monoclonal antibody enzyme-linked immunosorbent assay. We have purified and partially characterized a pilin, referred to as the 25-kilodalton (kDa) protein. Aggregates of this protein contain a heat-labile epitope which is recognized by a monoclonal antibody used in the BPF-specific enzyme-linked immunosorbent assay. The protein has a molecular weight of approximately 25,000, is insoluble in most detergents, and fractionates with outer membrane vesicles after LiCl extraction. Biochemical analysis of the 25-kDa protein shows it to have an amino acid composition similar but not identical to that of the H. influenzae type b pilin. The sequence of 20 N-terminal amino acids of the 25-kDa protein shows almost complete homology with the N terminus of the H. influenzae type b pilin and the types 1 and P pilins of Escherichia coli. Transmission electron microscopic analysis of the purified protein shows the presence of filamentous structures similar in morphology to those of H. influenzae pili. Reactivity between the 25-kDa protein and the BPF-specific monoclonal antibody is demonstrated by Western blotting (immunoblotting) and colloidal gold-enhanced immunoelectron microscopy. Hemadsorption analysis shows that expression of this protein is associated with increases in piliated cells and enhanced binding of these cells to human erythrocytes. These studies indicate that expression of the 25-kDa protein is a characteristic unique to the BPF clone and suggest that this protein plays a role in the pathogenesis of BPF.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H. Assembly of a chemically synthesized peptide of Escherichia coli type 1 fimbriae into fimbria-like antigenic structures. J Bacteriol. 1987 Jun;169(6):2460–2465. doi: 10.1128/jb.169.6.2460-2465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandileone M. C., Ajello G. W., Bibb W. F., Vieira V. S., Sottnek F. O., Swaminathan B. Development of diagnostic tests for Haemophilus influenzae biogroup aegyptius, the etiologic agent of Brazilian purpuric fever. The Brazilian Purpuric Fever Study Group. Pediatr Infect Dis J. 1989 Apr;8(4):243–245. [PubMed] [Google Scholar]
- Båga M., Normark S., Hardy J., O'Hanley P., Lark D., Olsson O., Schoolnik G., Falkow S. Nucleotide sequence of the papA gene encoding the Pap pilus subunit of human uropathogenic Escherichia coli. J Bacteriol. 1984 Jan;157(1):330–333. doi: 10.1128/jb.157.1.330-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J., Coffield L. M., Scott J. R. A plasmid-encoded regulatory gene, rns, required for expression of the CS1 and CS2 adhesins of enterotoxigenic Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):963–967. doi: 10.1073/pnas.86.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Brazilian purpuric fever: Haemophilus aegyptius bacteremia complicating purulent conjunctivitis. MMWR Morb Mortal Wkly Rep. 1986 Sep 5;35(35):553–554. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Preliminary report: epidemic fatal purpuric fever among children--Brazil. MMWR Morb Mortal Wkly Rep. 1985 Apr 26;34(16):217–219. [PubMed] [Google Scholar]
- Claflin L., Williams K. Mouse myeloma--spleen cell hybrids: Enhanced hybridization frequencies and rapid screening procedures. Curr Top Microbiol Immunol. 1978;81:107–109. doi: 10.1007/978-3-642-67448-8_16. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Mayer L. W., Rumschlag H. S., Yakrus M. A., Jones W. D., Jr, Good R. C. Expression of proteins of Mycobacterium tuberculosis in Escherichia coli and potential of recombinant genes and proteins for development of diagnostic reagents. J Clin Microbiol. 1987 Jul;25(7):1176–1180. doi: 10.1128/jcm.25.7.1176-1180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Clements J. R., Dodd D. C. Isolation and characterization of a monoclonal antibody directed against type 1 fimbriae organelles from Escherichia coli. Infect Immun. 1983 Oct;42(1):333–340. doi: 10.1128/iai.42.1.333-340.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Elleman T. C. Pilins of Bacteroides nodosus: molecular basis of serotypic variation and relationships to other bacterial pilins. Microbiol Rev. 1988 Jun;52(2):233–247. doi: 10.1128/mr.52.2.233-247.