Abstract
The intrinsic primary afferent neurons (IPANs) of the guinea pig enteric nervous system express Nav1.9 sodium channels that produce a persistent TTX-resistant current having a low activation threshold and slow gating kinetics. These neurons receive slow EPSPs induced mainly by the activation of neurokinin 3 receptors (NK3r). Here, we demonstrate that senktide, a specific NK3r agonist, potentiates the Nav1.9 current (INav1.9) in IPANs. Using whole-cell patch-clamp recordings from IPANs in duodenum longitudinal muscle/myenteric plexus preparations, we show that short (1–5 s) and long (up to 1 min) applications of senktide, increase the INav1.9 peak current up to 13-fold. The effect, blocked by a NK3r antagonist SB235375 is transient, lasting ∼2 min and is due to a negative shift of the activation voltage by ∼20 mV and of fast inactivation by ∼10 mV. As a consequence, the window current resulting from the product of the activation and fast inactivation curves is shifted and enlarged. The transient effect of senktide is likely to be due to the fast desensitization of NK3r. Protein kinase C (PKC) activation with phorbol or oleoyl acetylglycerol also increases INav1.9, although persistently, by inducing similar voltage-dependent changes. Current-clamp experiments showed that INav1.9 modulation by senktide lowers action potential threshold and increases excitability. The increase in INav1.9 by NK3r activation is also likely to amplify slow EPSPs generated in the IPANs. These changes in excitability potentially have a profound effect on the entire enteric synaptic circuit and ultimately on gut motility and secretion.
The enteric nervous system (ENS), embedded within the gut wall, contains all the basic components of polysynaptic reflex arcs, that is, primary afferent neurons, interneurons and motor neurons. The activation of these reflex arcs produces motility, secretomotor and vasomotor reflexes. Stimuli that indicate the mechanical state of the gastrointestinal tract and the presence, the consistency and the chemical nature of the luminal contents activate the reflexes. These sensory stimuli are transduced by the ENS primary afferent neurons, usually called intrinsic primary afferent neurons (IPANS; see Furness et al. 1995; Clerc & Furness 2004). IPANs transfer sensory information to interneurons or to motor neurons via slow EPSPs (Bornstein et al. 1991; Kunze et al. 1993).
We have previously established that among the 10 known Na+ channels, only three are expressed in adult guinea pig IPANs: Nav1.3, Nav1.7 and Nav1.9, (Rugiero et al. 2003; Sage et al. 2007; D. Sage, R. Levinson and N. Clerc, unpublished immunohistochemical data on Nav1.7 expression). Nav1.7 and Nav1.3, also expressed in other myenteric neuron types and in normal (Nav1.7) and axotomized (Nav1.3) nociceptive dorsal root ganglion (DRG) neurons are known to be sensitive to TTX and responsible for the upstroke of the action potential (Rush et al. 2007). Nav1.9 was detected within the soma and proximal axons of myenteric and submucosal IPANS (Rugiero et al. 2003; Padilla et al. 2007). The Nav1.9 current (INav1.9) is resistant to TTX and expressed exclusively in the IPANs (Rugiero et al. 2003). Important characteristics of INav1.9 are that it generates a persistent current due to a large window current and that it is activated around the resting potential. These characteristics have been revealed in both IPANs (Rugiero et al. 2003) and DRG nociceptive neurons (Dib-Hajj et al. 1998, 2002; Cummins et al. 1999; Coste et al. 2004, 2007).
An increase in the persistent Na+ current, due to Nav1.9 activation by G-protein pathways, is crucial in nociceptive sensory DRG neurons (Baker et al. 2003; Ostman et al. 2008; Maingret et al. 2008) as the potentiated Nav1.9 current acts as a threshold channel that tunes the voltage threshold for action potentials and generates plateau potentials (Ostman et al. 2008; Maingret et al. 2008). In these neurons, such a role has been proven by comparative experiments performed on wild-type and Nav1.9−/− mice, showing that GTP-γ-S alters the action potential threshold only when Nav1.9 is present (Ostman et al. 2008; Maingret et al. 2008). This effect of GTP-γ-S reflects the action of this specific activator of G-proteins on INav1.9 voltage-dependent properties (Maingret et al. 2008). The G-protein pathways that modulate the Nav1.9 current have been recently shown to be activated by a mixture of inflammatory mediators (Maingret et al. 2008) although each of the components of the mixture (ATP, bradykinin, noradrenaline, prostaglandin E2 and histamine) applied separately failed to modulate the current (Zheng et al. 2007; Maingret et al. 2008). Whether neurokinins, which are involved in enteric neurotransmission and in inflammatory processes, act on Nav1.9 is not known.
IPANs are organized in a self-reinforcing network (see Clerc & Furness, 2004), through which their cell bodies receive slow synaptic inputs arising mainly from other IPANs. These slow EPSPs are due to the activation of the neurokinin receptors NK1 (NK1r) and NK3 (NK3r) with a predominance of the NK3r component (Bertrand & Galligan, 1994; Alex et al. 2001; Johnson & Bornstein, 2004). Transmitter activation triggers slow EPSPs by inhibiting a calcium-dependent potassium conductance (gKCa), inhibiting a background (TASK1) gK and increasing a chloride conductance (gCl; North & Tokimasa, 1983; Bertrand & Galligan, 1994; Matsuyama et al. 2008). We hypothesized that NK3r activation might also influence IPANs by an action on Nav1.9 channels. We performed patch-clamp recordings on IPANs maintained in their normal tissue environment (which includes their synaptic relations with other neurons, and the presence of enteric glia, interstitial cells and longitudinal smooth muscle cells). We demonstrate that the Nav1.9 current is transiently but substantially increased by the activation of NK3r. We also show that this increase is caused by a negative shift of the INav1.9 window current.
Methods
All experiments were performed in accordance with the directives of the European Communities Council (86/609/EEC) and all efforts were made to minimize both animal suffering and numbers of animals used. Ninety-five Hartley strain guinea pigs weighing 280–400 g from the inbred colony of the ‘Institut Fédératif de Recherche Jean Roche, Marseille’ were killed by stunning and by severing the carotid arteries and spinal cord.
The duodenum was quickly removed and transferred to Krebs solution (in mm: 118 NaCl; 4.8 KCl; 1.2 MgCl2; 1 NaH2PO4; 25 NaHCO3; 2.5 CaCl2, and 11.1 d-glucose) equilibrated with 95% O2 and 5% CO2 and to which 1 μm atropine (Sigma, St Louis, MO, USA) and 3 μm nicardipine (ICN Biomedicals Inc., Aurora, OH, USA) were added in order to induce muscle relaxation.
Duodenum segments, 0.5–1 cm long, opened along the mesenteric line, were stretched and pinned mucosal side up on the silicone elastomer base (Sylgard, Dow Corning, MI, USA) of a Petri dish containing oxygenated standard Krebs solution (see above). The myenteric plexus was exposed by dissecting away the mucosa, the submucosal plexus and the circular muscle layer. The Petri dish was then placed on an inverted microscope stage and the longitudinal muscle/myenteric plexus (LMMP) preparation was continuously superfused with the oxygenated Krebs solution at room temperature. The upper surface of a ganglion was exposed to 0.01% protease type XIV (Sigma, St Louis, MO, USA) for 2–4 min and the neuron surface was cleaned by sweeping over the ganglion with a hair fixed at the tip of a microelectrode.
