Summary
Stimulation of the T-cell antigen receptor (TCR) leads to the activation of signaling pathways that are essential for T-cell development and the response of mature T cells to antigens. The TCR has no intrinsic catalytic activity, but TCR engagement results in tyrosine phosphorylation of downstream targets by nonreceptor tyrosine kinases. Three families of tyrosine kinases have long been recognized to play critical roles in TCR-dependent signaling. They are the Src, ζ-associated protein of 70 kDa (ZAP-70), and Tec families of kinases. More recently, the Abl tyrosine kinases have been shown to be activated by TCR engagement and to be required for maximal TCR signaling. Using T-cell conditional knockout mice deficient for Abl family kinases, Abl (Abl1) and Arg (Abl2), it was recently shown that loss of Abl kinases results in defective T-cell development and a partial block in the transition to the CD4+CD8+ stage. Abl/Arg double null T cells exhibit impaired TCR-induced signaling, proliferation, and cytokine production. Moreover, conditional knockout mice lacking Abl and Arg in T cells exhibit impaired CD8+ T-cell expansion in vivo upon Listeria monocytogenes infection. Thus, Abl kinase signaling is required for both T -ell development and mature T-cell function.
Keywords: T-cell receptor, Abl kinases, cytoskeleton, T-cell development, immunity
Introduction
T-cell differentiation, activation, and expansion require intimate interactions between T cells and antigen-presenting cells (APCs) to bring the T-cell receptor (TCR) in contact with surface-expressed antigenic complexes comprised of peptides bound to major histocompatibility complex proteins (MHCp) on APCs. TCR engagement at the T cell-APC contact site leads to rapid activation of a set of nonreceptor tyrosine kinases and the assembly of proximal signaling complexes, leading to remodeling of the actin cytoskeleton and activation of multiple pathways critical for cell proliferation, survival, adhesion, and migration. The trigger for TCR-dependent signaling cascades is the recruitment and activation of the Src family kinases Lck and Fyn, leading to the phosphorylation of tyrosine residues within the immunoreceptor tyrosine-based activation motifs (ITAMs) of the CD3 subunits of the TCR, including the CD3ζ tails (Fig. 1). The tyrosine-phosphorylated ITAMs recruit the ZAP-70 tyrosine kinase by binding to the tandem SH2 domains of ZAP-70, leading to kinase activation and phosphorylation of a number of signaling proteins including the membrane-associated adapter LAT (linker for activation of T cells) (1, 2). The phosphoryated LAT functions as a scaffold to recruit signaling molecules such as phospholipase C-γ (PLC-γ), Grb2-related adapter downstream of Shc (GADS), Src homology 2 (SH2) domain-containing leukocyte-specific phosphoprotein of 76 kDa (SLP76), Itk/Tec kinases, and the Vav guanine nucleotide exchange factor (GEF) (2) (Fig. 1). PLC-γ promotes calcium mobilization and activation of kinase cascades that regulate gene transcription, while Vav1 activates the Rac and Cdc42 GTPases leading to enhanced actin polymerization. The Tec family kinases function as modulators of TCR signaling and regulate phospholipase C-γ and calcium mobilization as well as remodeling of the actin cytoskeleton (3). This model of early signaling events downstream of TCR engagement has remained basically unchanged for over a decade. However, it appears that the model is more complex. We have shown that the Abl family of nonreceptor tyrosine kinases are activated in response to engagement of both the pre-TCR and TCR and have a role in transducing signals that affect both T-cell development and mature T-cell function in vitro and in vivo (4, 5). Our findings have provided a potential mechanistic explanation for the immune phenotypes promoted by targeted deletion or mutation of Abl in mice, including increased susceptibility to infection, splenic and thymic atrophy, and lymphopenia (6, 7). Notably, lymphopenia is also observed in mice deficient for several TCR signaling mediators such as Lck, ZAP-70, LAT, and SLP76 (8).
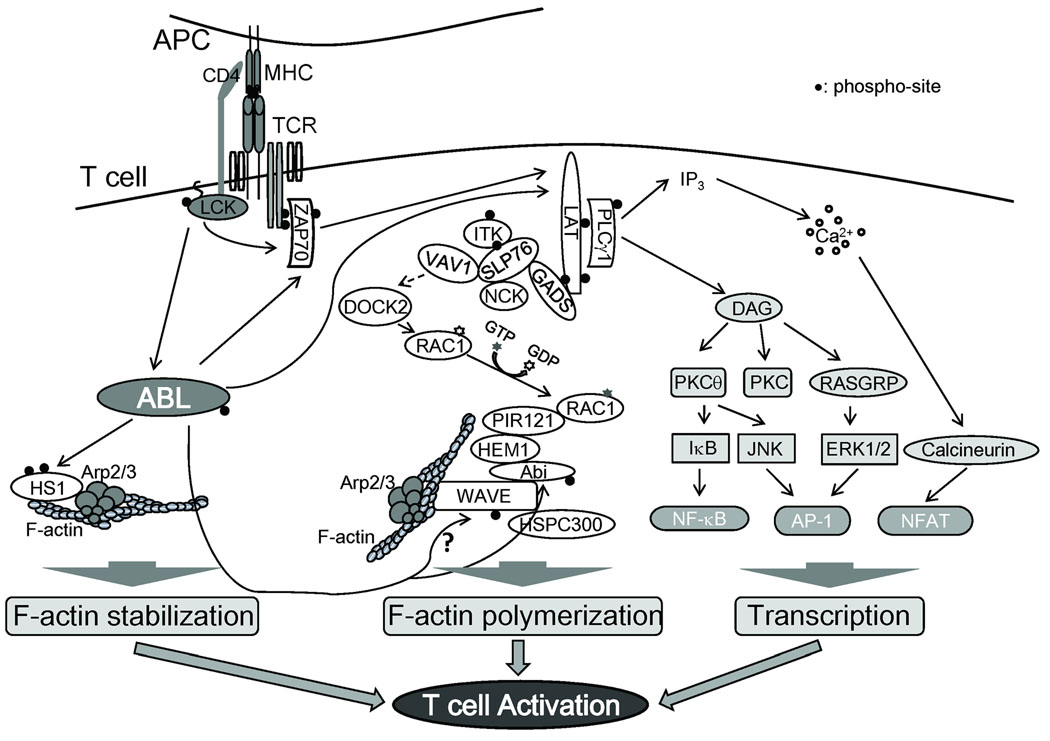
Fig. 1. Abl kinases are activated in response to TCR engagement and regulate downstream signaling events.
Abl kinases are activated in response to TCR stimulation in a pathway that requires Lck kinase activity. The activated Abl kinases promote the phosphorylation of ZAP-70 and LAT leading to activation of IL-2 transcription. Abl kinases also regulate actin polymerization through the Abi/Wave complex and HS1. The Wave complex promotes actin polymerization downstream of activated Rac leading to Arp2/3-mediated actin polymerization. The role of Abi and Wave phosphorylation by Abl is unclear. HS1 is also phosphorylated by Abl and regulates F-actin stabilization.
Abl family kinases: regulation and function
Abl kinases link diverse cell surface receptors to the regulation of cytoskeletal dynamics
The Abl nonreceptor tyrosine kinases, Abl (Abl1) and Arg (Abl2), regulate cytoskeletal reorganization, cell proliferation, survival and stress responses (reviewed in 9). Abl tyrosine kinases are activated by stimulation of growth factor receptor tyrosine kinases such as the platelet derived growth factor receptor (PDGFR) and the epidermal growth factor receptor (EGFR) (10–13), the insulin-like growth factor receptor (IGF-1R) (14), the agrin-stimulated MuSK receptor tyrosine kinase (15), and the ephrin-activated EphB4 receptor (16). Abl kinases are also activated downstream of receptors that lack intrinsic kinase activity such as the plexin A1-stimulated Semaphorin 6D (17) and integrins (18, 19). The activated Abl kinases link these diverse cell surface receptors to reorganization of the actin cytoskeleton and regulation of chemotaxis, migration, and invasion. Additionally, Abl kinases regulate PDGF-dependent cell proliferation (11, 20, 21).
We and others have shown that Abl kinases are also activated in response to the entry of microbial pathogens into mammalian cells (22, 23). Abl kinases regulate cytoskeletal reorganization during pathogen infection. We showed that Abl kinases are required for Shigella flexneri entry and also for intracellular motility, which involves regulation of actin comet elongation at one pole of the bacterium through Abl-dependent phosphorylation of the N-Wasp actin nucleator-promoting factor (24).
