Abstract
It is proposed that an important function of leptin is to confine the storage of triglycerides (TG) to the adipocytes, while limiting TG storage in nonadipocytes, thus protecting them from lipotoxicity. The fact that TG content in nonadipocytes normally remains within a narrow range, while that of adipocytes varies enormously with food intake, is consistent with a system of TG homeostasis in normal nonadipocytes. The facts that when leptin receptors are dysfunctional, TG content in nonadipocytes such as islets can increase 100-fold, and that constitutively expressed ectopic hyperleptinemia depletes TG, suggest that leptin controls the homeostatic system for intracellular TG. The fact that the function and viability of nonadipocytes is compromised when their TG content rises above or falls below the normal range suggests that normal homeostasis of their intracellular TG is critical for optimal function and to prevent lipoapoptosis. Thus far, lipotoxic diabetes of fa/fa Zucker diabetic fatty rats is the only proven lipodegenerative disease, but the possibility of lipotoxic disease of skeletal and/or cardiac muscle may require investigation, as does the possible influence of the intracellular TG content on autoimmune and neoplastic processes.
Leptin was discovered by positional cloning of a single gene mutation in the ob/ob mouse (1), a well-characterized model of obesity and diabetes with endocrine and immunologic abnormalities (2). Because the obesity is caused by deficiency of the ob gene product, leptin, and can be corrected by replacement of the peptide (3–5), leptin generally is regarded as an antiobesity hormone. Yet, there are theoretical and factual reasons for doubt that this is its primary function. First, there is little evidence of evolutionary pressure to prevent obesity; on the contrary, obesity can be a survival asset, a defense against famine, as proposed in the “thrifty gene” theory of Neel (6). Second, more than half of the population in the United States is overweight despite higher plasma leptin levels than the nonobese minority (7, 8)—hardly the credentials of a hormone that prevents obesity. Consequently, while leptin deficiency certainly causes obesity, it seems unlikely that prevention of obesity is its primary physiologic role.
A Novel Physiologic Role for Leptin
Regulating Fatty Acid Metabolism in Nonadipocytes.
If prevention of obesity is not the primary function of leptin, what is its physiologic mission? Here, we propose that an important physiologic function of leptin is to regulate in nonadipocytes the intracellular homeostasis of fatty acids (FA) and triglycerides (TG) so as to maintain a sufficient supply of FA for essential cell functions while avoiding TG overload.
Long-chain fatty acids provide the building blocks for biologic membranes, the anchors for membrane proteins, and the source of lipid-containing messengers. Those tissues thus far examined contain a small intracellular reserve of FA stored as TG (9, 10), perhaps to provide a committed source of FA for maintenance of structural and signaling components. We postulate that if this ready reserve of TG was diverted to use as a fuel, cell function and viability would be compromised. Because the TG storage capacity of nonadipocytes is very limited, the use of FA as a fuel required an extracellular storage facility in unique cells specialized to stockpile large quantities of TG and to export FA on demand. These cells are the adipocytes. The evolution of adipocytes provided nonadipocytes with unlimited access to FA for use as a fuel, while preserving their small intracellular TG stockpile for the maintenance of crucially important intracellular “housekeeping” functions.
Impact of Adipocytes.
The virtually limitless fuel storage capacity provided by adipocytes radically changed the evolutionary hierarchy and the dimensions of life on earth, freeing organisms to move about on the planet with its constantly changing food supply. Now enormous of quantities TG could be stored in anticipation of extended periods of food deprivation. For example, adipocytes allow the desert rat to survive months of summer famine by becoming obese during the rainy season when food is plentiful; obese Polynesians could traverse 2,000 miles of Pacific Ocean in outrigger canoes, bound for the Hawaiian Islands with minimal food supply on board. This type of temporary obesity was not a disease, but rather a means of preloading fuel to meet an anticipated need.
Full exploitation of the enormous storage capacity provided by the advent of the adipocyte required a major increase in caloric intake. In addition, it was critical that the surplus TG be stored primarily in adipocytes and not be distributed equally to all cells. It therefore was essential that, during periods of TG deposition, adipocytes secrete a signal that would block the overaccumulation of TG in nonadipocytes and thus confine fat storage to fat cells. We propose that the signal is leptin, and that its function is to create for adipocytes a monopoly on fat storage, so as to maintain the constancy of intracellular TG in nonadipocytes.
