Abstract
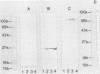
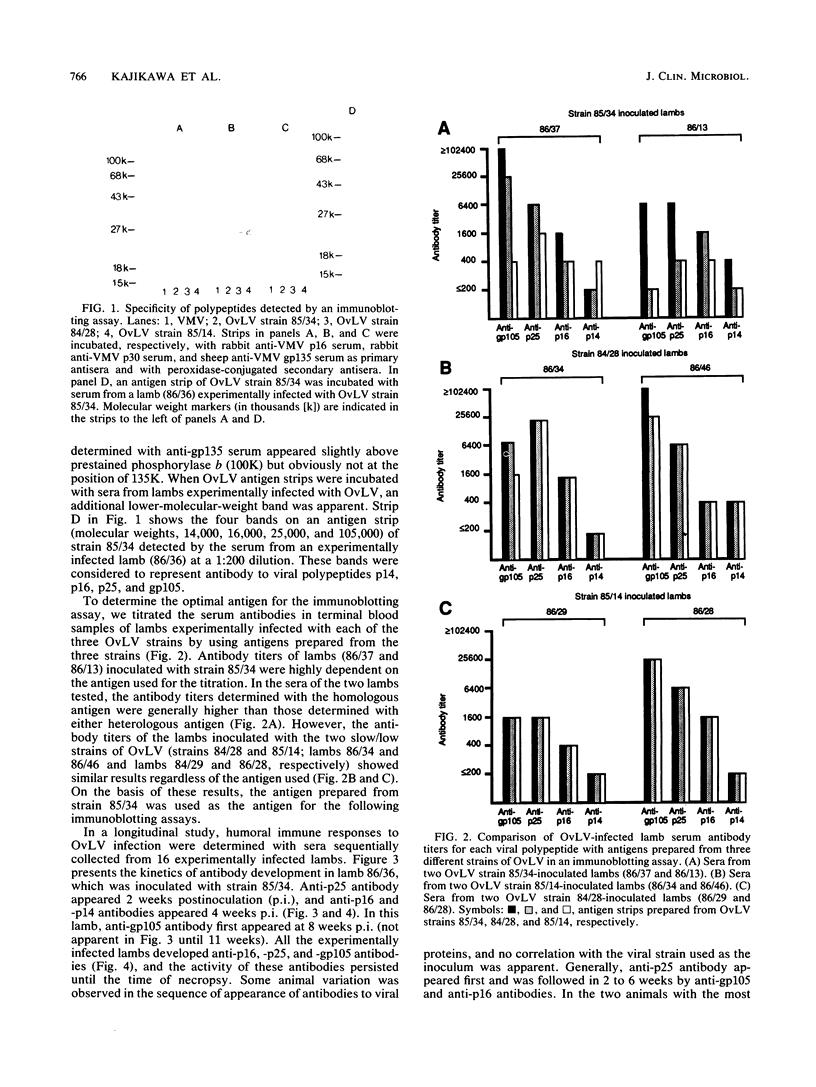
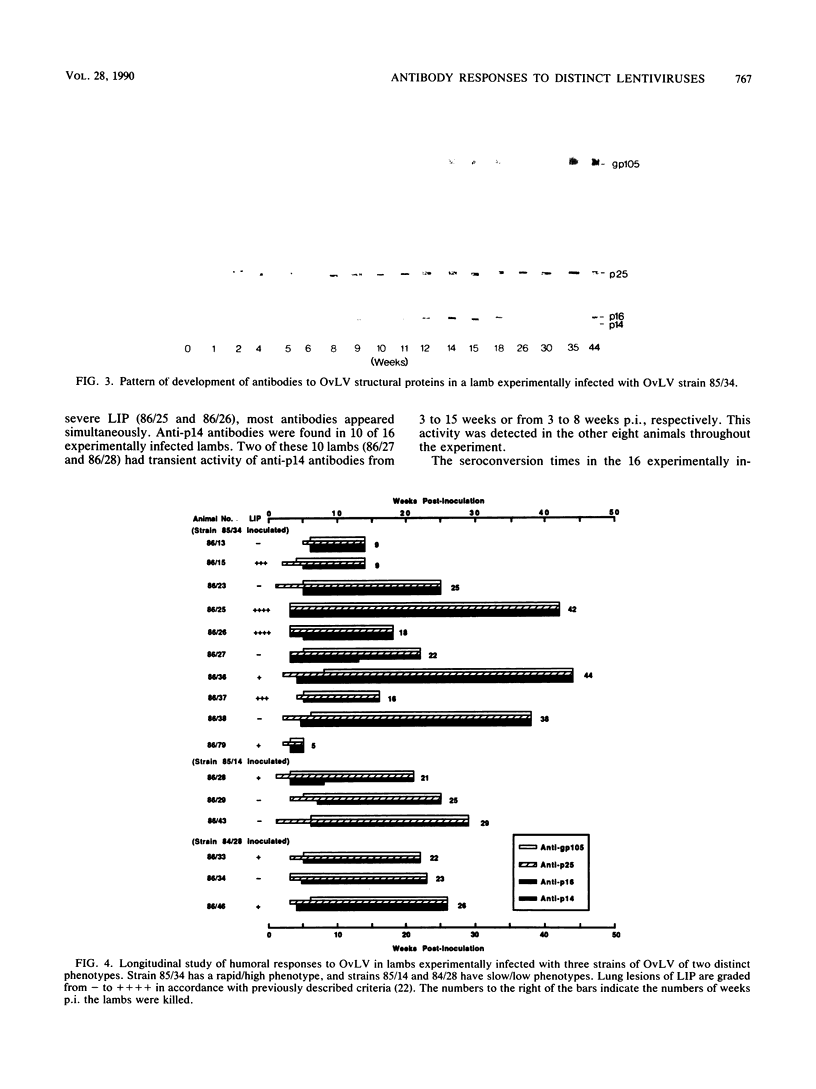
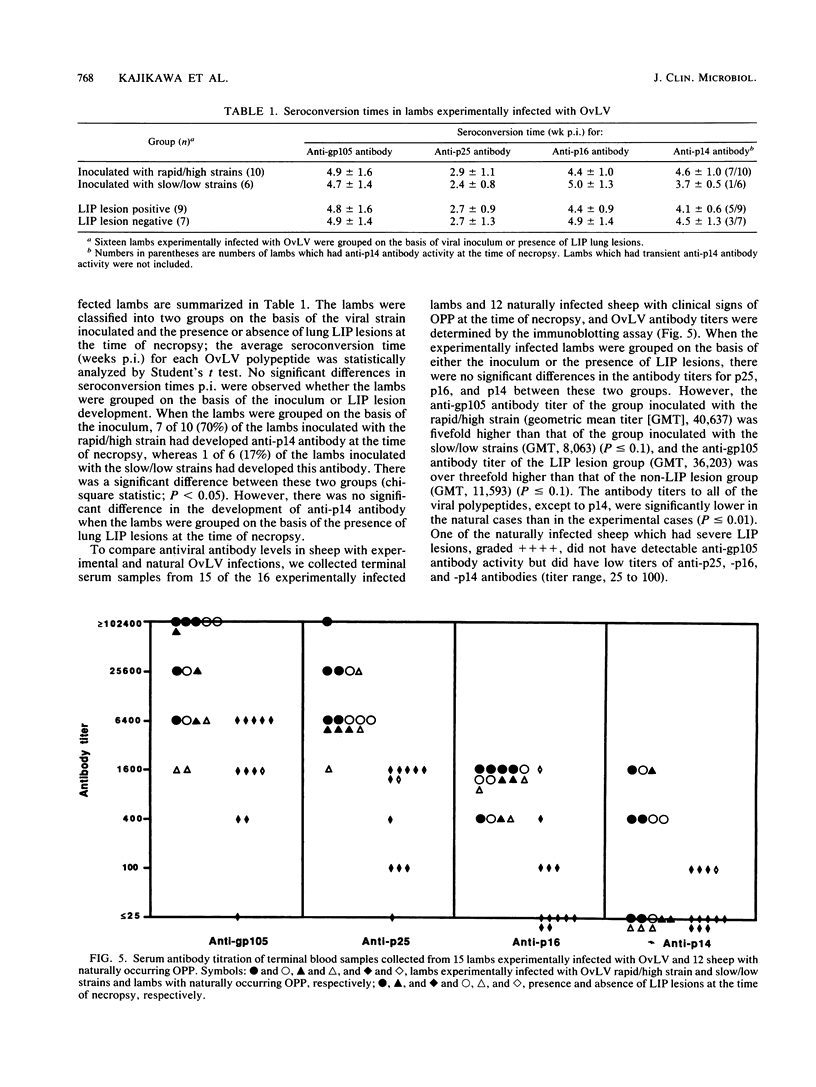
To define the immune responses against phenotypically and pathogenically distinct lentiviruses, we used an immunoblotting assay to study antibodies to viral proteins of ovine lentivirus (OvLV) in 16 experimentally and 12 naturally infected sheep. Two distinct phenotypes of OvLV were used to experimentally infect lambs: strain 85/34, a "rapid/high" isolate which rapidly induced lysis in infected primary macrophage cultures and replicated to relatively high titers, and strains 84/28 and 85/14, "slow/low" isolates which induced slowly progressive syncytia with minimal lysis in vitro and replicated only to low titers in the same cell type. Serum antibodies against four major viral structural proteins, gp105, p25, p16, and p14, were detected. In a longitudinal study of experimentally infected lambs, the antibody to p25 (major gag protein) usually appeared first (average, about 3 weeks postinoculation [p.i.]) and was followed in about 2 weeks by p16, p14, and gp105 almost simultaneously. Six of 16 animals did not develop anti-p14 antibody by the time of necropsy at 9 to 29 weeks p.i. Two of 10 lambs which developed antibody to p14 had the antibody only transiently from 3 to 8 or 13 weeks p.i. and lost it by the time of necropsy at 21 or 22 weeks p.i. In contrast, antibodies to the other three structural proteins remained fairly constant until the time of necropsy. There were differences in the antibody responses of the experimentally infected lambs to the two phenotypes of OvLV. Seven of 10 (70%) lambs which were inoculated with the rapid/high strain developed antibody to p14, whereas only 17% of the lambs inoculated with the slow/low strains had antibody to this protein. In the longitudinal study, no decline was observed in the activity of any specific antibody such as that which occurs with anti-p24 antibody in human immunodeficiency virus infection, except in the case of anti-p14 antibody in two lambs. There were no significant differences in antibody titers against p25, p16, and p14 in final blood samples between rapid/high virus- and slow/low