We reported more recently a novel role for Abl kinases in the regulation of cell-cell adhesion in epithelial cells and fibroblasts (25). Abl kinases are activated by cadherin-mediated adhesion signals and are required for the formation and maintenance of intercellular adhesions by regulating the activities of the Rac and Rho guanosine triphosphatases (GTPases), leading to cadherin-dependent rearrangements of the actin cytoskeleton (25). Thus, Abl kinases function to link an increasing number of cell surface receptors to signaling pathways required for cytoskeletal reorganization and other cellular processes such as proliferation and survival (Fig. 2).
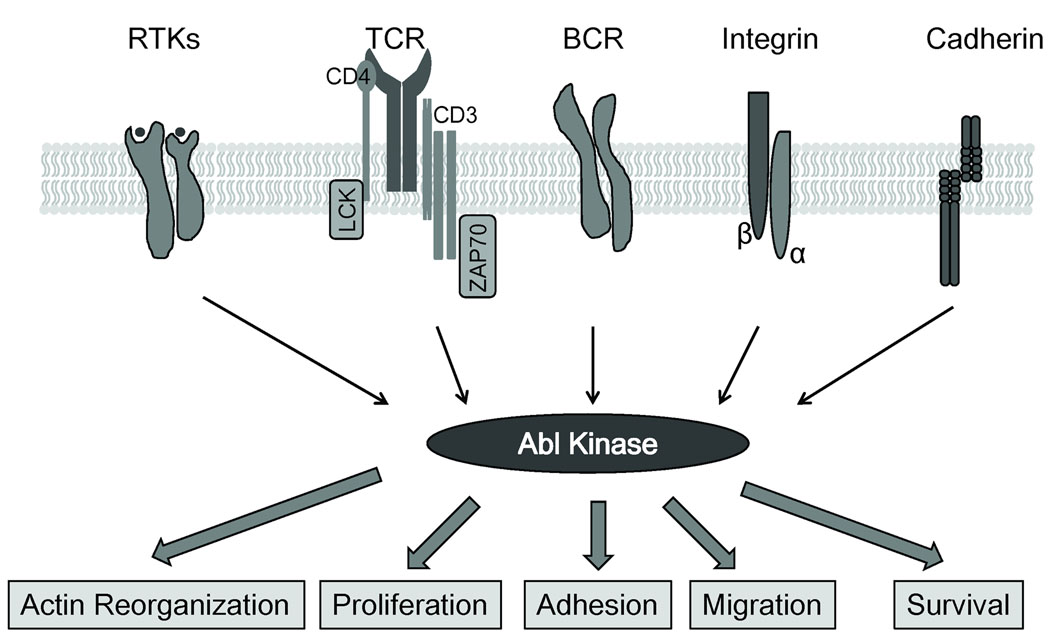
Fig. 2. Multiple cell surface receptors promote Abl kinase activation and signaling.
Abl kinases are activated by ligand-stimulated receptor tyrosine kinases (RTKs), adhesion receptors such as integrins and cadherins, and immunoreceptors such as the BCR and TCR. The activated Abl kinases signal to modulate cell proliferation, survival, or cytoskeletal processes in multiple cells types.
Structure of Abl family kinases
The Abl family of nonreceptor tyrosine kinases in vertebrates consists of two proteins, Abl (Abl1) and Arg (Abl2) (26, 27). Two predominant isoforms of Abl and Arg are generated by alternative splicing of the first exon, and one of the isoforms (1b/IV) contains a myristoylation site (27, 28). Abl and Arg are multi-domain proteins with conserved domains that include three highly conserved Src kinase homology (SH) domains at the N-terminus (Fig. 3). The SH3 domain recognizes proline-rich sequences, while the SH2 domain binds to tyrosine phosphorylated peptides (9). The kinase or SH1 domain is the most highly conserved domain between mammalian Abl and Arg, as well as among Abl kinases in mammals, flies, and worms (29, 30). The N-terminal sequences are critical for regulating the activity of the kinase domain through intramolecular interactions that maintain an auto-inhibited kinase domain conformation. Analysis of the crystal structure of the Abl kinase domain and upstream N-terminal sequences showed that autoinhibition of the Abl kinase is maintained by intramolecular interactions involving the SH3 domain with the linker that connects the SH2 and the kinase domain, and with the small lobe of the kinase domain (31). The Abl SH2 domain interacts with the large lobe of the kinase domain (31). Additionally, the myristoyl group at the N-terminus of Abl appears to contribute to the assembly of the autoinhibited conformation of the Abl kinase (32). Mutation of the myristoylation site was shown to enhance Abl kinase activity and a crystal structure of the isolated Abl kinase domain in complex with a myristoylated peptide showed that the myristoylated group bound within a deep hydrophobic pocket in the kinase C-terminal lobe, suggesting that the myristoylated group contributes to stabilize the closed conformation of the Abl kinase (32).
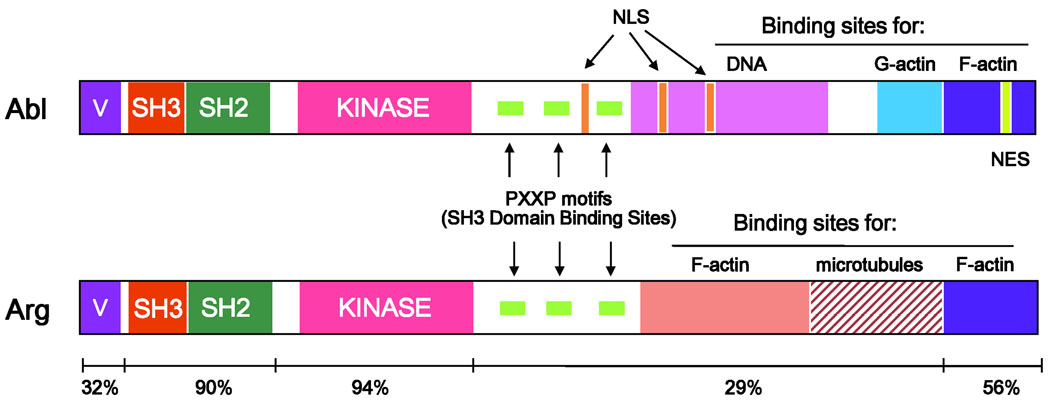
Fig. 3. Structural domains of Abl and Arg.
The Abl and Arg kinases are highly homologous in their SH3, SH2, and SH1 (kinase) domains in the N-terminal half. The C-terminus contains proline-rich (PXXP) motifs that recruit SH3-containing proteins. Both Abl and Arg have a C-terminal calponin-homology F-actin binding domain. Arg also has an internal talin-like F-actin binding domain and a microtubule binding domain. Abl has a DNA binding domain, three nuclear localization sequences (NLS), and one nuclear export sequence (NES). A G-actin binding domain is present in Abl just upstream of the C-terminal F-actin binding domain.
The C-terminal sequences following the kinase domain are less well conserved between Abl and Arg, but both kinases exhibit conservation in proline-rich sequences that provide binding sites for SH3 domain-containing proteins (9, 33). A unique feature of the Abl family of tyrosine kinase is the presence of actin-binding domains, which distinguish these kinases from other nonreceptor tyrosine kinases. Abl and Arg share a C-terminal calponin homology F-actin-binding domain (34, 35). In the Abl kinase, a globular (G)-actin binding domain precedes this F-actin-binding domain (34). In contrast, Arg lacks the G-actin-binding domain but displays a microtubule-binding (MT) domain and a second talin-like F-actin-binding domain that is characterized by an I/LWEQ sequence (35, 36). The Abl C-terminus also contains three nuclear localization signals (NLS), one nuclear export signal (NES), and a DNA-binding domain (37–39), which are absent in Arg. The presence of NLS and NES motifs in Abl but not Arg is consistent with the nuclear and cytoplasmic localization of Abl and the predominant cytoplasmic localization of Arg.
Mechanisms of Abl kinase activation
Abl and Arg are activated by multiple extracellular and intracellular stimuli that modulate intra- and inter-molecular interactions thereby promoting the inactive or active conformations of the Abl and Arg kinases. The inactive conformation of Abl is maintained not only by autoinhibitory intramolecular interactions, but also by intermolecular interactions. An example of the latter is the inhibitory effect of phosphatidyl inositol bisphosphate (PIP2) on the kinase activity of Abl and Arg (10, 12). Decreasing PIP2 cellular levels by PLC-γ1-mediated hydrolysis stimulated by PDGF resulted in increased Abl and Arg kinase activities (10, 12). In addition to inositol lipids, a number of proteins such as Pag/peroxiredoxin-1 have been reported to inhibit Abl activity, possibly by stabilizing the autoinhibited conformation of the kinase (reviewed in 9). Activation of Abl can occur by the binding of SH3 or SH2 domain ligands or proteins containing the corresponding target sequences (9, 40).