Validation of the Hypothesis
Evidence for a System of TG Homeostasis in Nonadipocytes.
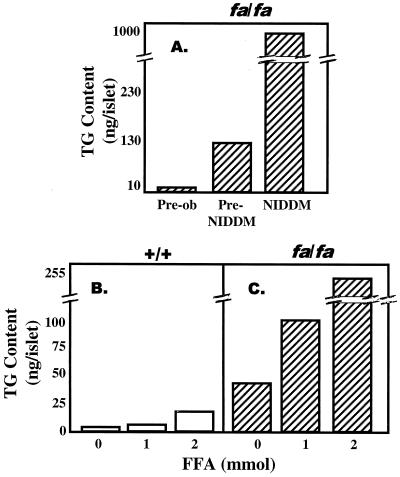
There is compelling evidence for the existence of a system of TG homeostasis in nonadipocytes. Whereas the TG content of adipocytes varies with caloric intake and can range from a small fraction of the lean body mass to hundreds of pounds, the TG content of normal nonadipocytes is confined to within a narrow range irrespective of caloric intake. This fact argues strongly for a regulated system of fat homeostasis inside nonadipocytes. Furthermore, when one cultures normal nonadipocytes, such as islets, in 1 or 2 mM FA, TG content rises only slightly (Fig. 1B), because FA-induced up-regulation of peroxisome proliferator-activated receptor α (PPARα) and the enzymes of oxidation, acyl CoA oxidase and carnitine palmitoyl transferase 1, direct the FA into oxidative rather than lipogenic pathways (11), thereby disposing of unwanted FA and preventing their accumulation as TG (12). Conversely, islets from leptin-insensitive Zucker diabetic fatty (ZDF, fa/fa) rats with defective leptin receptors exhibit a marked increase in TG accumulation at the same concentrations of FA (Fig. 1C) (13). These findings are evidence that a homeostatic system does exist and that it is dysfunctional in the absence of leptin.
Figure 1.
TG content of islets. (A) TG content of islets isolated from obese ZDF (fa/fa) at age of 4 weeks (preobese), 8 weeks (obese, prediabetic, Pre-NIDDM), and 12 weeks (obese, diabetic, NIDDM). (B) TG content of islets isolated from normal lean ZDF (+/+) rats and cultured in 0, 1, or 2 mM FA. (C) TG content of islets isolated from age-matched obese prediabetic ZDF (fa/fa) rats and cultured in 0, 1, or 2 mM FA.
Evidence that Intracellular TG Homeostasis in Nonadipocytes Is Regulated by Leptin Action.
If, in fact, leptin protects nonadipocytes from excessive accumulation of TG during periods of overfeeding, one would anticipate that leptin biosynthesis and secretion would increase in proportion to food intake. In fact, food intake is associated with a rise in adipocyte leptin mRNA (14, 15). We submit that the hyperleptinemia at the onset of obesity provides protection of nonadipocytes from TG overload until such time as nonadipocytes develop leptin resistance and late lipotoxic complications of obesity begin.
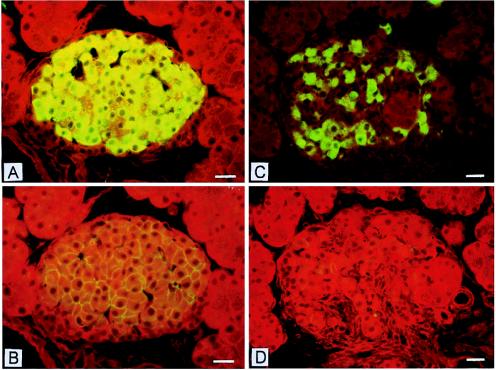
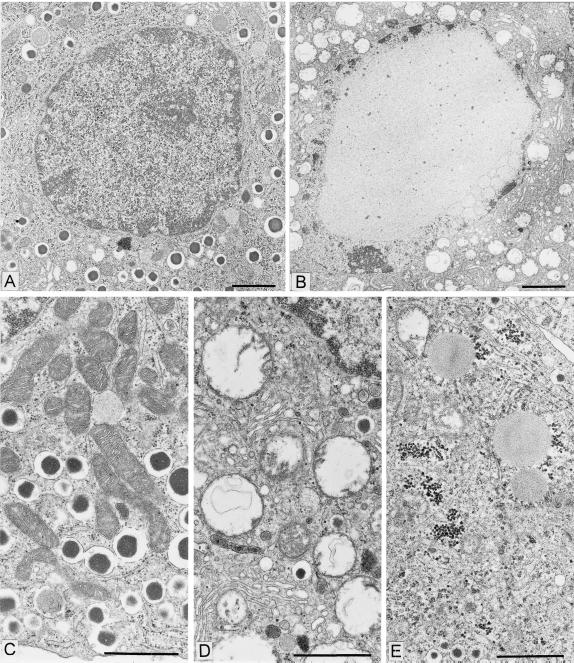
If leptin is directly responsible for regulation of TG homeostasis in nonadipocytes, loss of leptin action resulting from a mutation in the leptin receptor (OB-R) should result in excess fat deposition in and damage to nonadipocytes that is reversed by transgenic overexpression of wild-type OB-R. ZDF rats have a Glu-269 → Pro mutation in the extracellular domain of their OB-R isoforms (16, 17), and they are leptin resistant (18). The TG content of their nonadipocyte tissues is markedly increased (Fig. 1A) (10, 13). Longitudinal studies of islets of leptin-resistant ZDF rats indicate that the TG content of their nonadipocytes rises progressively in parallel with the increase in adipocyte TG (10, 19), consistent with the prediction that without leptin action nonadipocytes are unprotected from overaccumulation of fat (Fig. 1 A and C). As fat content in islets increases, β cells at first become hyperplastic and insulin production rises (20, 21). But once the fat content exceeds ≈50 times normal, the hyperplastic β cells undergo lipoapoptosis (22) and diabetes appears. This finding implies that lack of leptin action in these nonadipocytes tissue caused the fat overload, which impaired the function and ultimately the viability of β cells. In addition to the functional abnormalities, there are extensive morphologic changes (Figs. 2 and 3), including disorganization of the islet architecture (Fig. 2C), loss of the high Km glucose transporter of β cells, GLUT-2 (Fig. 2D) (23), and a high percentage of mitochondrial abnormalities (Fig. 3 B and D), consistent with the view that leptin action on β cells (and perhaps other nonadipocyte tissues) serves to maintain a normal TG content and to guard against fat overload and lipotoxicity.