virus-infected groups. However, the rapid/high virus-infected group developed a fivefold-higher geometric mean titer of anti-env product (gp 105) antibody than did the slow/low virus-infected group (P </= 0.1). Antibody titers to all major structural proteins, except p14, in the naturally infected sheep were markedly lower than those in experimentally induced OvLV infections (P </= 0.01). The failure of the slow/low virus-infected group to develop anti-p14 antibody may suggest diminished viral replication in vivo or a failure of the host to recognize p14 in the slow/low virus-infected group. Since the geometric mean antibody titer to gp105 was threefold higher in lambs with lymphoid interstitial pneumonia than in those without lesions and since no differences were observed in the titers of other antiviral antibodies between these groups, we found no evidence to suggest that levels of such antibodies correlated with protection from OvLV-induced disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosgiraud C., Rucheton M., Coste J., Lemaire J. M., Nicolas J. A., Simeon de Buochberg M., Beaubatie L. Détection d'anticorps et d'antigènes du virus de Visna-Maedi par immunotransfert. Ann Rech Vet. 1989;20(2):187–193. [PubMed] [Google Scholar]
- Chou M. J., Lee T. H., Hatzakis A., Mandalaki T., McLane M. F., Essex M. Antibody responses in early human immunodeficiency virus type 1 infection in hemophiliacs. J Infect Dis. 1988 Apr;157(4):805–811. doi: 10.1093/infdis/157.4.805. [DOI] [PubMed] [Google Scholar]
- Cutlip R. C., Jackson T. A., Laird G. A. Prevalence of ovine progressive pneumonia in a sampling of cull sheep from western and midwestern United States. Am J Vet Res. 1977 Dec;38(12):2091–2093. [PubMed] [Google Scholar]
- Cutlip R. C., Jackson T. A., Lehmkuhl H. D. Lesions of ovine progressive pneumonia: interstitial pneumonitis and encephalitis. Am J Vet Res. 1979 Oct;40(10):1370–1374. [PubMed] [Google Scholar]