Activation of the Abl kinases is also regulated by tyrosine phosphorylation. Tyrosine phosphorylation is not detectable on endogenous Abl and Arg kinases (9, 41), but activation of the Abl kinases by a variety of extracellular and intracellular stimuli results in the phosphorylation of several tyrosines, including tyrosine (Y) 245 in the SH2 domain-kinase linker and Y412 in the activation loop of the kinase domain (41, 42). Phosphorylation of these and other tyrosines occurs either through autophosphorylation by the Abl kinases in trans or phosphorylation by Src family kinases (11, 20, 40, 41). Maximal activation of the Abl kinases involves disruption of inhibitory interactions by binding to target proteins and tyrosine phosphorylation of specific sites by the Abl kinases themselves or other tyrosine kinases, including Src and the PDGF receptor (12, 41). Thus, the Abl family kinases integrate diverse signals that promote binding to target proteins and result in different levels of tyrosine phosphorylation, thereby producing a continuum of kinase activity levels from low to high. Oncogenic forms of the Abl family kinases are produced by retroviral transduction (v-Abl) or chromosomal translocation events (BCR-ABL). These fusion partners provide domains that promote oligomerization of the chimeric proteins leading to trans-phosphorylation and enhanced tyrosine kinase activity (9). Abl1 was originally identified as the cellular homolog of the v-Abl oncogene product of the Abelson murine leukemia virus, which produces a gag-Abl fusion protein with constitutively elevated tyrosine kinase activity (26, 43). The Abl kinase is best known for its role in human chronic myelogenous leukemia (CML) as a result of a chromosomal translocation event that fuses ABL with the BCR gene to produce the BCR-ABL oncoprotein (44). The BCR coiled-coil domain in BCR-ABL and the gag sequences in v-Abl promote oligomerization and constitutive trans phosphorylation of the Abl kinase. Other fusion proteins have been identified that link Abl or Arg to sequences containing oligomerization domains. These include the TEL-ABL and TEL-ARG fusion proteins involved in rare cases of human CML and acute myelogenous leukemia (AML), respectively (45–47). The pointed domain of TEL mediates oligomerization of the fusion proteins, which display constitutive tyrosine kinase activity. Two other ABL1 fusion proteins have been identified in rare cases of acute T-lymphoblastic leukemia (T-ALL). These are the NUP214-ABL (48) and EML1-ABL (49). Both NUP214 and EML1 contain oligomerization domains: NUP214 contains two coiled-coil domains and EML1 has one coiled coil domain. Interestingly, whereas the majority of the Abl and Arg fusion proteins localize to the cytoplasm or cytoskeleton, NUP214-ABL localizes to the nuclear pore complex (50).
Pharmacological inhibitors of the Abl kinases: mechanism of inhibition, specificity and phenotypes
A number of pharmacological inhibitors of the Abl kinases have been developed, which are currently used for the treatment of CML. The most widely used inhibitor is imatinib mesylate (Gleevec, STI571, CGP 57148B), which selectively inhibits Abl and Arg, as well as the receptor tyrosine kinases PDGFR and Kit (51–53). Structural studies of the Abl kinase domain complexed with imatinib revealed that this compound binds to the inactive conformation of Abl and obstructs adenosine triphosphate (ATP) binding (54). Imatinib was shown to selectively inhibit in vitro growth and in vivo leukemogenesis of BCR-ABL-positive cells (55) and was shown initially to be effective in the treatment of CML patients that were refractory to interferon-based therapy (56). Subsequent clinical trials showed that greater than 90% of CML patients treated with imatinib at doses of 400 mg daily achieve complete hematologic responses and disease progression is arrested in these patients (57). Imatinib was approved in 2001 by the Food and Drug Administration for the treatment of CML. However, a high percentage of CML patients in the accelerated and blast phases of the disease become resistant to imatinib treatment (58). The major cause of resistance is the emergence of BCR-ABL mutants that are insensitive or have reduced sensitivity to inhibition by imatinib (59, 60). Treatment of these patients with higher doses of imatinib (800 mg/daily) resulted in toxicity with severe pancytopenia in over the half of CML patients that failed to respond to the drug at a dose of 400 mg/daily (61). Additionally, cardiotoxicity has been reported in a small number of patients treated with imatinib (62). Some of these patients developed left ventricular dysfunction and even congestive heart failure. It was suggested that imatinib-induced cardiotoxicity might be due to activation of the endoplasmic reticulum (ER) stress response in cardiomyocytes as a result of inhibition of the endogenous Abl kinase (62). Second-generation ABL kinase inhibitors have been used to circumvent imatinib-resistant CML. Among these are nilotinib and dasatinib, which are active against some imatinib-resistant BCR-ABL mutants (63). However, both of these drugs exhibit negligible activity against the frequent BCR-ABL T315I mutation in CML patients. In this regard, it was recently reported that the Aurora kinase inhibitor PHA-739358 is effective against imatinib-resistant BCR-ABL mutations including T315I (64).
Imatinib treatment has also been linked to immunosuppression (65, 66). It is notable that higher doses of imatininb than those required to inhibit Abl kinases can inhibit Lck, a key regulator of T-cell development and activation (67). Moreover, imatinib has also been shown to inhibit c-Fms, the receptor for macrophage colony-stimulating factor (M-CSF), which correlates with growth inhibition of monocytes and macrophages (68). Thus, because imatinib targets several kinases, it is necessary to employ alternative strategies such as conditional knockout mice lacking specific kinases in distinct cell types to define the relevant molecular targets of imatinib and to elucidate the role of Abl kinase inactivation in various cellular processes.
Phenotypes of mice lacking Abl and Arg
Both Abl and Arg are ubiquitously expressed and their levels vary across cell types and tissues (9). Although Abl and Arg exhibit overlapping expression in many tissues, Arg is most highly expressed in brain, thymus, spleen, and muscle (69). Consistent with the widespread expression of Abl, targeted disruption of the Abl1 gene in mice resulted in pleiotropic phenotypes including runtedness, high perinatal lethality, susceptibility to infections, and immune deficiencies in homozygous null Abl mice (6, 7). In contrast, mice that are homozygous null for Arg (Abl2) are healthy and do not present with the immune-deficient phenotypes associated with homozygous loss of Abl (69). Evidence for overlapping roles of Abl and Arg was obtained by intercrossing abl−/− and arg−/− mice, which revealed that Abl family kinases are required for normal mouse development. Mice with targeted deletion of both abl and arg genes die around embryonic day 10.5, and the embryos display bleeding in the pericardial sac and peritoneal hemorrhaging (69). Abl−/−Arg+/− mice die between embryonic day 15.5 and 16. However, approximately 60% of Abl+/−Arg−/− mice are viable and can be used for analysis of partial loss of Abl/Arg function (69). Complete loss of both Abl kinases results in neurulation defects, including defective neural tube closure, and the neuroepithelial cells of Abl/Arg null mice have striking cytoskeletal abnormalities including disruption of the apical latticework, and the presence of ectopic, actin-rich structures at the basolateral surface. Tissue-specific, conditional knockout mice will be required to ascertain whether Abl and Arg exhibit overlapping as well as unique functions during mouse development, as well as during pathological responses to various stimuli.
Abl kinases signal downstream of the B-cell receptor
A clue that Abl kinases might have a role in immunoreceptor signaling was provided by analysis of mice that were singly deficient for Abl1 (6, 7). Two distinct abl1 mutant mice were generated and both displayed similar phenotypes. One was a complete null allele (abl−/−) with no Abl protein expression (7). The second strain (ablm1) targeted the C-terminal sequences of Abl encompassing the DNA-binding and actin-binding domains, while retaining the N-terminal sequences that include the SH3, SH2, and kinase domains as well as the three proline-rich sequences downstream of the kinase domain (6). Surprisingly, the phenotypes of these mice were similar and suggest that the functions encoded by the Abl C-terminus are critical for lymphocyte function. Both knockout strains displayed immune deficits, including increased susceptibility to infections and reduced size of thymus and spleen. The Abl-deficient mice showed decreased B and T-cell numbers, with reductions in pro-B and pre-B cells, as well as peripheral B lymphocytes in the abl−/− mice. It appears that cell autonomous effects in B-lymphocyte function as well as defects in the stromal microenvironment may contribute to the immune deficits of the complete null abl−/−mice. In one study, bone marrow transfer from abl−/− mice to irradiated wildtype recipients did not recapitulate the lymphopenic phenotypes of the Abl-deficient donors (7). However, in another study, Abl-deficient pro-B cells from the ablm1/m1 mice exhibited decreased proliferation when cultured on wildtype stromal cells, and the phenotypes of pro-B and pre-B cells could be reconstituted in wildtype irradiated hosts following bone marrow transfer from the ablm1/m1 mice (6, 70). Thus, abnormalities of the Abl-deficient lymphocytes as well as the Abl-deficient stroma may both contribute to the immune deficiencies characteristic of Abl-deficient mice.