Figure 2.
Insulin and GLUT-2 immunostaining in islets of nondiabetic and diabetic ZDF rats. (A and B) Consecutive sections of a nondiabetic female rat islet. The intense cytoplasmic insulin immunofluorescence is seen in the densely packed insulin cells in the islet’s center (A). GLUT-2 staining elicits a delicate immunofluorescent rim at the periphery of insulin cells (B). (Bar = 20 μm.) (C and D) Consecutive sections of a diabetic male rat islet. Insulin-immunofluorescent cells are in reduced numbers as compared with A and appear in cords separated by tracks of connective tissue (C). The GLUT-2 antibody elicits no staining at the periphery of insulin cells (D). (Bar = 20 μm.)
Figure 3.
Nuclear and cytoplasmic lesions in insulin-secreting cells of diabetic ZDF rats. (A) Low-power electron micrograph of a control insulin cell. This equatorial section shows the nucleus, the nucleolus, and the peripheral zone of heterochromatin on the inner side of the nuclear envelope; the section also shows a large area of cytoplasm containing a population of dense core secretory granules, a few mitochondria, and various membrane profiles belonging to the endoplasmic reticulum and Golgi apparatus compartments. (Bar = 2 μm.) (B) Low-power electron micrograph of an insulin cell in a diabetic ZDF rat. In this plane of section comparable to that in A, the nucleus appears markedly altered with a near complete disappearance of the euchromatin region replaced by a finely granular clear substance. Clumped chromatin masses indicative of classical apoptotic reaction also are observed in rare cells. The cytoplasm is markedly depleted in secretory granules and a large number of vacuoles represent the remnants of damaged mitochondria (see D). (Bar = 2 μm.) (C) Medium-power electron micrograph showing the typical morphology of the secretory granules, mitochondria, and endoplasmic reticulum compartments in a control insulin cell. (Bar = 1 μm.) (D) Field of an insulin cell in a diabetic ZDF rat showing a marked dilatation of the mitochondria and widespread rupture of the cristae. The RE-Golgi compartments appear relatively unaffected. (Bar = 1 μm.) Mitochondrial damage was quantitated. In the older (10–12 wk) and more hyperglycemic diabetic ZDF rats, the number of altered mitochondria was on average 98% of the total mitochondria present (three rats, three islets per rat, eight cells per islet, 1,475 mitochondria evaluated). One rat in the diabetic ZDF population (8 wk, moderately hyperglycemic), was completely “unreactive” with respect to mitochondrial damage (two islets, 555 mitochondria evaluated). In the control rat population, whether older or younger, the percentage of altered mitochondria was never above 4% (six rats, three islets per rat, eight cells per islet, 4,515 mitochondria evaluated). (E) Field of an insulin cell in a ZDF rat showing a cytoplasmic region with two altered mitochondrial profiles, but with several lipid droplets and glycogen deposits. On random sections lipid droplets were rare. When serially sectioned, several β cells that did not show lipid inclusion on one section contained lipid on the next consecutive sections. (Bar = 1 μm.)