Whole-cell patch-clamp recording of in situ myenteric neurons
Electrical activities were recorded using the whole-cell patch-clamp configuration (Rugiero et al. 2002, 2003) using either a List EPC-7 or an Axopatch 200B amplifier (Axon Instruments, Molecular Devices, Sunnyvale, CA, USA). Pipettes were pulled from borosilicate glass capillaries with a Sutter P-97 puller (Sutter Instruments, Novato, CA, USA) and had resistances of 1.8–2.5 MΩ. The experiments were controlled, and data recorded, using an in-house software or pCLAMP 9 (Axon Instruments, Molecular Devices). Currents were low-pass filtered at 2 kHz and sampled at 10–44 kHz. After seal rupture, series resistance (4–10 MΩ) was compensated (∼70%) and periodically monitored. Because whole-cell currents never exceeded 2 nA, errors in voltage owing to inadequate compensation should never have exceeded a few millivolts. Data were leak-subtracted using either the P/6 subtraction procedure of pCLAMP 9 or scaled current sweeps derived from small hyperpolarizing voltage commands. The pipette potential was zeroed before seal formation and voltages were not corrected for liquid junction potentials (5–6 mV). Recordings were performed at room temperature.
Voltage-clamp experiments were performed using both standard and inverse Na+ gradient (Osorio et al. 2005). In standard gradient conditions, the extracellular solution was as follows (mm): 140 NaCl, 4 KCl, 1 MgCl2, 2.5 CaCl2, 10 Hepes and 11 d-glucose. The intra-pipette solution contained (mm): 4 NaCl, 140 CsCl, 2 MgCl2, 1 CaCl2, 10 Hepes and 2 EGTA (pH 7.3). Internal CsCl was chosen instead of CsF, which is often used for recording INav1.9 in DRG neurons (Cummins et al. 1999; Coste et al. 2004). Fluoride would have masked the effect of NK3r activation because it shifts INav1.9 voltage dependence in both IPANs and DRG neurons (Rugiero et al. 2003; Coste et al. 2004). In these conditions the theoretical reversal potential for Na+ was +89 mV. Note that ATP was not added to the internal milieu in order to avoid muscle contractions that result from pipette leakage during seal formation. In inverse Na+ gradient conditions, the extracellular solution was (mm): 5 NaCl, 210 sucrose, 4 KCl, 1 MgCl2, 2.5 CaCl2, 10 Hepes and 11 d-glucose; the pipette solution consisted of (mm): 140 NaCl, 10 CsCl, 2 MgCl2, 1 CaCl2, 10 Hepes and 2 EGTA (pH 7.3). The theoretical reversal potential for Na+ in inverse gradient was −83 mV. Isolation of the Nav1.9 current was achieved by adding CdCl2 (0.5 mm), 4-AP (1 mm), TEACl (5 mm), CsCl (2 mm) and TTX (300 nm) to the extracellular solution.
Current-clamp experiments were performed using the solutions described for the standard Na+ gradient conditions for voltage-clamp recording. However, in some current-clamp experiments, TEA, 4-AP and TTX were omitted from the extracellular solution whereas CsCl2 (2 mm), CdCl2 (0.5 mm) and methanandamide (10 μm; Sigma, St Louis, MO, USA), a selective TASK1 blocker (Maingret et al. 2001), were present. For these experiments, KCl replaced CsCl in the pipette. Thus action potentials could be recorded in quasi-physiological conditions.
Extracellular solutions were locally applied via a gravity superfusion system delivering the solutions at a rate of 1 ml min−1 and terminated by a manifold, whose tip (300 μm diameter) was positioned at about 800 μm from the neuron of interest.
Pharmacology
Senktide (4 μm; Sigma), a selective NK3r agonists and substance P (1 μm, Sigma), were added to the extracellular solution and applied locally in order to avoid activation of NK3r in the whole preparation. The selective NK3r antagonist SB235375 (1 μm; a gift from Gareth Sanger, GlaxoSmithKline, Harlow, Essex, UK) was diluted into the Krebs solution bathing the LMMP preparation in order to incubate the whole preparation. The PKC activators 1-oleoyl-2-acetyl-sn-glycerol (OAG; Sigma, St Louis, MO, USA) and phorbol 12,13-dibutyrate (PdBu; Sigma, St Louis, MO, USA), initially dissolved in dimethylsulfoxide (DMSO), were diluted in the extracellular solution and used at 1 μm. The PKC inhibitor chelerythrine chloride (1 μm; Sigma, St Louis, MO, USA) was added to the intrapipette solution as described by Kotecha et al. (2003).
Data analysis and modelling
Data were analysed using pCLAMP 9 (Axon Instruments, Molecular Devices, Orleans, CA, USA), Sigma plot (Systat Software, Inc. San Jose, CA, USA) and PRISM 4.0 (GraphPad, San Diego, CA, USA). Conductance–voltage curves were calculated from the peak current according to the equation GNav1.9=INav1.9/(V−ENa) were V is the test pulse potential and ENa the reversal potential extrapolated from the current–voltage relationship. The activation curves (G–V) were fitted using the Boltzmann function G/Gmax= 1/(1 + exp[(V−V1/2)/p)], where G/Gmax is the normalized Nav1.9 conductance, V1/2 is the potential of half-maximum activation and p is the steepness factor. Data fittings with exponential functions, I=ΣAi exp(−t/τi) +C, were carried out using Clampfit.
To simulate the Nav1.9 current, equations predicting the values for the activation and fast inactivation of the current were developed. We applied a modified Hodgkin–Huxley equation of the following form:
| (1) |
where
| (2) |
and m and h are the probabilities of the activation and inactivation particles, respectively to be in the permissive position. Kinetics of INav1.9 were derived from new data and from previously published data (Rugiero et al. 2003; Maingret et al. 2008). m∞(V) was derived directly from the GNav1.9 activation curve. h∞(V) was derived directly from the GNav1.9 steady-state fast inactivation curve. The transitions of the inactivating particle h were modelled according the following first-order kinetic scheme from which it follows:
| (3) |
where
| (4) |
and
| (5) |
Numerical values for the rate constants α and β were derived from experimental values of time constants of fast inactivation, and recovery from fast inactivation (not illustrated), and from the h∞ curve by applying eqns (4) and (5). After obtaining the analytical functions describing the voltage dependence of the rate constants, the time course of INav1.9 activated in response to voltage steps was numerically reconstructed on the basis of eqns (1) and (2), and eqn (3) in its differential form.
In time course plots of the senktide effects, T= 0 refers to the beginning of senktide application and INav1.9 was normalized to peak amplitude. The normalized currents obtained every 5 s were then averaged and expressed as mean ±s.e.m. Statistical analysis was performed using Wilcoxon and Mann–Whitney tests for paired and unpaired tests, respectively (Graph Pad InStat 3 software) and the Kruskal–Wallis test for the multiple comparison tests. P < 0.05 was considered as statistically significant.
Results
NK3 receptor stimulation transiently increased Nav1.9 current
Whole-cell patch-clamp recordings were made from non-dissociated myenteric neurons of guinea pig duodenum (Rugiero et al. 2002). As previously described, in guinea pig IPANs, Nav1.9 is selectively expressed in the absence of Nav1.8 (Rugiero et al. 2003). INav1.9 was thus routinely isolated with TTX added to the bath solution. It was studied using a normal driving force for Na+ and a CsCl-based pipette solution (see Methods). Typically, INav1.9 had a threshold of activation at about −40 mV, peaked at −10 mV and exhibited a non-inactivating current component that was prominent between −40 and +20 mV (Fig. 1A and Ba). Its voltage of half-maximum activation (V1/2) was −19.2 ± 1.7 mV (n= 7; Fig. 1Bb).