- Cutlip R. C., Lehmkuhl H. D., Schmerr M. J., Brogden K. A. Ovine progressive pneumonia (maedi-visna) in sheep. Vet Microbiol. 1988 Jul;17(3):237–250. doi: 10.1016/0378-1135(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Gaskin J. M., Perk K. Morphological and immunological comparison of caprine arthritis encephalitis and ovine progressive pneumonia viruses. J Virol. 1981 Sep;39(3):914–919. doi: 10.1128/jvi.39.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. Lentivirus diseases of domesticated animals. J Comp Pathol. 1988 Nov;99(4):401–419. doi: 10.1016/0021-9975(88)90059-x. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Lange J. M., Paul D. A., Dawson G. J. Antigenemia and antibody titers to core and envelope antigens in AIDS, AIDS-related complex, and subclinical human immunodeficiency virus infection. J Infect Dis. 1987 Mar;155(3):558–560. doi: 10.1093/infdis/155.3.558. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Pomerantz R. J., Kaplan J. C. Pathogenesis of infection with human immunodeficiency virus. N Engl J Med. 1987 Jul 30;317(5):278–286. doi: 10.1056/NEJM198707303170505. [DOI] [PubMed] [Google Scholar]
- Houwers D. J., Nauta I. M. Immunoblot analysis of the antibody response to ovine lentivirus infections. Vet Microbiol. 1989 Feb;19(2):127–139. doi: 10.1016/0378-1135(89)90078-3. [DOI] [PubMed] [Google Scholar]
- Huffman E. M., Kirk J. H., Winward L., Gorham J. R. Serologic prevalence of ovine progressive pneumonia in a western range flock of sheep. J Am Vet Med Assoc. 1981 Apr 1;178(7):708–710. [PubMed] [Google Scholar]
- Isaksson B., Albert J., Chiodi F., Furucrona A., Krook A., Putkonen P. AIDS two months after primary human immunodeficiency virus infection. J Infect Dis. 1988 Oct;158(4):866–868. doi: 10.1093/infdis/158.4.866. [DOI] [PubMed] [Google Scholar]
- Joshi V. V., Oleske J. M., Minnefor A. B., Saad S., Klein K. M., Singh R., Zabala M., Dadzie C., Simpser M., Rapkin R. H. Pathologic pulmonary findings in children with the acquired immunodeficiency syndrome: a study of ten cases. Hum Pathol. 1985 Mar;16(3):241–246. doi: 10.1016/s0046-8177(85)80009-5. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Cabradilla C. D., Getchell J. P., Narayanan R., Braff E. H., Chermann J. C., Barré-Sinoussi F., Montagnier L., Spira T. J., Kaplan J. Antibodies to the core protein of lymphadenopathy-associated virus (LAV) in patients with AIDS. Science. 1984 Jul 20;225(4659):321–323. doi: 10.1126/science.6330889. [DOI] [PubMed] [Google Scholar]