The premise that Abl-deficient mice exhibit B-cell autonomous defects was further supported by our finding that proliferation in response to B-cell antigen receptor (BCR) stimulation is markedly reduced in splenic B cells isolated from abl−/− mice compared to wildtype controls (71). Moreover, we found that Abl kinase activity and protein levels are elevated in the cytosol of B-cell lines following activation of the BCR. We found that the BCR coreceptor CD19 is a target for Abl (71). CD19 is tyrosine phosphorylated following B-cell activation, which results in the recruitment of SH2 domain-containing proteins including Abl (71). CD19 interacts with Abl in cells and is a substrate of the Abl tyrosine kinase. The Abl kinase phosphorylated CD19 on tyrosine 490, which is located between the binding sites for the SH2 domains of the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and the Src family kinases. It is possible that recruitment of an SH2 domain-containing protein to the phosphorylated Y490 may regulate the binding of PI3K and Src to CD19. Thus, following BCR stimulation, CD19 is tyrosine phosphorylated and recruits a number of SH2-domain-containing proteins including Abl, which in turn might be activated via a Src/CD19 amplification loop, leading to enhanced CD19 phosphorylation by Abl and the subsequent recruitment of additional signaling proteins.
Abl kinases signal downstream of the TCR
The possibility that Abl kinases might have a role in TCR signaling was first suggested by the finding that mice lacking the Abl1 tyrosine kinase exhibited thymic atrophy, lymphopenia, and increased susceptibility to infections (6, 7). However, because the Abl1 kinase is expressed in all tissues and Abl1-deficient mice exhibited pleiotropic phenotypes, it remained unclear whether the immune phenotypes of the Abl1 knockout mice were cell autonomous and whether Abl1 directly regulated T-cell function. Moreover, the Abl-related Arg (Abl2) tyrosine kinase is co-expressed with Abl1 in thymus and spleen (69), and these kinases may have overlapping functions in lymphocytes. To directly examine whether endogenous Abl kinases are activated in response to TCR stimulation, we analyzed the phosphorylation status of the Abl substrate CrkL using a phosphospecific antibody directed against the conserved tyrosine phosphorylation site Y207 (5). This site is conserved in the Crk/CrkL family of adapters and phosphorylation of this site has been shown to be dependent on the activity of the Abl kinases in a number of cell types under various stimulation conditions (11, 15, 23). We showed for the first time that TCR stimulation results in activation of the endogenous Abl kinases and that this activation requires in part the activity of the Src family kinase Lck (5). Further, we showed that the ζ-associated protein of 70 kDa (ZAP-70) tyrosine kinase and the transmembrane adaptor LAT are targets of the Abl kinase. Inhibition of Abl kinase activity with STI571 (imatinib mesylate) or decreased Abl kinase expression in primary T cells derived from Abl1+/−Abl2−/− mice results in reduced TCR-induced ZAP-70 phosphorylation on tyrosine 319, decreased phosphorylation of LAT tyrosine 132, and decreased phosphorylation of PLCγ tyrosine 783 (5). Moreover, our analysis of T-cell conditional knockout mice that are homozygous null for both Abl and Arg showed that Abl/Arg double null primary T cells exhibited impaired TCR-dependent activation of ZAP-70, LAT, PLCγ1, Shc, Jnk, and NF-κB (4). The specific roles for the Abl-dependent phosphorylation of these signaling proteins remain to be determined. Tyrosine 319 of ZAP-70 is found in the SH2-kinase linker region, and this site has been shown to be phosphorylated by Lck, Fyn, and Abl kinases (5, 72). ZAP-70 tyrosine 319 and the adjacent tyrosine 315 participate in an autoinhibitory switch that regulates ZAP-70 kinase activity (72, 73). Tyrosines 315 and 319 in the SH2-kinase linker are involved in aromatic-aromatic interactions with the kinase domain that stabilize the autoinhibited ZAP-70 conformation (73). Phosphorylation of these tyrosines by Lck, Abl, or by ZAP-70 itself in the active ZAP-70 conformation is likely to function in the recruitment of SH2 domain-containing proteins. Future studies are needed to determine the role of Abl kinases in the regulation of ZAP-70 activity and downstream signaling. We showed that Abl kinases phosphorylate PLCγ1 on tyrosines 771 and 783 in vitro and tyrosines 771 and 1003 in vivo (10). Phosphorylation of tyrosine 783 is essential for PLCγ1 activation, whereas phosphorylation of tyrosine 771 may be involved in negative regulation of PLCγ1 (74). Activation of Abl kinases in response to PDGF stimulation in fibroblasts required PLCγ1 activity, and in turn, Abl-dependent phosphorylation of PLCγ1 in these cells resulted in negative regulation of PLCγ1 activity, possibly through the phosphorylation of tyrosine 771 (10). Abl-mediated PLCγ1 phosphorylation on tyrosine 783 in response to TCR stimulation might modulate PCLγ1 activation and downstream signaling leading to ERK activation (5).
We showed for the first time that Abl kinase activity is required for maximal TCR-dependent signaling leading to transcription of the IL-2 promoter in vitro in Jurkat T cells (5). Inhibition of Abl kinases leads to reduced activation of the CD28RE/AP and NFTA response elements of the IL-2 promoter (Fig. 1). Further, expression of an activated Abl mutant (Abl-PP) is sufficient to drive activation of the NFAT reporter element within the IL-2 promoter, and activate transcription of the IL-2 promoter. Abl kinases are also required for maximal cell proliferation and IL-2 production in response to TCR stimulation of primary T cells (5). Thus, the long-established model of TCR signaling which previously included three types of non-receptor tyrosine kinases (Lck, ZAP-70, and Tec), must be expanded to include the Abl kinases downstream of TCR activation.
Abl kinases modulate T-cell development and signal downstream of the pre-TCR
Thymocyte proliferation, survival, and differentiation are tightly controlled by signaling from the pre-TCR (75). A subset of protein kinases and adapters implicated in mature TCR signaling are known to play critical roles downstream of the pre-TCR (76). To address whether the Abl kinases are involved in T-cell development and function in vivo, we successfully deleted the Abl kinases in T cells by crossing the Lck-Cre transgenic mice with mice carrying lox-P-flanked abl1 sequences in the abl2−/− background. These mice lack Abl and Arg in T cells, showed reduced numbers of thymocytes, and displayed a partial block in the transition to the CD4+CD8+ double positive (DP) stage (4). Immature thymocytes lack expression of the CD4 and CD8 cell surface receptors and proceed from the CD4−CD8− double negative (DN) stage through a CD4+CD8+ DP stage to become mature CD4+ or CD8+ single positive (SP) T cells. The transition from DN into DP cells is regulated by signaling through the pre-TCR, while differentiation from DP cells to mature T cells requires a functional TCR α/β (77). The DN thymocytes can be subdivided into four developmental stages (DN1, DN2, DN3, and DN4) based on the expression of the CD25 and CD44 cell surface molecules (76, 77). These stages progress in the following order: CD25−CD44+ (DN1), CD25+CD44+ (DN2), CD25+CD44− (DN3), and CD25−CD44− (DN4). TCR β gene rearrangements are initiated at the DN2 stage and continue during the DN3 stage. The re-arranged TCR β–chain heterodimerizes with the pre-Tα chain to form the pre-TCR at the cell surface. Signals derived from the pre-TCR at the DN3 stage are important for maturation to the DN4 stage and are implicated in proliferative expansion, survival, allelic exclusion of the TCR β locus, and induction of TCR α rearrangement (77). Thymocyte development in the Abl/Arg conditional knockout mice was analyzed by the expression of CD25 and CD44 among the DN populations. Thymocytes from Abl/Arg conditional knockout mice consistently presented a higher percentage of CD25+CD44−(DN3) cells with a corresponding decrease in DN4 stage cells compared to wildtype mice, suggesting that loss of Abl kinases impaired the DN3 to DN4 transition during thymocyte development. Successful TCR β gene rearrangement and expression of the pre-TCR occurs at DN3 stage, and functional signaling through pre-TCR is critical for thymocyte development. There was no significant difference in surface expression of TCR β in all four thymocyte sub-populations between wildtype and Abl/Arg mutant mice. Thus, Abl kinases do not appear to affect TCR β-chain rearrangement and surface expression. Defective transition from DN3 to DN4 might result from impaired signaling downstream of the pre-TCR. Therefore, in vivo injections with anti-CD3ε antibody, which activates the pre-TCR and accelerates thymocyte maturation, were carried out with wildtype and Abl/Arg mutant mice. As expected, wildtype mice exhibited accelerated maturation through the DN3 stage with few cells remaining at the DN2 and DN3 stages. In contrast, a relatively high number of DN3 cells accumulated in the Abl/Arg double null thymocytes (4). In this regard, knockout mice lacking Lck (78) or ZAP-70/Syk (79) kinases have arrested thymocyte development with a partial or complete block at the DN3 stage of T cell development.