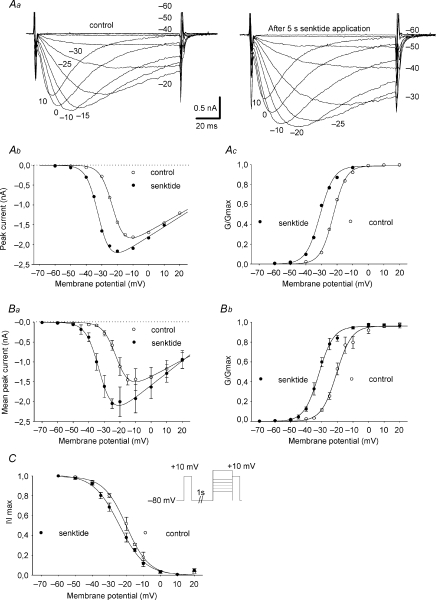
Figure 1. Increase of the Nav1.9 current by activating NK3r. Recordings were made from enteric IPANs using whole-cell patch-clamp electrodes.
A: family of Nav1.9 current traces in control conditions. Nav1.9 currents were isolated by including TTX (300 nm) in the extracellular solution and were evoked by stepping from −60 to +20 mV in 5 or 10 mV increments from a holding potential of −80 mV. Ba and b: mean peak current (n= 8; a) and normalized conductance (G/Gmax; b) obtained in control conditions and plotted versus membrane potential. In Bb, G/Gmax was fitted by a single Boltzmann function giving V1/2=−19.1 ± 1.3 mV and a slope factor p= 4.4 ± 0.4 mV. Ca and b: transient increase of Nav1.9 current induced by a 5 s application of senktide (4 μm). Ca: Nav1.9 currents evoked successively by depolarizing steps to −30 mV from −80 mV applied every 5 s. Cb: superimposed Nav1.9 current traces evoked before (1) and after (2 and 3) a 5 s senktide application at the times indicated in Ca. D: Nav1.9 currents evoked by depolarizing steps to −30 mV from a holding potential of −80 mV. Normalized peak Nav1.9 currents, obtained as in Ca, were plotted against time, with T= 0 referring to the beginning of the 5 s senktide application (n= 4). Ea and b: selective involvement of NK3r in Nav1.9 current increase. Ea: mean normalized Nav1.9 peak currents evoked by depolarizing steps to −20 from −80 mV applied every 5 s, before and after 5 s senktide applications, in the continued presence of the NK3r antagonist SB235375 (1 μm; n= 8). Eb: histogram showing the percentage of current increase in response to 5 s applications of senktide in the absence (n= 4) or presence of SB235375 (n= 8). Mann–Whitney test: P= 0.004. Fa and b: transient increase of Nav1.9 current induced by a 5 s application of substance P (1 μm). Fa: Nav1.9 currents evoked successively by depolarizing steps to −10 mV from −80 mV applied every 5 s. Fb: superimposed Nav1.9 current traces evoked before (1) and after (2) the substance P application at the times indicated in Fa.
Activation of NK3r was induced using senktide (4 μm), a specific NK3r agonist. Brief applications of senktide for 1–5 s produced a marked increase in Nav1.9 current amplitude evoked by depolarizing steps to −30 mV from a holding potential of −80 mV (Fig. 1Ca and b). On average, Nav1.9 current underwent an increase up to ∼13−fold in amplitude at −30 mV within 10–15 s of the onset of the senktide application (Fig. 1C and D and Fig. 2Ba and b). After a similar delay, the endogenous transmitter substance P (1 μm) also induced a large increase (3.94 ± 0.32 fold, n= 5) of the amplitude of the current evoked by depolarizing steps to −10 mV from a holding potential of −80 mV as illustrated in Fig. 1Fa and b. INav1.9 potentiation by senktide was prevented by pre-treating LMMP preparations with the NK3r antagonist SB235375 (1 μm for 30 min), demonstrating selective mediation by NK3r (Fig. 1Ea and b).
Figure 2. Time course of NK3r-mediated increase in Nav1.9 current.
Aa and b: the duration of the senktide application did not influence the time course of decline in the NK3r-mediated effect on Nav1.9 current. Aa: normalized Nav1.9 currents evoked by depolarizing steps to −30 mV from a holding potential of −80 mV and recorded when senktide was applied for 1 s (n= 7), 15 s (n= 6), 30 s (n= 4) and 60 s (n= 5). Average time constants, τ, of the decay of the senktide effects, determined by monoexponential fits, ranged from 16.9 to 29.2 s. Ab: absence of correlation between the time constants of the decay of the senktide effects and the durations of senktide applications. B: desensitization of the responses to senktide in neurons exposed to 2 successive applications of senktide (1 s, St1 and St2) separated by a 5 min interval. Inset: histogram showing the mean increase induced by St1 and St2 in Nav1.9 currents evoked by depolarizing steps to −30 mV from a holding potential of −80 mV (mean peak INav1.9 in response to St1: from 0.2 ± 0.10 to 2.2 ± 0.6 nA; mean peak INav1.9 in response to St2: from 0.2 ± 0.06 to 0.8 ± 0.3 nA; n= 6; Wilcoxon test: P= 0.0313).
As shown in Fig. 1Ca and D, potentiation of INav1.9 by 5 s senktide applications was transient and the current returned progressively to its initial amplitude with a decay time constant (τ) of 27.7 s. Similar decay time constants, from the time of maximal amplitude, were measured when the exposure to the agonist was shortened (1 s) or increased up to 1 min (Fig. 2A), suggesting desensitization of NK3r. Consistently, in a sample of six neurons in which 1 s exposure to senktide was applied twice, with a 5 min interval, the second response was reduced by ∼65% compared with the first response (Fig. 2B).
Effect of the holding potential on Nav1.9 current potentiation
G-protein-coupled receptors inhibit TTX-sensitive Na+ channel currents and this modulation depends on the holding potential (Cantrell et al. 1999; Carr et al. 2003). We thus examined whether facilitation of INav1.9 by NK3r activation was dependent on the holding potential. To test this, senktide was applied for 5 s and INav1.9 was evoked at −30 mV from holding potentials ranging from −60 to −90 mV. As shown in Fig. 3Aa, the senktide-mediated increase in INav1.9 amplitude was not significantly different at these different holding potentials. However, the decay time constant became much slower at −90 mV (τ= 61 s; n= 7, Fig. 3Aa, b and c) compared with those determined at −60 mV (τ= 20.7 s, n= 7; Fig. 3Ab) and −80 mV (τ= 27.7 s; n= 4; Fig. 1D). More hyperpolarized holding potentials (−100 mV) were tested in two neurons exposed to 5 s senktide applications, giving decay time constants of 71.4 s and 73.5 s.
Figure 3. Voltage dependence of the change in Nav1.9 current by NK3r activation.