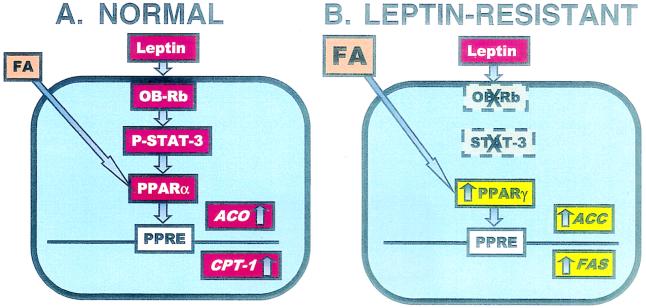
The foregoing in vivo findings can be duplicated in vitro. When islets from ZDF rats with defective OB-R are cultured in 2 mM FA (Fig. 1C), TG accumulation is 19 times greater than in normal islets (Fig. 1B). This difference is attributed to the high expression level of the enzymes of FA synthesis (acetyl CoA carboxylase and FA synthetase) and esterification (glycerol-Po4-acyl transferase) and of their transcription factor, PPAR-γ (Fig. 4B), coupled with reduced expression of the enzymes of FA oxidation, acyl CoA oxidase, and carnitine palmitoyl transferase, and their transcription factor, PPAR-α (24).
Figure 4.
(A) Depiction of the putative homeostatic system in normal nonadipocytes (islets). FA stimulate PPARα, acyl CoA oxidase (ACO), and carnitine palmitoyl transferase 1 (CPT-1) expression, increasing their oxidation and minimizing TG formation. (B) In nonadipocytes (islets) of leptin-resistant ZDF (fa/fa) rats, this homeostatic system is nonfunctional. PPARα is low and expression of PPARγ and the lipogenic enzymes, acetyl CoA carboxylase (ACC), and FA synthetase (FAS) is increased, leading to TG accumulation (10). PPRE, PPAR response element.
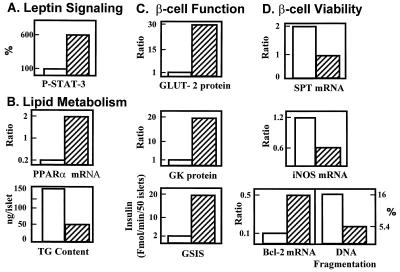
If the full phenotype of diabetic islets of fa/fa rats is the direct result of impaired leptin action, overexpression of wild-type OB-R in islets of OB-R-defective, diabetic fa/fa rats should restore leptin’s lipopenic action, and, in so doing, correct the various morphologic and functional derangements. The transfer by adenovirus of the OB-Rb cDNA to islets of diabetic fa/fa rats does, in fact, correct all identified islet abnormalities that incapacitate and ultimately kill β cells (25–28) (Fig. 5).
Figure 5.
Effect of overexpression of wild-type OB-Rb or β-galactosidase cDNA in islets of obese, diabetic (fa/fa) ZDF rats on (A) leptin signaling, phosphorylated STAT-3; (B) lipid metabolism, PPARa mRNA and TG content; (C) β-cell function, GLUT-2 protein, glucokinase (GK) protein, and glucose-stimulated insulin secretion (GSIS); (D) β-cell viability, serine-palmitoyl transferase (SPT) mRNA, inducible NO synthetase (iNOS) mRNA, Bcl-2 mRNA, and DNA fragmentation. See text for further description. Values for OB-Rb-overexpressing islets are expressed as % of β-galactosidase-overexpressing controls.