Abl kinases are required for linking pre-TCR stimulation to the activation of downstream signaling molecules. Pharmacological inhibition of Abl kinases in a pre-T-cell line derived from a spontaneous SCID mouse thymoma that stably expresses functionally rearranged TCR β with endogenous pre-TCRα chains, followed by TCR stimulation showed reduced phosphorylation of ZAP-70 (Tyr319)/Syk (Tyr352), LAT (Tyr132), and PLCγ1 (Tyr783), as well as the adapter Shc (Tyr239/240) compared to control cells (4). Together these results suggest that Abl kinases are involved in pre-TCR signaling and modulate T-cell development in mice.
Abl kinases regulate TCR-independent basal signaling and suppress Recombination-activating gene expression
Ligand-independent signaling by the pre-TCR has been shown to promote developmental progression of immature T cells (75, 77). It has been proposed that in the absence of extracellular stimulation, pre-TCR and TCRs exhibit continuous low level signaling (known as basal or tonic signaling) that regulates gene expression. It was reported that basal signaling in thymocytes contributes to the regulation of recombination-activating gene (Rag) expression and that this signaling is dependent on the function of the LAT and SLP-76 adapter proteins and the Abl and Erk kinases (80).
The Rag-1 and Rag-2 genes are activated during T-cell development, and the corresponding proteins regulate genetic recombination and cell surface expression of TCRs. Expression of the Rag genes was shown to be markedly activated in T cells deficient for LAT and SLP-76 and also in cells treated with chemical inhibitors that suppress the kinase activities of Erk and Abl (80). It appears that Abl functions downstream of LAT in this tonic signaling pathway, leading to the repression of Rag expression and that constitutive Abl kinase activity requires phosphorylation of tyrosine 132 in LAT (80). Erk activation is also dependent on LAT, and Erk appears to function independently of Abl in the regulation of Rag gene expression (80). Notably, we have shown that TCR-stimulated LAT tyrosine phosphorylation is dependent on Abl kinase activity and protein, thereby placing LAT downstream of Abl kinases in ligand-stimulated TCR signaling. It is possible that the LAT multiprotein complex recruits Abl kinases and Abl-target proteins and that the activities of complex components are interdependent, thereby making it difficult to determine whether specific proteins in this complex function upstream or downstream of one another.
Role for Abl kinases in cytoskeletal remodeling processes in T cells: immunological synapse formation, migration, and adhesion
Interaction of T cells with APCs results in dynamic reorganization of the T-cell actin cytoskeleton and segregation of the TCR and other signaling proteins at the interface with the APC (81). The polarized rearrangement of the cytoskeleton and signaling complexes leads to the formation of the immunological synapse (IS). Recent studies suggest that Abl kinases play a role in promoting actin polymerization at the IS (82).
Imaging studies have revealed that the T-cell synapse is a radially symmetrical structure that is comprised of three concentric compartments, the supra-molecular activation clusters (SMACs) (83). The central SMAC (c-SMAC) is enriched in TCRs, whereas the peripheral SMAC (p-SMAC) is enriched in integrins such as LFA-1, the Lck tyrosine kinase, and cytoskeletal proteins such as talin. Outside of the p-SMAC is the distal SMAC (d-SMAC), which is enriched with the CD45 tyrosine phosphatase, CD43, and protein kinase C-ζ. The localization of the SMAC components is highly dynamic. Microscopy studies with planar bilayers show that TCR microclusters appear to form in the d-SMAC and translocate through the p-SMAC to the c-SMAC (84). TCR signaling is required for synapse formation and stability, and in turn, a mature IS which is sustained for several hours, is required for T-cell activation (1). The recruitment of TCRs, integrins, co-receptors, and signaling molecules at the IS promotes sustained TCR signaling and increased adhesion. It appears that at early time points following T cell-APC conjugation, signaling initiates at the pSMAC under conditions of both high and low antigenic stimulation (85). However, signaling also occurs at the c-SMAC at later time points and weak antigenic stimuli trigger sustained protein tyrosine phosphorylation predominantly in the cSMAC, as measured by staining with antibodies against phosphotyrosine and phosphorylated ZAP-70 (85). Importantly, the cSMAC is also a region of signaling termination, where internalization and degradation of the TCR and other receptors occurs (83, 85). Other functions of the IS are to direct cytokine secretion and to set up an axis of asymmetry with the segregation of protein complexes into the proximal and distal poles, which may lead to segregation of signaling pathways important for asymmetric cell division and subsequent T-cell fate differentiation (83).
Actin polymerization in response to TCR engagement at the T cell-APC contact site requires the activity of a number of proximal signaling molecules, including kinases such as Lck, ZAP-70, and Itk, as well as the LAT adapter protein (1, 81). More recently, it was shown that inhibition of Abl kinase with imatinib or suppression of Abl expression by RNA interference, although dispensable for the formation of conjugates between T cells and activated B cells, inhibited the accumulation of actin at the T cell-B cell interface in vitro (82). Abl was also shown to be required for other cytoskeletal responses such as chemokine-induced T-cell migration and spreading on anti-TCR-coated coverslips (82).
Which molecules mediate the effects of Abl on these various cytoskeletal processes? Actin polymerization events in T cells have been shown to be mediated by Wave, Wiskott-Aldrich syndrome protein (WASP), and HS1/cortactin, and all of these proteins have been linked to the Abl kinases in various cell types (24, 82, 86–88). Actin nucleation through Arp2/3 produces the branched actin networks observed in lamellipodia and drives actin polymerization during cell migration and cell-cell junction formation (89, 90). Actin nucleation by Arp2/3 is enhanced by nucleation-promoting factors, which include the WASP family: WASP, N-WASP, Wave1, Wave2, and Wave3 (91). The widely expressed cortactin and its hematopoietic ortholog HS1 are weak activators of Arp2/3-dependent actin polymerization and prolong the half-life of branched actin structures (92, 93). We and others showed that the Abl interactor protein (Abi), which binds to Wave in a multiprotein complex regulates actin polymerization in response to T cell-APC engagement (94, 95). The Wave proteins link activated Rac to Arp2/3-mediated actin polymerization by forming a complex that contains the Rac-binding protein Sra1 (also known as PIR121), Hem1 or Hem2 (also known as Nap1), and the Abl-interactor proteins, Abi1 and Abi2. The Abi proteins function to directly connect Hem/Nap1 proteins to Wave. Depletion of Wave and Abi decreases T cell- B cell conjugate formation and impairs actin accumulation at the IS (94, 95). Moreover, we showed that partial loss of Abi proteins in mice (Abi1+/−Abi2−/−) impairs TCR-induced cell proliferation and IL-2 production of primary T cells (95). Thus, the Wave/Abi complex plays a critical role in the polymerization of actin during IS formation, which is essential for T-cell activation. In contrast, the Wave-related WASP protein is not required to polymerize F-actin at the T cell-APC interface or for initial IS formation and TCR-mediated integrin activation (1). However, WASP appears to be required for re-establishment of the IS following symmetry breaking of migratory, naive T cells (96).
Recent reports link the Abl kinases to HS1 and Wave2 in T cells (82, 86) (Fig 1 and Fig 4). Similar to the effects of Abl inhibition or depletion, HS1-deficient T cells have decreased actin polymerization at the T cell-B cell contact site, while conjugate formation is unaffected by loss of either Abl or HS1 (82, 97). However, the effects of Abl deletion in T cells are more profound than those of HS1 deletion. Abl-deficient T cells exhibit marked defects during spreading on anti-TCR-coated coverslips and show decreased persistence in the formation of lamellipodial protrusions (82). In contrast, TCR-stimulated actin polymerization during spreading and lamellipodial protrusions can occur in HS1-deficient cells, but these cells are defective in maintaining these actin-dependent structures (97). In this regard, the immune phenotypes of Abl-deficient mice are more severe than those of HS1 knockout mice (4, 6, 7, 98). In contrast to Abl- and Abl/Arg-deficient mice, development of the lymphoid system is normal in HS1-deficient mice. However, Abl protein and kinase activity are required for maximal tyrosine phosphorylation of HS1 and the related cortactin (82, 86) (Fig. 1). A recent study has shown that distinct tyrosine phosphorylation sites on HS1 are required for chemotaxis and for adhesion to intercellular adhesion molecule-1 (ICAM-1) in natural killer (NK) cells (99). It would be interesting to examine the role of Abl-mediated phosphorylation of HS1 in T cells during distinct cytoskeletal processes in T cells. However, because Abl-deficient T cells exhibit a more profound defects than HS1-deficient T cells, additional Abl targets are likely to mediate the Abl-dependent phenotypes.