Aa–c: effect of hyperpolarizing the holding potential on senktide responses. Aa: histogram showing that the peak amplitude of the Nav1.9 current reached after NK3r activation is not significantly affected by changing the holding potential (Kruskal–Wallis test : P= 0.06). Ab and Ac: normalized Nav1.9 currents evoked by depolarizing steps to −30 mV from a holding potential of either −60 mV (b; n= 7) or – 90 mV (c; n= 6). The time constants of the decay of the senktide effect, determined by fitting the decay period by a monoexponential function, increased significantly at −90 mV (Mann–Whitney test: P= 0.0023). Ba and b: the increase in the Nav1.9 current depends on the depolarizing step amplitude. Ba: Nav1.9 currents evoked at 5 s intervals by paired depolarizing steps to −30 mV (upper traces and inset) and 0 mV (lower traces and inset) from −80 mV. Insets: superimposed traces 1 and 2 obtained before and 15 s after 1 s senktide application. Bb: histogram comparing the percentage of peak current increase of Nav1.9 currents evoked by depolarizing steps to different potentials. Mann–Whitney test: −20 mV (n= 9)/+20 mV (n= 17): P= 0.0183; −30 mV (n= 24)/0 mV (n= 15): P < 0.0001; −30 mV (n= 24)/+20 mV (n= 17): P < 0.0001.
NK3r-mediated changes in Nav1.9 current are voltage dependent
The relative increase in INav1.9 amplitude induced by NK3r activation varied strongly according to the test potential (Fig. 3Ba and b). Only a 2-fold increase in INav1.9 was seen at a depolarizing test pulse of 0 mV (Fig. 3Ba and b) whereas an increase up to 13-fold was typically observed at a test pulse of −30 mV (Fig. 3Ba and b). These data show that the effects of senktide were dependent on the open probability of Nav1.9 channels, suggesting that senktide potentiates INav1.9 through a change in the voltage-dependent gating of Nav1.9 channels.
In order to quantify the changes that affect specifically the activation and fast inactivation, we compared the steady-state activation and inactivation properties of INav1.9 before and during senktide application. However, because the effects of senktide were transient, activation and inactivation protocols were applied between the 10 and 100 s after the onset of senktide application, when the effects of senktide were still manifest but declining. The activation curves were determined for each cell in the presence and absence of senktide, using 100 ms-graded depolarizations from a holding potential of −80 mV (Fig. 4Aa). The current–voltage relationships and the corresponding peak-conductance relationships of INav1.9 show that the half-activation voltage was shifted toward more hyperpolarized potentials upon exposure to senktide (Fig. 4Ab and c). Boltzmann functions fitted to the conductance–voltage relationships constructed using normalized data from seven neurons gave an average negative shift of ∼10 mV, with no change in the slope factor (Fig. 4Ba and b).
Figure 4. Changes in voltage-dependent properties of Nav1.9 current during the senktide effects.
Aa–c: senktide negatively shifts the voltage dependence of Nav1.9 activation. Aa: families of Nav1.9 current traces obtained before (left panel) and after 5 s application (right panel) of senktide. Currents were evoked every 10 s by stepping from −60 to 10 mV from a holding potential of −80 mV. Nav1.9 current–voltage relationships (Ab) and corresponding activation curves (G/Gmax, Ac) determined from the traces shown in Aa. Ac: the data points are fitted with a Boltzmann function giving V1/2 values of −22.4 mV and −31 mV in the absence and presence of senktide, respectively. Ba and b: mean peak current (a) and normalized conductance (G/Gmax; b) plotted versus membrane potential, in the presence or absence of senktide. In Bb, V1/2control (−19.2 ± 1.7 mV) and V1/2senktide (−31 ± 0.8 mV) are significantly different (Wilcoxon test: P= 0.00156; n= 7). p was unchanged (pcontrol= 5.5 ± 0.9 mV and psenktide= 4.9 ± 0.7 mV). C: senktide negatively shifts the voltage dependence of Nav1.9 fast inactivation. Fast inactivation was evoked at +10 mV in response to 200 ms conditioning voltage steps as shown in the inset and normalized peak Nav1.9 currents were plotted against conditioning potentials. The data points were fitted with a Boltzmann function giving the following parameters: V1/2control=−20.6 ± 1.6 mV, p=−6.6 ± 0.5 mV; V1/2senktide=−24.4 ± 1.3 mV, p=−6.0 ± 0.4 mV (n= 6). V1/2control and V1/2senktide are significantly different (Wilcoxon test: P= 0.0313).
Inactivation curves were also constructed by measuring the peak current in response to a test pulse to +10 mV that was preceded by 200 ms conditioning voltage steps to various potentials between −80 and +10 mV. As illustrated in Fig. 4C, the half-inactivation potential was only slightly shifted to the left in the presence of senktide.
To quickly explore the full extent of voltage-dependent changes in INav1.9, voltage ramp protocols were then used. Relatively slow ramps (100 mV s−1) were selected since they allow the recording of persistent Na+ currents (Fleidervish & Gutnick, 1996). Figure 5Aa shows the currents evoked by such a protocol in a representative IPAN, both in control conditions and after a 5 s application of senktide. Before NK3r activation, the continuous current–voltage relationship thus obtained showed a negative slope region associated with Nav1.9-mediated inward current that peaked at about −20 mV. After senktide application, the Nav1.9 peak current was strongly increased and negatively shifted to ∼−35 mV. In a sample of nine neurons, the apparent threshold of the Nav1.9 current, taken from the deviation from the linear slope region, was shifted from −42.1 ± 3.4 mV in control conditions to −55.7 ± 4.1 mV in the presence of senktide (Fig. 5Aa and b). When evoked 100 s after senktide application, the ramp current shifted backward and the net inward current decreased, which illustrates the transient effect of senktide. To attest that the shift was not amplified by an inadequate remote control of the voltage, similar experiments were performed with an inverse Na+ gradient. A similar negative shift was obtained in both conditions (Fig. 5Aa and b, and Bb), although the INav1.9 increase was much higher in standard (Fig. 1Ca and b, and D) than in inversed Na+ gradient (Fig. 5Ba and b). Comparison of current–voltage relationships determined in the presence and absence of senktide yielded a 10 mV negative shift of the outwardly flowing Nav1.9 current. These values compare favourably with those obtained with voltage ramp commands in standard Na+ gradient (Fig. 5Aa) and those derived from the ‘steady-state’ activation curves (Fig. 4).
Figure 5. Persistent Nav1.9 current is transiently increased and negatively shifted by NK3r activation.
Aa: whole-cell currents evoked by a slow voltage ramp (100 mV s−1) from −80 to 0 mV. The current traces have been recorded in control conditions (1), at the peak of the effects of a 5 s senktide application, and 100 s after the application of senktide (3) in normal Na+. Each sweep was separated by an 8 s interval in order to avoid cumulative inactivation. Note that the net inward current was strongly increased and negatively shifted by approximately 15 mV by senktide (arrow). Ab: difference currents obtained from the traces shown in Aa. The arrow shows the backward shift of the net inward current during the 95 s interval between traces 2 and 3. Ba and b: Nav1.9 currents recorded in an inverse transmembrane Na+ gradient. Ba: increase in outwardly flowing Nav1.9 current evoked at −20 mV by the application of senktide (holding potential =−80 mV). Bb: increase and negative shift of the net outward current induced by a slow voltage ramp (100 mV s−1) applied before (1) and 5 s after senktide application (2).