- Lairmore M. D., Akita G. Y., Russell H. I., DeMartini J. C. Replication and cytopathic effects of ovine lentivirus strains in alveolar macrophages correlate with in vivo pathogenicity. J Virol. 1987 Dec;61(12):4038–4042. doi: 10.1128/jvi.61.12.4038-4042.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Poulson J. M., Adducci T. A., DeMartini J. C. Lentivirus-induced lymphoproliferative disease. Comparative pathogenicity of phenotypically distinct ovine lentivirus strains. Am J Pathol. 1988 Jan;130(1):80–90. [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Rosadio R. H., DeMartini J. C. Ovine lentivirus lymphoid interstitial pneumonia. Rapid induction in neonatal lambs. Am J Pathol. 1986 Oct;125(1):173–181. [PMC free article] [PubMed] [Google Scholar]
- Lange J. M., Coutinho R. A., Krone W. J., Verdonck L. F., Danner S. A., van der Noordaa J., Goudsmit J. Distinct IgG recognition patterns during progression of subclinical and clinical infection with lymphadenopathy associated virus/human T lymphotropic virus. Br Med J (Clin Res Ed) 1986 Jan 25;292(6515):228–230. doi: 10.1136/bmj.292.6515.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J. M., Paul D. A., Huisman H. G., de Wolf F., van den Berg H., Coutinho R. A., Danner S. A., van der Noordaa J., Goudsmit J. Persistent HIV antigenaemia and decline of HIV core antibodies associated with transition to AIDS. Br Med J (Clin Res Ed) 1986 Dec 6;293(6560):1459–1462. doi: 10.1136/bmj.293.6560.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J. M., van den Berg H., Dooren L. J., Vossen J. M., Kuis W., Goudsmit J. HTLV-III/LAV infection in nine children infected by a single plasma donor: clinical outcome and recognition patterns of viral proteins. J Infect Dis. 1986 Jul;154(1):171–174. doi: 10.1093/infdis/154.1.171. [DOI] [PubMed] [Google Scholar]
- Leis J., Baltimore D., Bishop J. M., Coffin J., Fleissner E., Goff S. P., Oroszlan S., Robinson H., Skalka A. M., Temin H. M. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988 May;62(5):1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Kaminsky L. S., Morrow W. J., Steimer K., Luciw P., Dina D., Hoxie J., Oshiro L. Infection by the retrovirus associated with the acquired immunodeficiency syndrome. Clinical, biological, and molecular features. Ann Intern Med. 1985 Nov;103(5):694–699. doi: 10.7326/0003-4819-103-5-694. [DOI] [PubMed] [Google Scholar]