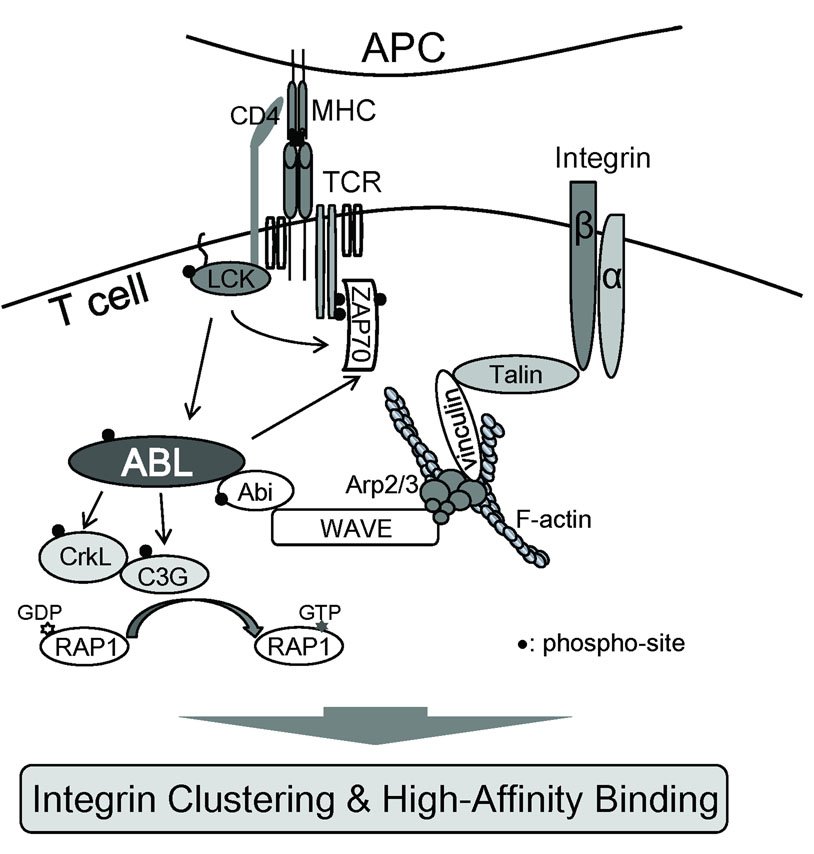
Fig. 4. Abl kinases regulate TCR-mediated integrin activation and adhesion.
Abl kinases interact with the Wave protein complex and regulate Rap1 activation via CrkL-C3G resulting in inside-out integrin activation and adhesion. Wave also regulates TCR-mediated integrin clustering and high affinity binding through the recruitment of a complex containing Arp2/3, vinculin, and talin.
Among other potential Abl targets in T cells is Wave2 (Fig 1 and Fig 4). It was observed that in Abl-deficient T cells, Wave localization to the IS was impaired (82). The mechanism whereby loss of Abl affects Wave localization remains to be determined. One possibility is that Rac activation might be impaired in the absence of Abl activity and thereby would impair Wave recruitment to the IS. However, it appears that Rac activity was unaffected by treatment with imatinib. Depletion or complete loss of Abl and Arg proteins could affect multiple cellular targets that might indirectly affect Wave recruitment to the IS. In contrast to these findings, another study reported that Wave2 recruits Abl to the membrane in response to TCR ligation (88). These contrasting results remain unexplained and future experiments are needed to identify the factors that contribute to the localization of Abl kinases and the Wave complex to the IS. Moreover, although Abl interacts with the Wave complex, it is likely that the interaction is indirect. It is unclear whether Wave2 is phosphorylated by Abl in T cells. It was reported that Wave2 is tyrosine phosphorylated in an Abl-dependent manner in fibroblasts and B16F1 cells in response to PDGF stimulation (87). However, others have failed to detect tyrosine phosphorylation of Wave2 in T cells (82).
Abl kinases also are implicated in the regulation of integrin-mediated adhesion to fibronectin and vascular cell adhesion molecule-1 (VCAM-1) (88). Stimulation of the TCR by MHCp complexes on APCs promotes the activation of T-cell integrins, an event that is required for the formation of stable T cell-APC conjugates (1). This TCR-stimulated process is known as inside-out signaling and results in clustering of integrins on the plasma cell membrane (avidity) and changes in integrin conformation (affinity). The leukocyte function-associated antigen-1 (LFA-1) (αLβ2) and very late antigen 4 (VLA4) (α4β1) integrins are involved in regulation of T cell-APC conjugate formation and localize to the pSMAC within the IS. LFA-1 has a major role during conjugate formation by binding to ICAM-1 on the surface of the APC, and functions as a costimulatory molecule for T-cell activation. The α4β1 integrin binds to the extracellular matrix protein fibronectin and to VCAM-1 and regulates T-cell extravasation. TCR-stimulated integrin activation requires the activity of Lck, LAT, Vav1, and Wave2, but not WASP (1). The Rap1, RhoA, Rac, and Cdc42 GTPases are also important in the regulation of TCR-dependent integrin activation.
Abl kinases were shown to interact with the Wave complex, and similar to Wave2, depletion of Abl resulted in impaired activation of Rap1 and reduced integrin-mediated adhesion (88) (Fig. 4). Previously, Wave2 was shown to regulate high-affinity integrin binding through the recruitment of vinculin and talin to the IS (100). Wave2 regulates TCR-mediated activation of Rap1 through a pathway involving Abl and the activation of the CkrL-C3G complex (88). C3G is a guanine nucleotide exchange factor (GEF) for Rap1 and associates with CrkL, a known substrate of the Abl kinases. Moreover, the CrkL-C3G complex has been shown to function downstream of both BCR-ABL and Abl in various cell types (101, 102). Knockdown of Abl, Wave2, CrkL, or C3G result in decreased integrin-mediated adhesion and decreased IL-2 production (88) (Fig. 4). Analysis of these signaling proteins tagged with different fluorescent proteins will be required to examine the dynamics of their recruitment to the IS in response to TCR stimulation by APCs.
Rap1 regulates multiple processes in addition to integrin-mediated adhesion (103). Rap1 was first reported to antagonize Ras signaling and induce T-cell anergy (104). TCR-mediated T-cell stimulation in the absence of CD28 costimulation resulted in anergy, which correlated with elevated Rap1 activity and decreased Ras signaling (104, 105). In contrast, studies using transgenic mice expressing the activated Rap1A-V12 mutant (106) or mice with a targeted disruption of the Rap1A gene (107, 108) have shown that Rap1A does not antagonize T-cell activation but rather Rap1A positively regulates T cells by promoting integrin-mediated adhesion. Moreover, T-cell proliferation and IL-2 production are decreased in Rap1A-deficient mice in response to CD3 stimulation alone or CD3/CD28 costimulation (107). However, the phenotypic consequences of Rap1A deficiency in T cells are mild compared to that of the Abl/Arg T-cell-conditional knockout mice. In contrast to the Abl/Arg T-cell-conditional knockout mice, the Rap1A−/− mice do not exhibit defective T-cell development (4, 107). Moreover, the decreased IL-2 production in the Rap1A-deficient mice is very mild compared to that observed in mice lacking Abl and Arg in T cells. Thus, it is unlikely that Rap1A mediates the defects in T-cell proliferation, IL-2 production, and T-cell development characteristic of the Abl/Arg T-cell-deficient mice. Rap1-independent pathways involving other GTPases such as Rac and Rho might be targeted by the Abl kinases in T cells. In this regard, we have shown that Abl kinases activate Rac and inhibit Rho downstream cadherin-mediated adhesion (25).