To obtain further insight into the consequences of NK3r activation we then examined the changes in INav1.9 kinetics during the effects of senktide. As shown in Fig. 6A and B, both activation (Fig. 6Ab) and inactivation (Fig. 6Ac) kinetics of INav1.9 were accelerated at the peak of the senktide effects. The kinetics returned to their initial values within ∼2 min in the continued presence of senktide (Fig. 6Ad, Ba and b). The fact that the time courses of these kinetic changes paralleled those of the amplitude changes (Fig. 2A) prompted us to use the change in time constants as an index of the instantaneous voltage-dependent changes induced by NK3r activation. The activation time constant (τact) reached 12 ms at −30 mV, in control conditions (Fig. 6Ca), whereas the fast inactivation time constant (τinact) ranged from 20 to 60 ms at 0 to −20 mV and appeared to decrease steadily with increasing potentials in this voltage window (Fig. 6A and Cb). Pooled estimates of τact and τinact of Nav1.9 current, determined in control conditions for a sample of neurons, were fitted with functions derived from the Hodgkin–Huxley (HH) formalism and then compared with those obtained at the peak of the senktide effect (Fig. 6Ca and b). Data comparison showed that the voltage dependences of τact and τinact were negatively shifted by 20 and 8.5 mV, respectively (Fig. 6Ca and b).
Figure 6. Probing the extent of the voltage-dependent shift at the peak of the senktide effect.
Aa–d: current kinetics before and after senktide application: Aa: successive sweeps of Nav1.9 currents evoked by 100 ms test pulses to −20 mV applied every 5 s from a holding potential of −80 mV. Ab–d: superimposed Nav1.9 current traces recorded in control (1), and at the beginning (2) or at the end (3) of the senktide application as numbered in Aa. Activation (τact) and inactivation (τinact) kinetics were fitted by using monoexponential functions (thick continuous traces). Note the acceleration of the activation and inactivation kinetics at the peak of the senktide effect (τact= 8.5 ms for trace 1 and 5 ms for trace 2 in Ab; τinact= 80 ms for trace 1 and 34 ms for trace 2 in Ac). These accelerations were transient as shown in Ad. Ba and b: time course of the changes in τact (a) and τinact (b) induced by senktide. Data from A. Ca and b: summary showing activation (a) and fast inactivation (b) time constants over a range of membrane potentials in control conditions and at the peak of the senktide effect. The data obtained in control conditions were fitted to Gaussian functions (filled symbols). The control Gaussian fits were then translated towards the points obtained at the peak of the senktide effect (open symbols). This procedure allows the prediction of a shift of the voltage dependence of −20 and −8.5 mV for the activation and the fast inactivation, respectively. Each data point represents the mean ±s.e.m. of 6 experiments.
To further clarify the basis of the experimentally observed increase in INav1.9, a theoretical reconstruction of the biophysical properties of INav1.9 in guinea pig myenteric neurons was carried out. A simple HH model, utilizing single activation and inactivation gates was used to be consistent with the monoexponential time course of INav1.9 activation and fast inactivation (Coste et al. 2004, 2007; Maingret et al. 2008). For describing the voltage dependence of activation and fast inactivation of the conductance underlying INav1.9 in the presence and absence of senktide, the same parameters as obtained from the data in Fig. 4Bb (control) and Fig. 6 (maximal deduced senktide effect) were used in these simulations (Fig. 7Aa). The time constant of INav1.9 slow inactivation (Rugiero et al. 2003) was not incorporated into the model, because we were interested in examining rapid changes in INav1.9 gating and because slow inactivation did not affect the maximal amplifying effect of senktide (Fig 3Aa). The simulated shift and amplification of the window current compare well with those deduced from the experimentally obtained data (presented in Fig. 4Bb and C) as shown in Fig. 7Ab.
Figure 7. Simulated senktide effects on the Nav1.9 current.
Aa: plots of the voltage dependence of activation and fast inactivation before (black continuous lines) and 5 s after (grey continuous lines) NK3r stimulation. The dotted lines represent the product of the two fitting functions corresponding to the predicted voltage dependence of the resulting window current. In order to calculate the voltage dependence of activation and inactivation, the same parameters as obtained from the data of Fig. 4 (for control values) and Fig. 6 (for senktide effect values) were used. Fitting parameters for activation were: V1/2control=−20 mV, p= 5.5 mV and V1/2senktide=−40 mV, p= 4.6 mV and for fast inactivation: V1/2control=−20 mV, p= 6.6 mV and V1/2senktide=−28.5 mV, p= 7 mV. Ab: experimentally observed effects of senktide. Plots of the voltage dependence of activation and fast inactivation before (black continuous lines) and after application of senktide (grey continuous lines). The dotted lines represent the voltage dependence of the window current with and without senktide (compare with Aa). Fitting parameters for activation were: V1/2control=−20 mV, p= 5.5 mV and V1/2senktide=−31 mV, p= 5 mV and for fast inactivation: V1/2control=−20 mV, p= 6.6 and V1/2senktide=−25 mV, p=−6 mV. Data derived from Fig. 4Bb and C. B: time course showing the increase in simulated Nav1.9 current induced by senktide as depicted in A. The currents were activated by depolarizing steps from −80 to −25 mV applied every 5 s. The imposed time constants for the leftward and backward shifts were 5 and 25 s, respectively. Inset: normalized Nav1.9 current traces obtained in control conditions (black trace, 1) and at the peak of the senktide effect (grey trace, 2).
Figure 7B illustrates the main properties of INav1.9 returned by the model, in simulated voltage-clamp experiments. The properties of reconstructed INav1.9 currents were reasonably close to those of the experimental currents. The senktide-mediated negative shift in activation and fast inactivation gating parameters caused a 3.5-fold increase in peak current evoked at −25 mV and was associated with a marked acceleration in both activation and inactivation time constants. As experimentally observed, the increase in reconstructed INav1.9 was associated with a ∼2-fold increase in the relative window conductance, consistent with the idea that the negative shift in activation is about twice as large as that of fast inactivation.
PKC activation mimics the effects of NK3r
The best-characterized consequence of NK-class receptor stimulation is the activation of PKC (Khawaja & Rogers, 1996). Therefore, we also tested the effects of PdBu (1 μm), a non-specific PKC agonist, and OAG (1 μm) an agonist of Ca2+-dependent PKCs, on Nav1.9 current. Both PdBu (Fig. 8A and B) and OAG (data not shown) increased INav1.9, although much more slowly than senktide (Fig. 8B). The fact that PKC activation is involved in the effects of NK3r was confirmed by the observation that modulation of INav1.9 by senktide was virtually abolished after 8 min of intracellular dialysis of the PKC antagonist chelerythrine (1 μm). The ratio INav1.9senktide/INav1.9control was significantly smaller in the presence (1.2 ± 0.1; n= 3) than in the absence (12 ± 5; n= 5; P= 0.035) of chelerythrine (not shown).
Figure 8. Activation of PKC mimics the effects of senktide on the Nav1.9 current.