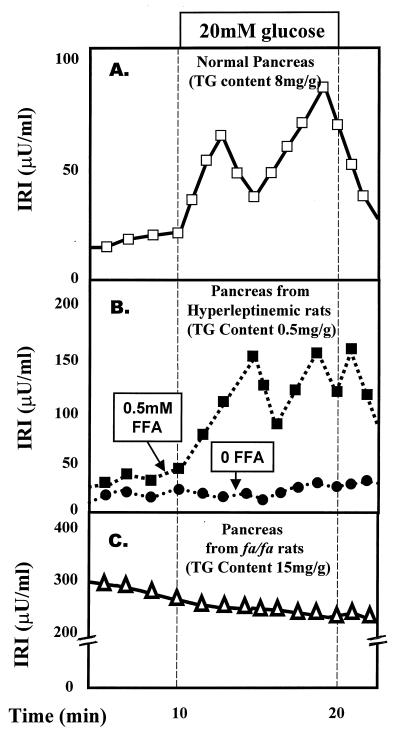
Finally, if the hypothesis that leptin regulates fat homeostasis in nonadipocytes is truly correct, an excess of leptin should profoundly deplete the fat reservoirs of normal rats with functioning OB-Rs; moreover, if preservation of the normal TG stores is important for cell function, fat depletion should result in functional abnormalities in these cells. The fat content of normal pancreas can be reduced from the normal level of 8 mg/g of wet weight to unmeasurable levels by adenovirus-mediated ectopic overexpression of the leptin gene (29). Fat-depleted islets are morphologically normal but are functionally “paralyzed,” with a low rate of basal insulin production and complete unresponsiveness to glucose and arginine stimulation (29); however, normal responses are restored within moments on adding FA (Fig. 6B). Similar results had been observed previously in fat-depleted islets of fasted rats treated with nicotinic acid (30).
Figure 6.
Effect of TG content of islets on β-cell function. (A) Insulin secretory response to glucose in perfused pancreata of normal rats. (B) Insulin response to glucose in pancreata from fat-depleted hyperleptinemic rats perfused with or without FA in the perfusate. (C) Insulin response to glucose of fat-laden pancreata from obese, diabetic (fa/fa) ZDF rats. IRI, immunoreactive insulin.
In summary, the hypothesis of leptin-regulated homeostatic control over nonadipocytes TG content is supported (i) by the relative constancy of fat content in normal nonadipocytes, (ii) by the profound depletion of nonadipocyte TG caused by hyperleptinemia, (iii) by the steatosis of nonadipocytes in rats with leptin insensitivity and (iv) by the fact that, insertion of the normal OB-R in the leptin unresponsive islets corrects leptin unresponsiveness and reduces the steatosis.
Disease Implications of Abnormal TG Homeostasis Lipotoxicity and Lipoapoptosis
Mechanism of Lipoapoptosis in Underleptinized Cells.
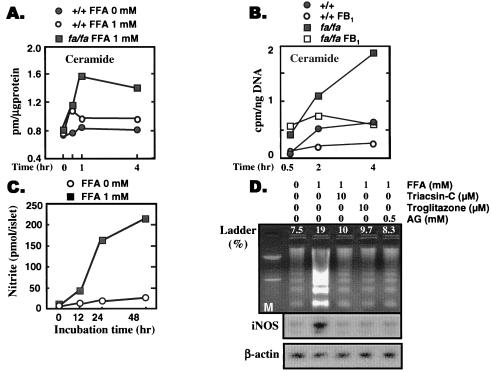
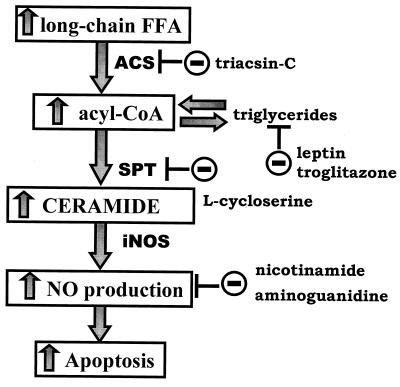
Lipoapoptosis in islets appears to be largely the result of increased de novo synthesis of ceramide (Fig. 7 A and B) (27). Ceramide synthesis begins with the condensation of palmitoyl CoA with serine. The reaction is catalyzed by serine-palmitoyl transferase (SPT). SPT is expressed at abnormally high levels in ZDF islets (27), which, together with the overabundance of long-chain FA, accounts for the increased ceramide formation (Fig. 7A) (22). Ceramide stimulates the expression of inducible NO synthase (31), which is increased in OB-R-defective fa/fa islets (32). The lipoapoptotic pathway, together with loci of potential interventions, is portrayed in Fig. 8.