- Narayan O., Cork L. C. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985 Jan-Feb;7(1):89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Silverstein A. M. Slow virus infection: replication and mechanisms of persistence of visna virus in sheep. J Infect Dis. 1977 May;135(5):800–806. doi: 10.1093/infdis/135.5.800. [DOI] [PubMed] [Google Scholar]
- Pedersen C., Nielsen C. M., Vestergaard B. F., Gerstoft J., Krogsgaard K., Nielsen J. O. Temporal relation of antigenaemia and loss of antibodies to core antigens to development of clinical disease in HIV infection. Br Med J (Clin Res Ed) 1987 Sep 5;295(6598):567–569. doi: 10.1136/bmj.295.6598.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quérat G., Barban V., Sauze N., Filippi P., Vigne R., Russo P., Vitu C. Highly lytic and persistent lentiviruses naturally present in sheep with progressive pneumonia are genetically distinct. J Virol. 1984 Nov;52(2):672–679. doi: 10.1128/jvi.52.2.672-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quérat G., Barban V., Sauze N., Vigne R., Payne A., York D., De Villiers E. M., Verwoerd D. W. Characteristics of a novel lentivirus derived from South African sheep with pulmonary adenocarcinoma (jaagsiekte). Virology. 1987 May;158(1):158–167. doi: 10.1016/0042-6822(87)90249-2. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Haller O., Vogt M., Lüthy R., Joller H., Oelz O., Popovic M., Sarngadharan M. G., Gallo R. C. Antibodies to HTLV-III in Swiss patients with AIDS and pre-AIDS and in groups at risk for AIDS. N Engl J Med. 1985 Jan 31;312(5):265–270. doi: 10.1056/NEJM198501313120502. [DOI] [PubMed] [Google Scholar]
- Stanley J., Bhaduri L. M., Narayan O., Clements J. E. Topographical rearrangements of visna virus envelope glycoprotein during antigenic drift. J Virol. 1987 Apr;61(4):1019–1028. doi: 10.1128/jvi.61.4.1019-1028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne R., Filippi P., Quérat G., Sauze N., Vitu C., Russo P., Delori P. Precursor polypeptides to structural proteins of visna virus. J Virol. 1982 Jun;42(3):1046–1056. doi: 10.1128/jvi.42.3.1046-1056.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]