Aa and b: families of Nav1.9 current traces evoked by stepping from −60 to 10 mV (a) or 20 mV (b) from a holding potential of −80 mV. Aa: control conditions. Ab: 3 min after bath application of PdBu (1 μm). B: time course of the effects of PdBu (1 μm). The Nav1.9 current was evoked by depolarizing steps to −20 from −80 mV applied every 5 s. The times at which recordings in A were taken are indicated by arrows. Ca–c: effect of PKC activators on the mean peak current (Ca, PdBu) and its activation (Cb, PdBu and Cc, OAG). In Cb and Cc, showing G/Gmax plotted versus membrane potential, the half-activation voltage V1/2 is more negative (V1/2PdBu: −29 ± 1.5 mV; n= 4; V1/2OAG: −23.6 ± 4.5 mV; n= 3) in the presence of PdBu or OAG than in control conditions (V1/2controlPdBU: −19 ± 1.3 mV; n= 4; V1/2controlOAG: −16.5 ± 5.5 mV; n= 3) whereas the slope factor remains unchanged (mean pPdBu= 4.2 ± 0.9; mean pcontrolPdBu= 4.9 ± 0.8; n= 4; mean pOAG= 4.8 ± 0.8; mean pcontrolOAG= 5 ± 1.2; n= 3). Da and b: effect of PdBu on the voltage dependence of the fast inactivation. Da: voltage dependence of inactivation of the Nav1.9 current evoked at +20 mV in response to 500 ms conditioning voltage steps at −30 and −50 mV in the presence and the absence of PdBu. Db: mean normalized Nav1.9 peak current plotted against the conditioning potential in the presence and the absence of PdBu (mean inactivation V1/2control=−19.2 ± 1.3 mV; mean inactivation V1/2PdBu=−28 ± 3 mV; mean pcontrol= 7.8 ± 0.9; mean pPdBu= 7.4 ± 2; n= 4).
To determine whether PKC-mediated potentiation of INav1.9 mimicked the effect of NK3r activation on the voltage-dependent activation of Nav1.9, we compared the steady-state activation and inactivation properties of INav1.9 before and after the effects of PdBu had stabilized (∼3 min). This technique was adequate because, in contrast to NK3r activation, PKC activation by PdBu slowly increases the INav1.9 current. The effect of PdBu on the activation is described by the fact that Boltzmann functions fitted to the conductance–voltage relationships constructed using normalized data from four neurons gave an average negative shift of ∼10 mV (Fig. 8Ca and b), with no change in the slope factor (Fig. 8Cb). A similar shift was also obtained after a 3–4 min exposure to OAG (Fig. 8Cc). PdBu also affected the fast inactivation voltage dependence (Fig. 8Da) as it shifted the half-inactivation potential by ∼9 mV (Fig. 8Db) without changing the slope factor (Fig. 8Db). This shows that, for both the activation and fast inactivation processes, the effects of PKC activation are qualitatively comparable to those of NK3r activation.
The modulation of INav1.9 by senktide lowers action potential threshold and induces plateau potentials
Current-clamp experiments were performed to determine the functional impact of INav1.9 potentiation on IPAN excitability. In a first set of experiments, internal Cs+ was still present in order to occlude NK3r-mediated inhibition of K+ currents (Bertrand & Galligan, 1994) and the extracellular solution was as for recording in voltage clamp. Figure 9Aa shows representative responses to current injection in a neuron in which Nav1.9 had been identified. Brief depolarizing current injections from a steadily hyperpolarized membrane potential elicited a broad action potential-like event (Fig. 9Aa). A few seconds after the application of senktide, depolarizing current pulses evoked an action potential followed by a long-lasting plateau potential (Fig. 9Ab). In a second set of experiments, in which recordings were performed with KCl in the pipette and in the absence of TTX, TEA and 4-AP in the bath, but in the presence of CsCl2 (in order to block hyperpolarisation-activated current (Ih)) and CdCl2 (in order to block IKCa) senktide application lowered the threshold of action potential initiation, decreased the first spike latency and increased the firing frequency of IPANs (Fig. 9Ba and b). The fact that these effects were due to INav1.9 amplification and not to the closure of TASK1 channels, recently described in the IPANs (Matsuyama et al. 2008), was checked in a third set of experiments described in Fig. 10. We first checked that, when INav1.9 was isolated in the standard ionic conditions, it was not affected by methanandamide (10 μm), a selective TASK1 blocker (Maingret et al. 2001; Fig. 10A). Then, recordings were performed with KCl in the pipette and using an extracellular solution that contained no TTX, TEA, 4-AP and CsCl2. In these conditions, we found that methanandamide blocked a component of background current that presented TASK1-like characteristics (Fig. 10Ba) and that adding senktide revealed an inward current as a consequence of the amplification of INav1.9 (Fig. 10Bb). When evoking action potentials, we found that senktide application lowered the threshold of action potential initiation and increased the firing frequency of IPANs (Fig. 10Ca and b).
Figure 9. NK3r-mediated increase of the Nav1.9 current triggers long-lasting plateau depolarization and facilitates spiking.
Aa and b: recordings made using a CsCl-based pipette solution and TTX, CsCl and CdCl2 added to the bath. Representative current-clamp responses to 40 ms current pulses before (a) and after 10 s senktide application (b). Steady bias current (∼−75 pA) was injected to hold the neuron at −80 mV in both A and B. Injected current pulses were incremented in 20 pA steps. Note that current pulses elicited passive RC-circuit type responses and regenerative action potential-like responses in Aa whereas in Ab this regenerative response was followed by a long-lasting plateau depolarization. Ba and b: recordings made using a KCl-based pipette solution and TTX omitted from the bath. Membrane potential changes evoked by a series of current pulse injections before (a) and after senktide application (b). Note that senktide lowers the threshold of excitability and increases the number of action potentials in response to injected currents. The presence of Cd2+ in the bath prevented modulation of the slow after HyperPolarisation (sAHP) by senktide.
Figure 10. Facilitated spiking persisted when Nav1.9 modulation by NK3r was performed when TASK1 was blocked.
A: INav1.9 is not affected by methanandamide, a selective TASK1 blocker. INav1.9, recorded in the standard ionic conditions, was evoked by depolarizing steps from −80 mV to −20 mV. Ctr, control trace; Meth, trace obtained 30 s after starting an application of methanandamide (10 μm). Ba and b: ramp currents induced by voltage ramps from −100 to 0 mV, when recording with KCl in the pipette and with an extracellular solution containing no TTX, TEA, 4-AP or CsCl2 but with CdCl2. Ba: in the presence of methanandamide a component of background current that presents TASK1-like characteristics was blocked. Bb: adding senktide revealed an inward current as a consequence of the amplification of INav1.9. Note that senktide also activated a Na+-induced K+ current. Ca and b: membrane potential changes evoked by a series of current pulse injections before (Ca) and after senktide application (Cb), using recording conditions as in Ba and Bb. Note that senktide lowers the threshold of excitability and increases the number of action potentials in response to injected currents. The presence of Cd2+ in the bath prevented modulation of the sAHP by senktide.
Discussion
In this study, performed using patch-clamp recording on IPANs maintained in a portion of the ENS, we show that INav1.9 is potentiated by the activation of NK3r. The most striking process underlying the INav1.9 potentiation by NK3r is a rapid and transient shift of the INav1.9 window current toward more hyperpolarized potentials. The major consequence of this shift is a reduction of the voltage threshold of the action potentials which underlines the role of ‘threshold channels’ played by Nav1.9 channels in determining the excitability of IPANs.
In our recording conditions, an INav1.9 increase was induced by stimulating NK3r with senktide, a specific NK3r agonist, and blocked by SB235375, a specific NK3r antagonist. A similar effect was induced by substance P, the endogenous agonist. These results demonstrate unequivocally that INav1.9 is transiently but substantially potentiated by NK3r-selective activation.