Figure 7.
(A) Comparison of ceramide formation in islets from normal lean (+/+) ZDF rats or obese, prediabetic (fa/fa) ZDF rats cultured in 1 mM FA. (B) Comparison of [3H]ceramide formation from [3H]palmitate in islets of +/+ and fa/fa rats cultured in the presence or absence of fumonisin-B1 (FB1), an inhibitor of de novo ceramide synthesis. (C) NO formation in islets from obese, prediabetic (fa/fa) ZDF rats cultured in 0 or 1 mM FA. (D) The effect of an acyl CoA synthetase inhibitor, triacsin-C, and of the inhibitor of the inducible NO synthetase, aminoguanidine (AG) on DNA laddering induced by 1 mM FA. DNA laddering is an index of apophosis.
Figure 8.
The proposed pathway of lipoapoptosis and loci of intervention.
Lipotoxic Diabetes.
Islet cell function in the obese fa/fa rats is sufficient to prevent diabetes until the rate of β-cell apoptosis begins to exceed the rate of β-cell replacement. At this point, the reduced mass of insulin-producing cells can no longer meet the rising insulin needs that obesity causes. The result is lipotoxic diabetes. Lipotoxicity of β cells, identified as the probable cause of diabetes of the ZDF rat, seems to warrant designation as a true lipodegenerative disease. Indeed, β cells are notoriously vulnerable to damage by free radicals (33). It remains to be determined whether other diabetic syndromes in rodents and humans (34–40) also are caused by overloading of nonadipocyte tissues with TG (Table 1). In obesity syndromes caused by generalized leptin deficiency or resistance, ectopic fat deposition is likely caused by the failure of leptin to prevent excess TG storage in nonadipocytes. All such syndromes share the phenotype of hypertriglyceridemia, insulin resistance, and ultimately overt diabetes. In obesity caused by lesions of the ventromedial nucleus of the hypothalamus in rats, TG does not accumulate outside of the adipocytes, and the nonadipocytes appear to be sensitive to leptin (18).
Table 1.
Possible syndromes of ectopic fat deposition
| Leptin deficiency |
| ob/ob mouse? (2) |
| Human leptin deficiency? (40) |
| Lipodystrophy |
| SERBP-1 transgenic mouse? (35) |
| A-ZIP/F-1 transgenic mouse? (36) |
| aP2/DTA transgenic mouse? (37) |
| Human lipodystrophy? (38) |
| Leptin receptor defect |
| db/db mouse? (2) |
| Human OB-R defect? (40) |
| ZDF (fa/fa) rat (10) |
| Diet-induced obesity |
| Rat and human? |
? indicates that careful measurements of fat in nonadipocytes have not been reported but are predicted to be high.
Measurements of TG content are not yet available in diet-induced obesity of rats or humans (Table 1). It is likely that their hyperleptinemia will maintain a normal level of ectopic fat deposition during much of their obesity, but that resistance to leptin will appear later in the disease and permit a rise in nonadipocyte TG.
Compared to the obesity syndromes, we expect ectopic TG deposition to be much more severe in the lipodystrophic syndromes (Table 1). The lack of TG storage capacity should shunt TG to nonadipocytes, where, because of hypoleptinemia, ectopic fat accumulates to cause insulin resistance and β-cell lipotoxicity.
Lipotoxic Liver Disease.
Despite extensive clinical experience with fatty liver caused by alcohol, viral infection, and unknown causes (41, 42), little is known about the molecular pathophysiology of hepatic steatosis. However, the similarity between the disorganized, fibrotic islets of ZDF rats (Fig. 2A) and the pathology of end-stage fatty liver warrants further study to determine whether blockade of the lipoapoptotic pathway would favorably alter the course of the liver disease.
Liptoxicity of Muscle in the Elderly Obese.
More subtle lipotoxic disorders may involve aging skeletal and cardiac muscle, particularly in obese individuals. Age-related loss of skeletal muscle in the elderly occurs in association with an increasing adipocyte mass. The possibility that increased TG content, high NO generation, and apoptosis is a factor in loss of aging muscle cells may warrant serious study, because it may be preventable.
Lipotoxic Potentiation of Autoimmune Diseases.