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Schoolnik G. K., Kessler T. W., Goldmann D. A. Purification and characterization of Haemophilus influenzae pili, and their structural and serological relatedness to Escherichia coli P and mannose-sensitive pili. J Exp Med. 1985 Jan 1;161(1):145–159. doi: 10.1084/jem.161.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Aberrant migration of lipopolysaccharide in sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Eur J Biochem. 1983 Jul 1;133(3):685–688. doi: 10.1111/j.1432-1033.1983.tb07517.x. [DOI] [PubMed] [Google Scholar]
- Kaplan S. L., Mason E. O., Jr, Wiedermann B. L. Role of adherence in the pathogenesis of Haemophilus influenzae type b infection in infant rats. Infect Immun. 1983 Nov;42(2):612–617. doi: 10.1128/iai.42.2.612-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna J. G., Major G. N., Williams R. S. Methods for reducing non-specific antibody binding in enzyme-linked immunosorbent assays. J Immunol Methods. 1985 Dec 27;85(2):409–419. doi: 10.1016/0022-1759(85)90150-4. [DOI] [PubMed] [Google Scholar]
- Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986 Jun;5(6):1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Connor E., Penney D. A comparison of the adherence of fimbriated and nonfimbriated Haemophilus influenzae type b to human adenoids in organ culture. Infect Immun. 1988 Feb;56(2):484–489. doi: 10.1128/iai.56.2.484-489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mayer L. W., Bibb W. F., Birkness K. A., Irino K., Weyant R. S., Reeves M. W., Swenson J. M. Distinguishing clonal characteristics of the Brazilian purpuric fever-producing strain. The Brazilian Purpuric Fever Study Group. Pediatr Infect Dis J. 1989 Apr;8(4):241–243. [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre P., Wheaton G., Erlich J., Hansman D. Brasilian purpuric fever in central Australia. Lancet. 1987 Jul 11;2(8550):112–112. doi: 10.1016/s0140-6736(87)92788-7. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Normark S., Falkow S., Schoolnik G. K. Mannose-sensitive and Gal-Gal binding Escherichia coli pili from recombinant strains. Chemical, functional, and serological properties. J Exp Med. 1983 Nov 1;158(5):1713–1719. doi: 10.1084/jem.158.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Whitney A. M., Schoolnik G. K., Zollinger W. D. Common epitopes of pilin of Neisseria meningitidis. J Infect Dis. 1988 Aug;158(2):332–342. doi: 10.1093/infdis/158.2.332. [DOI] [PubMed] [Google Scholar]
- Stull T. L., Mendelman P. M., Haas J. E., Schoenborn M. A., Mack K. D., Smith A. L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984 Dec;46(3):787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XVIII. 125I-labeled peptide mapping of the major protein of the gonococcal cell wall outer membrane. Infect Immun. 1979 Mar;23(3):799–810. doi: 10.1128/iai.23.3.799-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Scraba D. G., Paranchych W. Formation of 9-nm filaments from pilin monomers obtained by octyl-glucoside dissociation of Pseudomonas aeruginosa pili. J Bacteriol. 1982 Sep;151(3):1508–1513. doi: 10.1128/jb.151.3.1508-1513.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E., Reeves M. W., McKinney R. M., Graves L. M., Olsvik O., Bergan T., Feeley J. C. Production and characterization of monoclonal antibodies to toxic shock syndrome toxin 1 and use of a monoclonal antibody in a rapid, one-step enzyme-linked immunosorbent assay for detection of picogram quantities of toxic shock syndrome toxin 1. J Clin Microbiol. 1987 Mar;25(3):516–521. doi: 10.1128/jcm.25.3.516-521.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., van den Berghe N., Geelen-van den Broek L. Interaction of Haemophilus influenzae with human erythrocytes and oropharyngeal epithelial cells is mediated by a common fimbrial epitope. Infect Immun. 1988 Jul;56(7):1800–1806. doi: 10.1128/iai.56.7.1800-1806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]