We demonstrate that NK3r activation shifted the voltage dependence of INav1.9 to more negative potentials by using three complementary protocols: steady-state activation and inactivation curves, current responses to slow voltage ramps and analysis of the kinetics of both activation and fast inactivation during the transient changes in INav1.9 amplitude. Each protocol revealed that the voltage dependence of the activation shifted rapidly but transiently towards more hyperpolarized potentials. The maximal amplitude of the activation shift was ∼−20 mV. This shift was directly revealed by the ramp protocol and was similar in both standard and inverse Na+ gradient conditions, which shows that it is not due to an inadequate voltage clamp. The shift of the fast inactivation was smaller, and was estimated to be ∼−10 mV maximum by analysis of the fast inactivation kinetics. As deduced from our analysis of the simultaneous changes in kinetics of both the activation and fast inactivation (Fig. 6), both gating processes shifted in concordance, which is in agreement with the gating model of TTX-sensitive Na+ currents in which these gating processes are coupled (O'Leary et al. 1995). As confirmed by our modelling study, these non-symmetrical negative shifts substantially extended the window current resulting from the product of the activation and inactivation curves (from −60 to 5 mV at the maximal effect of NK3r activation, instead of −40 to 10 mV in control conditions). Another important consequence of these negative shifts is that the window current can reach 50% of the total Nav1.9 current (at −35 mV) whereas in control conditions, it cannot exceed 25% of the total Nav1.9 current (at −20 mV). Importantly, the calculated window current overlaps well with the persistent current evoked with voltage ramp.
The temporal pattern of the transient increase in INav1.9 is likely to reflect that of NK3r signalling pathways. This assertion is based on the fact that this pattern fits remarkably with that of slow EPSPs, known to be mainly induced by NK3r activation (Morita & North, 1985; Bertrand & Galligan, 1994, 1995; Alex et al. 2001; Johnson & Bornstein, 2004). The temporal pattern of INav1.9 facilitation is also in perfect synchrony with the trafficking of PKC, in particular PKCɛ, after NK3r activation (Poole et al. 2008). PKC trafficking after NK3r activation is an adequate temporal index because (1) PKC signalling pathways are responsible for a large part of the inhibition of gKCa and background gK, the main conductances involved in slow EPSPs in IPANs (Bertrand & Galligan, 1995; Matsuyama et al. 2008); (2) the determinant role of PKC in INav1.9 increase, demonstrated by Baker (2005) in DRG neurons is confirmed in the IPANs by the fact that PdBu and OAG, on the one hand, and chelerythrine, on the other hand, respectively mimic and block senktide effects (present study). Our time course analysis of INav1.9 potentiation by NK3r activation shows that INav1.9 peaked in phase with slow EPSPs, that is usually in up to 12–15 s (Morita & North, 1985; Bertrand & Galligan, 1994, 1995; Johnson & Bornstein, 2004). A similar temporal pattern was found for translocation of PKCɛ and the downstream-activated protein kinase D (PKD) from the cytoplasm to the plasma membrane after IPANs exposure to 1 μm senktide: PKCɛ and PKD were predominantly associated with the plasma membrane within 10 s and after 10–30 s of NK3r activation, respectively (Poole et al. 2008). The decrease of the INav1.9 current is also similar to the duration of the slow repolarization of the slow EPSPs (Morita & North, 1985; Bertrand & Galligan, 1994, 1995; Johnson & Bornstein, 2004) and temporally parallels the complete return of PKCɛ to the cytoplasm (2 min) after its translocation to the plasma membrane induced by NK3r activation (Poole et al. 2008).
Increasing the agonist application time did not change the time course of decay of the INav1.9 current because NK3r desensitizes and internalizes quickly (see below). In agreement with our results, slow depolarizations induced by substance P (SP) on neurons of ganglia of the sphincter of Oddi, were almost abolished when SP was applied 10 min after superfusion of the neurons with 1 μm senktide (Manning & Mawe, 2001). Precise data on the early time course of NK3r desensitization and internalization are not available although in vivo experiments show that NK3r expressed by vasopressinergic neurons in the hypothalamic paraventricular nucleus are significantly internalized 5 min after senktide treatment (Haley & Flynn, 2007). Internalization might occur more quickly since, in IPANs, after exposure to 1 μm senktide, PKCɛ remains associated with vesicle-like structures within the cytoplasm for between 2 and 10 min whereas PKD was completely cytoplasmic by 2 min (Poole et al. 2008). A similar time course has been described for NK1r in myenteric neurons, and the temporal characteristics of the desensitization processes of this NK receptor have been well described (McConalogue et al. 1998, 1999). In particular, SP-induced Ca2+ mobilization experiments showed that NK1r desensitization does occur even when using low SP concentrations (100 nm). In these conditions, however, the extent of desensitization depends on the duration of SP exposure: myenteric neurons exposed to 100 nm SP for 5 min and then perfused with SP-free medium for 5 min, exhibit a strong desensitization to a second 100 nm SP exposure. Desensitization was minimal when the first exposure was reduced to 1 min (McConalogue et al. 1998).
Although the temporal pattern of INav1.9 increase globally reflects that of NK3r signalling pathways, the decrease of INav1.9 current markedly slows down when the holding potential is hyperpolarized. This result might indicate that the decay of INav1.9 modulation is tuned by the slow inactivation that is half-operating at −56 mV and totally over at −100 mV (Rugiero et al. 2003).
Our results obtained by recording in current-clamp conditions reveal the role of the coupling between NK3r and INav1.9 in IPANs. They show that increase of the persistent INav1.9 by NK3r stimulation is crucial in determining the voltage threshold of action potentials in IPANs. In these neurons, action potentials are triggered by the TTX-sensitive α subunits Nav1.7 and Nav1.3, the only Na+ channels other than Nav1.9 that are present in the guinea pig ENS (Sage et al. 2007; D. Sage, R. Levinson & N. Clerc, unpublished observations). In addition, NK3r activation lowers the INav1.9 threshold to produce regenerative responses in response to depolarizing pulses, and allows INav1.9 to generate long-lasting plateau potentials. Our results are in agreement with those we obtained previously using an inflammatory soup (bradykinin, ATP, histamine, PGE2 and noradrenaline) that potentiated INav1.9 and increased excitability of mouse DRG neurons (Maingret et al. 2008). The involvement of Nav1.9 in generating plateau potentials and in increasing excitability was proven by comparing recordings obtained in wild-type and Nav1.9-null mutant mice. INav1.9 potentiation by NK3r activation is also likely to amplify slow EPSP produced in the IPANs. As IPANs are synaptically interconnected, forming a self-reinforcing network, and mediate reflex responses to mechanical and chemical stimuli (see Furness et al. 2004; Clerc & Furness, 2004), any change in excitability of these neurons can have a profound effect on the entire circuit and ultimately on gut motility and secretion, as it has been directly shown (Ferens et al. 2007).