Autoimmune destruction of β cells in insulin-dependent diabetes mellitus is believed to involve ceramide (43). Although the ceramide is thought to be derived from sphingomyelin breakdown, rather than from de novo synthesis, depletion of islet fat by leptin or troglitazone markedly inhibits interleukin 1β-induced cytotoxicity (44), suggesting the possibility of “metabolic immunosuppression” to prevent autoimmune destruction of β cells. The implication of these findings is that intracellular FA content may influence the vulnerability of certain cells to cytokine-induced apoptosis.
Lipotoxic Potentiation of Oncogenesis.
The proliferative effect of high levels of long-chain FA on β cells (20) raises the possibility that exposure to TG has mitogenic effects that increase the risk of neoplasia in other cells. Considerable evidence suggests a link between a high-fat diet and cancer of the colon (45). FA synthase is expressed in all colorectal neoplasms and adenomas at higher levels than in mature colorectal mucosa (46). Recently, there have been reports that PPAR activation may provide a molecular link between a high-fat diet and increased risk of colorectal cancer (47–49).
Acknowledgments
We thank Tess Perico and Susan Kennedy for expert secretarial work and Kay McCorkle for medical illustration skills. We are grateful to Drs. M. S. Brown and J.-P. Giacobino for critical examination of the manuscript. This work was supported by the National Institutes of Health (DK02700-37), National Institutes of Health/Juvenile Diabetes Foundation Diabetes Interdisciplinary Research program, Department of Veterans Affairs Institutional Support (SMI 821-109), Sankyo, Novo Nordisk, and Swiss National Science Foundation (L.O.).
ABBREVIATIONS
- FA
fatty acids
- TG
triacylglycerol
- PPAR
peroxisome proliferator-activated receptor
- ZDF
Zucker diabetic fatty
- OB-R
leptin receptor
- GLUT-2
glucose transporter-2
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Coleman D L. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 3.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 4.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 5.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 6.Neel J V. Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 7.Maffei M, Halaas J, Ravussin E, Prattey R E, Lee G H, Zhang Y, Fei H, Kim S, Lalloone R, Ranganathan S, et al. Nat Med. 1995;11:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 8.Considine R V, Sinha M K, Heiman A, Kriaciunas A, Stephens T W, Nyce M R, Ohannesian J P, Marco C C, McKee L J, Bauer T L, Caro J F. N Engl J Med. 1996;334:292–325. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 9.Koyama K, Chen G, Lee Y, Unger R H. Am J Physiol. 1997;273:E708–E713. doi: 10.1152/ajpendo.1997.273.4.E708. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Hirose H, Ohneda M, Johnson J H, McGarry J D, Unger R H. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y T, Shimabukuro M, Wang M-Y, Lee Y, Higa M, Milburn J L, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1998;95:8898–8903. doi: 10.1073/pnas.95.15.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimabukuro M, Koyama K, Chen G, Wang M-Y, Trieu F, Lee Y, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y, Hirose H, Zhou Y-T, Esser V, McGarry J D, Unger R H. Diabetes. 1997;46:408–413. doi: 10.2337/diab.46.3.408. [DOI] [PubMed] [Google Scholar]
- 14.Frederich R C, Lollman B, Hamann A, Napolitano-Rosen A, Kahn B B, Flier J D. J Clin Invest. 1995;96:1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saladin R, DeVos P, Guerre-Milo M, Leturque A, Girard J, Staels B, Auwerx J. Nature (London) 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 16.Phillips M S, Liu Q, Hammond H A, Dugan V, Hey P J, Caskey C J, Hess J F. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 17.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 18.Koyama K, Shimabukuro M, Chen G, Wang M-Y, Lee Y, Kalra P S, Dube M G, Kalra S P, Newgard C B, Unger R H. J Clin Invest. 1998;102:728–733. doi: 10.1172/JCI3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unger R H. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 20.Milburn J L, Jr, Hirose H, Lee Y H, Nagasawa Y, Ogawa A, Ohneda M, BeltrandelRio H, Newgard C B, Johnson J H, Unger R H. J Biol Chem. 1995;270:1295–1299. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 21.Hirose H, Lee Y H, Inman L R, Nagasawa Y, Johnson J H, Unger R H. J Biol Chem. 1996;271:5633–5637. doi: 10.1074/jbc.271.10.5633. [DOI] [PubMed] [Google Scholar]