Abl kinases are required for both humoral and cellular immunity in mature T cells in vivo
In vitro studies have shown that Abl kinases are activated by TCR stimulation and that the activated Abl kinases regulate multiple signaling pathways leading to T-cell proliferation, development, adhesion, migration, and IS formation. What is the role of Abl kinases in immune function in vivo? Analysis of the role of Abl kinases in mature T cells showed that the total number of T cells was greatly reduced in mice lacking the Abl and Arg kinases and that Abl/Arg double null T cells exhibit reduced proliferation and cytokine production in response to TCR stimulation (4). Notably, T cells lacking Abl kinases were deficient in both IL-2 and IFNγ production but displayed similar levels of IL-4 (4). Significantly, antibody production in response to T-cell-dependent antigen was severely impaired in Abl/Arg conditional null mice (4). As expected, wildtype and mutant mice secreted equal amounts of IgM in response to T-cell-independent antigen. A striking result was the finding that mice lacking Abl kinases were compromised in their ability to produce IFN-positive CD8+ T cells upon Listeria monocytogenes infection (4). The number of IFNγ-positive CD8+ T cells in the Abl/Arg-conditional knockout mice was reduced by greater than 80% compared to wildtype controls following infection. Also, the number of specific CD8+ T cells binding to the H-2Kb peptide was also significantly reduced (73% reduction) in the Abl/Arg-null mice compared to wildtype. Thus, expansion of specific CD8+ T cells upon Listeria infection was impaired in Abl/Arg-null mice. The total number of IFNγ-positive CD4+ T cells in Abl/Arg-null mice was ~50% of the wildtype, and the total number of CD4+CD154+ DP cells in the Abl/Arg mutant mice was also ~50% of the wildtype (4). Thus, Abl kinases are required for the production of effector CD8+ T cells and to a lesser extent CD4+ and CD4+CD154+ T cells in response to Listeria infection. Together these findings reveal that Abl kinases regulate T-cell development and activation and that loss of Abl kinases specifically in T cells results in compromised immunity. These results are consistent with the reported immunosuppressive effects of STI571/imatinib treatment in some CML patients. A subset of CML patients receiving standard imatinib therapy develop varicella zoster virus infection as a consequence of reactivation of latent viral infection (109). This effect is likely due to defects in cell-mediated immunity as a result of low numbers of CD4+ T cells (109). The availability of mouse models lacking Abl family kinases in the T-cell compartment should allow for greater insights into the pathways regulated by Abl kinases and the identification of specific targets that are required for mediating the effects of Abl in distinct cellular processes. Future studies will reveal whether Abl kinases play a role in pathologies associated with immune deficiency in mice and humans.
Acknowledgements
We thank Dr. Patricia A. Zipfel for multiple contributions in the discovery of the roles of Abl family kinases in the immune system. Our work was supported by National Institutes of Health Grant AI056266.
References
- 1.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 2.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–116. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Rodriguez J, Readinger JA, Viorritto IC, Mueller KL, Houghtling RA, Schwartzberg PL. Tec kinases, actin, and cell adhesion. Immunol Rev. 2007;218:45–64. doi: 10.1111/j.1600-065X.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 4.Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. Defective T cell development and function in the absence of Abelson kinases. J Immunol. 2007;179:7334–7343. doi: 10.4049/jimmunol.179.11.7334. [DOI] [PubMed] [Google Scholar]
- 5.Zipfel PA, Zhang W, Quiroz M, Pendergast AM. Requirement for Abl kinases in T cell receptor signaling. Curr Biol. 2004;14:1222–1231. doi: 10.1016/j.cub.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzberg PL, et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991;65:1165–1175. doi: 10.1016/0092-8674(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 7.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 8.Mak TW, Penninger JM, Ohashi PS. Knockout mice: a paradigm shift in modern immunology. Nat Rev Immunol. 2001;1:11–19. doi: 10.1038/3509551. [DOI] [PubMed] [Google Scholar]
- 9.Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 10.Plattner R, et al. A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-gamma1. Nat Cell Biol. 2003;5:309–319. doi: 10.1038/ncb949. [DOI] [PubMed] [Google Scholar]
- 11.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plattner R, Koleske AJ, Kazlauskas A, Pendergast AM. Bidirectional signaling links the Abelson kinases to the platelet-derived growth factor receptor. Mol Cell Biol. 2004;24:2573–2583. doi: 10.1128/MCB.24.6.2573-2583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008;27:1095–1105. doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
- 15.Finn AJ, Feng G, Pendergast AM. Postsynaptic requirement for Abl kinases in assembly of the neuromuscular junction. Nat Neurosci. 2003;6:717–723. doi: 10.1038/nn1071. [DOI] [PubMed] [Google Scholar]
- 16.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 17.Toyofuku T, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez SE, Krishnaswami M, Miller AL, Koleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 2004;14:36–44. doi: 10.1016/j.tcb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JM, Baskaran R, Taagepera S, Schwartz MA, Wang JY. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc Natl Acad Sci U S A. 1996;93:15174–15179. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furstoss O, Dorey K, Simon V, Barila D, Superti-Furga G, Roche S. c-Abl is an effector of Src for growth factor-induced c-myc expression and DNA synthesis. Embo J. 2002;21:514–524. doi: 10.1093/emboj/21.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plattner R, Pendergast AM. Activation and signaling of the Abl tyrosine kinase: bidirectional link with phosphoinositide signaling. Cell Cycle. 2003;2:273–274. [PubMed] [Google Scholar]
- 22.Backert S, Feller SM, Wessler S. Emerging roles of Abl family tyrosine kinases in microbial pathogenesis. Trends Biochem Sci. 2008;33:80–90. doi: 10.1016/j.tibs.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Burton EA, Plattner R, Pendergast AM. Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 2003;22:5471–5479. doi: 10.1093/emboj/cdg512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton EA, Oliver TN, Pendergast AM. Abl kinases regulate actin comet tail elongation via an N-WASP-dependent pathway. Mol Cell Biol. 2005;25:8834–8843. doi: 10.1128/MCB.25.20.8834-8843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci U S A. 2007;104:17686–17691. doi: 10.1073/pnas.0703077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goff SP, Gilboa E, Witte ON, Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980;22:777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- 27.Kruh GD, Perego R, Miki T, Aaronson SA. The complete coding sequence of arg defines the Abelson subfamily of cytoplasmic tyrosine kinases. Proc Natl Acad Sci U S A. 1990;87:5802–5806. doi: 10.1073/pnas.87.15.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Neriah Y, Bernards A, Paskind M, Daley GQ, Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986;44:577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- 29.Deng X, et al. Caenorhabditis elegans ABL-1 antagonizes p53-mediated germline apoptosis after ionizing irradiation. Nat Genet. 2004;36:906–912. doi: 10.1038/ng1396. [DOI] [PubMed] [Google Scholar]
- 30.Henkemeyer MJ, Bennett RL, Gertler FB, Hoffmann FM. DNA sequence, structure, and tyrosine kinase activity of the Drosophila melanogaster Abelson proto-oncogene homolog. Mol Cell Biol. 1988;8:843–853. doi: 10.1128/mcb.8.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagar B, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112:859–871. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 32.Hantschel O, et al. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell. 2003;112:845–857. doi: 10.1016/s0092-8674(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 33.Ren R, Ye ZS, Baltimore D. Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 1994;8:783–795. doi: 10.1101/gad.8.7.783. [DOI] [PubMed] [Google Scholar]
- 34.Van Etten RA, Jackson PK, Baltimore D, Sanders MC, Matsudaira PT, Janmey PA. The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J Cell Biol. 1994;124:325–340. doi: 10.1083/jcb.124.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Miller AL, Mooseker MS, Koleske AJ. The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin-binding domains to bundle F-actin.[comment] Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14865–14870. doi: 10.1073/pnas.251249298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller AL, Wang Y, Mooseker MS, Koleske AJ. The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. J Cell Biol. 2004;165:407–419. doi: 10.1083/jcb.200308055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao YJ, Wang JY. Binding of A/T-rich DNA by three high mobility group-like domains in c-Abl tyrosine kinase. J Biol Chem. 1996;271:22823–22830. doi: 10.1074/jbc.271.37.22823. [DOI] [PubMed] [Google Scholar]
- 38.Taagepera S, et al. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci U S A. 1998;95:7457–7462. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen ST, Jackson PK, Van Etten RA. The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. Embo J. 1996;15:1583–1595. [PMC free article] [PubMed] [Google Scholar]