References
- Alex G, Kunze WA, Furness JB, Clerc N. Comparison of the effects of neurokinin-3 receptor blockade on two forms of slow synaptic transmission in myenteric AH neurons. Neuroscience. 2001;104:263–269. doi: 10.1016/s0306-4522(01)00064-1. [DOI] [PubMed] [Google Scholar]
- Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol. 2005;567:851–867. doi: 10.1113/jphysiol.2005.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol. 2003;548:373–382. doi: 10.1113/jphysiol.2003.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Galligan JJ. Contribution of chloride conductance increase to slow EPSC and tachykinin current in guinea-pig myenteric neurones. J Physiol. 1994;481:47–60. doi: 10.1113/jphysiol.1994.sp020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Galligan JJ. Signal-transduction pathways causing slow synaptic excitation in guinea pig myenteric AH neurons. Am J Physiol Gastrointest Liver Physiol. 1995;269:G710–720. doi: 10.1152/ajpgi.1995.269.5.G710. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. J Neurosci. 1991;11:505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Scheuer T, Catterall WA. Voltage-dependent neuromodulation of Na+ channels by D1-like dopamine receptors in rat hippocampal neurons. J Neurosci. 1999;19:5301–5310. doi: 10.1523/JNEUROSCI.19-13-05301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Clerc N, Furness JB. Intrinsic primary afferent neurones of the digestive tract. Neurogastroenterol Motil. 2004;16(Suppl 1):24–27. doi: 10.1111/j.1743-3150.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- Coste B, Crest M, Delmas P. Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J Gen Physiol. 2007;129:57–77. doi: 10.1085/jgp.200609665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Osorio N, Padilla F, Crest M, Delmas P. Gating and modulation of presumptive NaV1.9 channels in enteric and spinal sensory neurons. Mol Cell Neurosci. 2004;26:123–134. doi: 10.1016/j.mcn.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci. 1999;19:RC43. doi: 10.1523/JNEUROSCI.19-24-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj S, Black JA, Cummins TR, Waxman SG. NaN/Nav1.9: a sodium channel with unique properties. Trends Neurosci. 2002;25:253–259. doi: 10.1016/s0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferens D, Baell J, Lessene G, Smith JE, Furness JB. Effects of modulators of Ca2+-activated, intermediate-conductance potassium channels on motility of the rat small intestine, in vivo. Neurogastroenterol Motil. 2007;19:383–389. doi: 10.1111/j.1365-2982.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Gutnick MJ. Kinetics of slow inactivation of persistent sodium current in layer V neurons of mouse neocortical slices. J Neurophysiol. 1996;76:2125–2130. doi: 10.1152/jn.1996.76.3.2125. [DOI] [PubMed] [Google Scholar]
- Furness JB, Johnson PJ, Pompolo S, Bornstein JC. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Motil. 1995;7:89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol. 2004;72:143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Haley GE, Flynn FW. Tachykinin NK3 receptor contribution to systemic release of vasopressin and oxytocin in response to osmotic and hypotensive challenge. Am J Physiol Regul Integr Comp Physiol. 2007;293:R931–937. doi: 10.1152/ajpregu.00196.2007. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Bornstein JC. Neurokinin-1 and -3 receptor blockade inhibits slow excitatory synaptic transmission in myenteric neurons and reveals slow inhibitory input. Neuroscience. 2004;126:137–147. doi: 10.1016/j.neuroscience.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, MacDonald JF. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Furness JB, Bornstein JC. Simultaneous intracellular recordings from enteric neurons reveal that myenteric AH neurons transmit via slow excitatory postsynaptic potentials. Neuroscience. 1993;55:685–694. doi: 10.1016/0306-4522(93)90434-h. [DOI] [PubMed] [Google Scholar]
- McConalogue K, Corvera CU, Gamp PD, Grady EF, Bunnett NW. Desensitization of the neurokinin-1 receptor (NK1-R) in neurons: effects of substance P on the distribution of NK1-R, Gαq/11, G-protein receptor kinase-2/3, and β-arrestin-1/2. Mol Biol Cell. 1998;9:2305–2324. doi: 10.1091/mbc.9.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConalogue K, Dery O, Lovett M, Wong H, Walsh JH, Grady EF, Bunnett NW. Substance P-induced trafficking of. β-arrestins. The role of β-arrestins in endocytosis of the neurokinin-1 receptor. J Biol Chem. 1999;274:16257–16268. doi: 10.1074/jbc.274.23.16257. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK 1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Coste B, Padilla F, Clerc N, Crest M, Korogod SM, Delmas P. Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism. J Gen Physiol. 2008;131:211–225. doi: 10.1085/jgp.200709935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BP, Mawe GM. Tachykinins mediate slow excitatory postsynaptic transmission in guinea pig sphincter of Oddi ganglia. Am J Physiol Gastrointest Liver Physiol. 2001;281:G357–364. doi: 10.1152/ajpgi.2001.281.2.G357. [DOI] [PubMed] [Google Scholar]
- Matsuyama H, Nguyen TV, Hunne B, Thacker M, Needham K, McHugh D, Furness JB. Evidence that TASK1 channels contribute to the background current in AH/type II neurons of the guinea-pig intestine. Neuroscience. 2008;155:738–750. doi: 10.1016/j.neuroscience.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Morita K, North RA. Significance of slow synaptic potentials for transmission of excitation in guinea-pig myenteric plexus. Neuroscience. 1985;14:661–672. doi: 10.1016/0306-4522(85)90317-3. [DOI] [PubMed] [Google Scholar]
- North RA, Tokimasa T. Depression of calcium-dependent potassium conductance of guinea-pig myenteric neurones by muscarinic agonists. J Physiol. 1983;342:253–266. doi: 10.1113/jphysiol.1983.sp014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary ME, Chen LQ, Kallen RG, Horn R. A molecular link between activation and inactivation of sodium channels. J Gen Physiol. 1995;106:641–658. doi: 10.1085/jgp.106.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio N, Alcaraz G, Padilla F, Couraud F, Delmas P, Crest M. Differential targeting and functional specialization of sodium channels in cultured cerebellar granule cells. J Physiol. 2005;569:801–816. doi: 10.1113/jphysiol.2005.097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman JA, Nassar MA, Wood JN, Baker MD. GTP up-regulated persistent Na+ current and enhanced nociceptor excitability require NaV1.9. J Physiol. 2008;586:1077–1087. doi: 10.1113/jphysiol.2007.147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla F, Couble ML, Coste B, Maingret F, Clerc N, Crest M, Ritter AM, Magloire H, Delmas P. Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: implication for intestinal reflex function and orofacial pain. Mol Cell Neurosci. 2007;35:138–152. doi: 10.1016/j.mcn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Poole DP, Amadesi S, Rozengurt E, Thacker M, Bunnett NW, Furness JB. Stimulation of the neurokinin 3 receptor activates protein kinase Cɛ and protein kinase D in enteric neurons. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1245–1256. doi: 10.1152/ajpgi.00521.2007. [DOI] [PubMed] [Google Scholar]
- Rugiero F, Gola M, Kunze WA, Reynaud JC, Furness JB, Clerc N. Analysis of whole-cell currents by patch clamp of guinea-pig myenteric neurones in intact ganglia. J Physiol. 2002;538:447–463. doi: 10.1113/jphysiol.2001.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugiero F, Mistry M, Sage D, Black JA, Waxman SG, Crest M, Clerc N, Delmas P, Gola M. Selective expression of a persistent tetrodotoxin-resistant Na+ current and NaV1.9 subunit in myenteric sensory neurons. J Neurosci. 2003;23:2715–2725. doi: 10.1523/JNEUROSCI.23-07-02715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage D, Salin P, Alcaraz G, Castets F, Giraud P, Crest M, Mazet B, Clerc N. Nav1.7 and Nav1.3 are the only tetrodotoxin-sensitive sodium channels expressed by the adult guinea pig enteric nervous system. J Comp Neurol. 2007;504:363–378. doi: 10.1002/cne.21450. [DOI] [PubMed] [Google Scholar]
- Zheng T, Kakimura J, Matsutomi T, Nakamoto C, Ogata N. Prostaglandin E2 has no effect on two components of tetrodotoxin-resistant Na+ current in mouse dorsal root ganglion. J Pharmacol Sci. 2007;103:93–102. doi: 10.1254/jphs.fp0061402. [DOI] [PubMed] [Google Scholar]