- 22.Shimabukuro M, Zhou Y-T, Levi M, Unger R H. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orci L, Ravazzola M, Baetens D, Inman L, Amherdt M, Peterson R G, Newgard C B, Johnson J H, Unger R H. Proc Natl Acad Sci USA. 1990;87:9953–9957. doi: 10.1073/pnas.87.24.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isseman I, Green S. Nature (London) 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 25.Wang M-Y, Koyama K, Shimabukuro M, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1998;95:714–718. doi: 10.1073/pnas.95.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M-Y, Koyama K, Shimabukuro M, Mangelsdorf D, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1998;95:11921–11926. doi: 10.1073/pnas.95.20.11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimabukuro M, Higa M, Zhou Y-T, Wang M-Y, Newgard C B, Unger R H. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 28.Shimabukuro M, Wang M-Y, Zhou Y-T, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1998;95:9558–9561. doi: 10.1073/pnas.95.16.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama K, Chen G, Wang M-Y, Lee Y, Shimabukuro M, Newgard C B, Unger R H. Diabetes. 1997;46:1276–1280. doi: 10.2337/diab.46.8.1276. [DOI] [PubMed] [Google Scholar]
- 30.Stein D T, Esser V, Stevenson B E, Lane K E, Whiteside J H, Daniels M B, Chen S, McGarry J D. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahan K, Sheikh F G, Khan M, Namboodiri A M, Singh I. J Biol Chem. 1997;273:2591–2600. doi: 10.1074/jbc.273.5.2591. [DOI] [PubMed] [Google Scholar]
- 32.Shimabukuro M, Koyama K, Lee Y, Unger R H. J Clin Invest. 1997;100:1750–1754. doi: 10.1172/JCI119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabinovitch A. J Lab Clin Med. 1992;119:455–456. [PubMed] [Google Scholar]
- 34.Montague C T, Farooqi I S, Whitehead J P, Soos M A, Rau H, Wareham N J, Sewter C P, Digby J E, Mohammed S N, Hurst J A, et al. Nature (London) 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 35.Shimomura I, Hammer R E, Richardson J A, Ikemoto S, Bashmakov Y, Goldstein J L, Brown M S. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moitra J, Mason M M, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbuam L, Lee E, Aoyama T, Eckhaus M, et al. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burant C F, Sreenan S, Hirano K, Tai T A, Lohmiller J, Lukens J, Davidson N O, Ross S, Graves R A. J Clin Invest. 1997;100:2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyahara R, Tsutamura C, Sugihara M. Hiroshima J Med Sci. 1965;14:31–39. [PubMed] [Google Scholar]
- 39.Chen H, Charlat O, Tartaglia L A, Woolf E A, Weng X, Ellis S J, Lakey N D, Culpepper J, Moore K J, Breitbart R E, et al. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 40.O’Rahilly S. Nature (London) 1998;392:330–331. doi: 10.1038/32769. [DOI] [PubMed] [Google Scholar]
- 41.Lieber C S, Spritz N, DeCarli L M. J Clin Invest. 1966;45:51–62. doi: 10.1172/JCI105323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fontan A, Battin J J, Kermarec J, Mortureux Y, Alberty J. Arch Fr Pediatr. 1965;22:1116–1117. [PubMed] [Google Scholar]
- 43.Obeid L M, Linardic C M, Karolak L A, Hannun Y A. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 44.Shimabukuro M, Koyama K, Lee Y, Unger R H. J Clin Invest. 1997;100:1750–1754. doi: 10.1172/JCI119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Correa P. Cancer Res. 1975;49:3395–3397. [PubMed] [Google Scholar]
- 46.Rashid A, Pizer E S, Moga M, Milgraum L Z, Zahurak M, Pasternack G R, Kuhajda F P, Hamilton S R. Am J Pathol. 1997;150:201–208. [PMC free article] [PubMed] [Google Scholar]
- 47.Saez E, Tontonoz P, Nelson M C, Alvarez J G, Ming U T, Baird S M, Thomazy V A, Evans R N. Nat Med. 1998;9:1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 48.Lefebvre A M, Chen I, Desreumaux P, Najib J, Fruchart J C, Geboes K, Briggs M, Heyman R, Auwerx J. Nat Med. 1998;9:1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 49.DuBois R N, Gutpa N, Brockman J, Reddy B S, Krakow S L, Lazar M A. Carcinogenesis. 1998;19:49–53. doi: 10.1093/carcin/19.1.49. [DOI] [PubMed] [Google Scholar]