- 40.Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 41.Tanis KQ, Veach D, Duewel HS, Bornmann WG, Koleske AJ. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol Cell Biol. 2003;23:3884–3896. doi: 10.1128/MCB.23.11.3884-3896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brasher BB, Van Etten RA. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J Biol Chem. 2000;275:35631–35637. doi: 10.1074/jbc.M005401200. [DOI] [PubMed] [Google Scholar]
- 43.Wang JY, Ledley F, Goff S, Lee R, Groner Y, Baltimore D. The mouse c-abl locus: molecular cloning and characterization. Cell. 1984;36:349–356. doi: 10.1016/0092-8674(84)90228-9. [DOI] [PubMed] [Google Scholar]
- 44.Groffen J, Stephenson JR, Heisterkamp N, de Klein A, Bartram CR, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984;36:93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- 45.Cazzaniga G, et al. The tyrosine kinase abl-related gene ARG is fused to ETV6 in an AML-M4Eo patient with a t(1;12)(q25;p13): molecular cloning of both reciprocal transcripts. Blood. 1999;94:4370–4373. [PubMed] [Google Scholar]
- 46.Golub TR, et al. Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol Cell Biol. 1996;16:4107–4116. doi: 10.1128/mcb.16.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iijima Y, et al. A new ETV6/TEL partner gene, ARG (ABL-related gene or ABL2), identified in an AML-M3 cell line with a t(1;12)(q25;p13) translocation. Blood. 2000;95:2126–2131. [PubMed] [Google Scholar]
- 48.Graux C, et al. Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nat Genet. 2004;36:1084–1089. doi: 10.1038/ng1425. [DOI] [PubMed] [Google Scholar]
- 49.De Keersmaecker K, et al. Fusion of EML1 to ABL1 in T-cell acute lymphoblastic leukemia with cryptic t(9;14)(q34;q32) Blood. 2005;105:4849–4852. doi: 10.1182/blood-2004-12-4897. [DOI] [PubMed] [Google Scholar]
- 50.De Keersmaecker K, et al. Kinase activation and transformation by NUP214-ABL1 is dependent on the context of the nuclear pore. Mol Cell. 2008;31:134–142. doi: 10.1016/j.molcel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Buchdunger E, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–104. [PubMed] [Google Scholar]
- 52.Buchdunger E, et al. Selective inhibition of the platelet-derived growth factor signal transduction pathway by a protein-tyrosine kinase inhibitor of the 2-phenylaminopyrimidine class. Proc Natl Acad Sci U S A. 1995;92:2558–2562. doi: 10.1073/pnas.92.7.2558. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Okuda K, Weisberg E, Gilliland DG, Griffin JD. ARG tyrosine kinase activity is inhibited by STI571. Blood. 2001;97:2440–2448. doi: 10.1182/blood.v97.8.2440. [DOI] [PubMed] [Google Scholar]
- 54.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 55.Druker BJ, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 56.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 57.Kantarjian H, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 58.Druker BJ, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 59.Gorre ME, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 60.Shah NP, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 61.Kantarjian HM, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–475. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 62.Kerkela R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 63.Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6:834–848. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- 64.Gontarewicz A, et al. Simultaneous targeting of Aurora kinases and Bcr-Abl kinase by the small molecule inhibitor PHA-739358 is effective against imatinib-resistant BCR-ABL mutations including T315I. Blood. 2008;111:4355–4364. doi: 10.1182/blood-2007-09-113175. [DOI] [PubMed] [Google Scholar]
- 65.Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104:1094–1099. doi: 10.1182/blood-2003-12-4266. [DOI] [PubMed] [Google Scholar]
- 66.Seggewiss R, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105:2473–2479. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 67.Fabian MA, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 68.Dewar AL, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–3132. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 69.Koleske AJ, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 70.Hardin JD, et al. Bone marrow B lymphocyte development in c-abl-deficient mice. Cell Immunol. 1995;165:44–54. doi: 10.1006/cimm.1995.1185. [DOI] [PubMed] [Google Scholar]
- 71.Zipfel PA, Grove M, Blackburn K, Fujimoto M, Tedder TF, Pendergast AM. The c-Abl tyrosine kinase is regulated downstream of the B cell antigen receptor and interacts with CD19. J Immunol. 2000;165:6872–6879. doi: 10.4049/jimmunol.165.12.6872. [DOI] [PubMed] [Google Scholar]
- 72.Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol Cell Biol. 2005;25:4924–4933. doi: 10.1128/MCB.25.12.4924-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007;129:735–746. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 74.Kim HK, et al. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 75.Hayday AC, Pennington DJ. Key factors in the organized chaos of early T cell development. Nat Immunol. 2007;8:137–144. doi: 10.1038/ni1436. [DOI] [PubMed] [Google Scholar]
- 76.Cantrell DA. Transgenic analysis of thymocyte signal transduction. Nat Rev Immunol. 2002;2:20–27. doi: 10.1038/nri703. [DOI] [PubMed] [Google Scholar]
- 77.von Boehmer H. Unique features of the pre-T-cell receptor alpha-chain: not just a surrogate. Nat Rev Immunol. 2005;5:571–577. doi: 10.1038/nri1636. [DOI] [PubMed] [Google Scholar]
- 78.Molina TJ, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 79.Cheng AM, et al. The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc Natl Acad Sci U S A. 1997;94:9797–9801. doi: 10.1073/pnas.94.18.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roose JP, et al. T cell receptor-independent basal signaling via Erk and Abl kinases suppresses RAG gene expression. PLoS Biol. 2003;1:E53. doi: 10.1371/journal.pbio.0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Huang Y, Comiskey EO, Dupree RS, Li S, Koleske AJ, Burkhardt JK. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood. 2008;112:111–119. doi: 10.1182/blood-2007-10-118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 84.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cemerski S, et al. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007;17:445–451. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 87.Leng Y, et al. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proc Natl Acad Sci U S A. 2005;102:1098–1103. doi: 10.1073/pnas.0409120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nolz JC, et al. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. J Cell Biol. 2008;182:1231–1244. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 90.Welch MD, Mullins RD. Cellular control of actin nucleation. Annu Rev Cell Dev Biol. 2002;18:247–288. doi: 10.1146/annurev.cellbio.18.040202.112133. [DOI] [PubMed] [Google Scholar]
- 91.Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14:303–311. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 92.Uruno T, Zhang P, Liu J, Hao JJ, Zhan X. Haematopoietic lineage cell-specific protein 1 (HS1) promotes actin-related protein (Arp) 2/3 complex-mediated actin polymerization. Biochem J. 2003;371:485–493. doi: 10.1042/BJ20021791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol. 2002;12:1270–1278. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 94.Nolz JC, et al. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zipfel PA, et al. Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr Biol. 2006;16:35–46. doi: 10.1016/j.cub.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 96.Sims TN, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 97.Gomez TS, et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taniuchi I, Kitamura D, Maekawa Y, Fukuda T, Kishi H, Watanabe T. Antigen-receptor induced clonal expansion and deletion of lymphocytes are impaired in mice lacking HS1 protein, a substrate of the antigen-receptor-coupled tyrosine kinases. Embo J. 1995;14:3664–3678. doi: 10.1002/j.1460-2075.1995.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butler B, Kastendieck DH, Cooper JA. Differently phosphorylated forms of the cortactin homolog HS1 mediate distinct functions in natural killer cells. Nat Immunol. 2008;9:887–897. doi: 10.1038/ni.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nolz JC, et al. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol Cell Biol. 2007;27:5986–6000. doi: 10.1128/MCB.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho YJ, Hemmeryckx B, Groffen J, Heisterkamp N. Interaction of Bcr/Abl with C3G, an exchange factor for the small GTPase Rap1, through the adapter protein Crkl. Biochem Biophys Res Commun. 2005;333:1276–1283. doi: 10.1016/j.bbrc.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 102.Radha V, Rajanna A, Mitra A, Rangaraj N, Swarup G. C3G is required for c-Abl-induced filopodia and its overexpression promotes filopodia formation. Exp Cell Res. 2007;313:2476–2492. doi: 10.1016/j.yexcr.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 103.Stork PJ, Dillon TJ. Multiple roles of Rap1 in hematopoietic cells: complementary versus antagonistic functions. Blood. 2005;106:2952–2961. doi: 10.1182/blood-2005-03-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 105.Reedquist KA, Bos JL. Costimulation through CD28 suppresses T cell receptor-dependent activation of the Ras-like small GTPase Rap1 in human T lymphocytes. J Biol Chem. 1998;273:4944–4949. doi: 10.1074/jbc.273.9.4944. [DOI] [PubMed] [Google Scholar]
- 106.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251–258. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 107.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, et al. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179:8322–8331. doi: 10.4049/jimmunol.179.12.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mattiuzzi GN, et al. Development of Varicella-Zoster virus infection in patients with chronic myelogenous leukemia treated with imatinib mesylate. Clin Cancer Res. 2003;9:976–980. [PubMed] [Google Scholar]